Abstract
Glucoamylase obtained from Rhizopus sp. is frequently preferred for certain applications of starch modification or saccharification. The predominant enzyme, which contains a starch-binding domain on the amino terminus, has been previously characterized from several species. Additionally, the cDNA encoding this protein was cloned and found to show 100% identity to a R. oryzae strain 99-880 that has recently been sequenced by the Broad Institute of Harvard and Massachusetts Institute of Technology. Analysis of this genome indicates coding regions for two additional glucoamylase genes, amyC and amyD, lacking a starch-binding domain. The two genes encode proteins that are approximately 50% identical to the catalytic region of the AmyA protein and 67% identical to each other. The predicted AmyC and AmyD proteins contain the highly conserved signature sequences of Family 15 glycoside hydrolases. The two genes appear to be transcriptionally expressed in cultures grown in fermentable and gluconeogenic carbon sources. The predicted 49.7-kD AmyC and 48.8-kD AmyD proteins were expressed in several different ways using Pichia pastoris. When the sequence for putative secretion signal was left intact, glucoamylase activity was detected in the crude cell extracts, but no activity was present in the growth medium. However, replacement of this region with the yeast alpha-secretion signal resulted in secretion of active glucoamylase that was able to degrade soluble starch.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Glucoamylase (α-1,4-glucan glucohydrolase, amyloglucosidase, EC 3.2.1.3) has long been of considerable importance to the fermentation and food industries for saccharification of starch/amylopectin. The enzyme consecutively hydrolyzes α(1-4) glycosidic bonds from the nonreducing ends of starch, resulting in the production of glucose. It also has a limited ability to produce glucose through the hydrolysis of amylopectin α(1-6) linkages. Many commercial glucoamylase enzyme preparations are derived from Aspergillus and Rhizopus sp., due to their near complete conversion of starch to glucose.
Glucoamylase has been isolated and characterized from a number of Rhizopus species [12–14]. Takahashi et al. [12, 13] identified three different glucoamylase enzymes with the ability to digest gelatinized starch in a commercial Rhizopus extract. However, only the largest of the three enzymes was able to bind raw starch. Partial protein sequencing later demonstrated the two smaller fragments were likely the result of limited proteolytic cleavage [12, 13].
It is now widely accepted that R. oryzae (syn. R. arrhizus) can be divided into two different taxonomic groups, Type-I and Type-II, based on differences in phenotypes (e.g., acid production), rDNA internal transcribed regions [1], and genomic structure [10]. A cDNA fragment encoding the predominant glucoamylase protein, AmyA, with a starch-binding domain (SBD) on the amino terminus was isolated from R. oryzae (syn. R. arrhizus) [2]. It appears that this gene is present in both subgroups of R. oryzae [8]. Additionally, R. oryzae strain NRRL 395, a Type-I strain, has a second glucoamylase gene highly homologous to amyA, except that it lacks the N-terminal SBD [8]. This second glucoamylase gene was found to be transcriptionally regulated in a manner similar to the amyB gene of Aspergillus [5]. Despite high homology to amyA in the catalytic region, no glucoamylase activity could be detected from the recombinant protein, leaving the function of this protein presently unclear.
Recently, the genome of R. oryzae strain 99-880 was sequenced as a part of the Fungal Genome Initiative [9]. Analysis of the genome confirms that it has a glucoamylase gene with 100% homology to that described by Ashikari et al. [2]. In addition, a search of the database uncovered two hypothetical genes with high similarity to family 15 glycoside hydrolases, with neither gene containing an N-terminal SBD. To determine whether the two hypothetical proteins are indeed active glucoamylase enzymes, we have amplified and cloned the processed genes from cDNA prepared from R. oryzae 99-880. The two genes, amyC and amyD, while containing the conserved signature sequences of family 15 glycoside hydrolases, are divergent from the glucoamylase genes previously isolated from R. oryzae. We demonstrate that both amyC and amyD encode active enzymes and appear to be transcriptionally expressed in a manner largely different from previously characterized Rhizopus glucoamylase genes.
Materials and Methods
Strains and Media
Media components and chemicals were obtained from Sigma Chemical (St. Louis, MO) unless otherwise noted. R. oryzae strain 99-880, deposited in the Fungal Genetic Stock Center as FGSC 9543, was the source of RNA for all experiments. Shake flask cultures of 99-880 were grown in 50-mL minimal RZ1 media [11] and the desired carbon source at 1% (wt/vol). Cultures were started by inoculation of approximately 1E+06 spores and then incubated at 30°C, 250 rpm. Escherichia coli strain TOP10 (Invitrogen, Carlsbad, CA) was used for construction and propagation of cDNA clones. The wild-type Pichia pastoris strain X-33 (Invitrogen) was used for protein expression studies.
Isolation of amyC and amyD cDNA
The two glucoamylase genes were isolated by polymerase chain reaction (PCR) amplification of cDNA prepared from RNA isolated from R. oryzae 99-880 cultured for 24 h, as above, in the presence of soluble potato starch. Primers were designed according to locus RO3G_00127.1, amyC, and locus RO3G_06101.1, amyD, of the Rhizopus oryzae Genome database [9]. The amyC cDNA was amplified using Pfu Taq polymerase (Stratagene, LaJolla, CA) with the forward primer, 5′-CGGAATTCATGTTTA TAGGGTATCTGTTACTAAC-3′ and reverse primer 5′-CCTCTAGA CCAGTAGATGTGATCAC-3′. The amyC cDNA was also amplified without the putative signal sequence as predicted by the SignalP program [3] using the 5′ primer: 5′-CGGAATTCACCCATATTTTTG ATATTGTG-3′. The amplification primers contain an engineered EcoRI site and XbaI site in the forward and reverse primers, respectively. In addition, the reverse primer lacks the native stop codon and is designed to be in frame with the C-terminal 6X His tag found in the Pichia pPICZ expression vectors. The amyD cDNA was amplified in a similar manner with the forward and reverse primers 5′-CGGAATTCATGTTTATAGG-3′ and 5′-CCTCTAGACCAAAAG CAACCGAGT-3′. The amyD cDNA was also amplified without the putative signal sequence as predicted by the SignalP program [3] using the same reverse primer as listed above and the following forward primer: 5′-CGGATTTCAGCAGTATCTTTAAAAAG-3′. The amplified cDNA was TA cloned into pCR2.1 and transformed into Escherichia coli TOP10 according to the manufacturer’s protocol (Invitrogen; Carlsbad, CA). Individual amyC and amyD cDNA clones were isolated and sequenced to confirm the fidelity of the sequence.
Expression of amyC and amyD in Pichia pastoris
The amyC and amyD pCR2.1 constructs were digested with EcoRI and XbaI and the resulting cDNA fragments were ligated into Pichia expression vector pPICZB for internal expression and pPICZalphaA for secreted expression of the enzymes. The amyC and amyD constructs used for secreted expression using expression vector pPICZalphaA do not contain the putative signal sequence. After sequencing to confirm the fidelity of the constructs, the amyC and amyD expression constructs were linearized with PmeI and transformed into P. pastoris strain X-33 (Invitrogen) using electroporation according to the manufacturer’s protocol. Transformants were cultured on YPDS + Zeocin plates (1% [wt/vol] yeast extract, 2% [wt/vol] peptone, 2% [wt/vol] dextrose, 1 M sorbitol, and 100 μg/mL Zeocin) at 30°C. After 3 days, 10 isolates of each transformation were chosen at random for expression studies. Transformants were initially grown in 3 mL cultures containing YPD + Zeocin media for 24 h at 250 rpm and 30°C. The cultures were harvested, washed, and transferred to 3 mL of methanol induction media (2.8% [wt/vol] yeast nitrogen base with ammonium sulfate, 100 mM potassium phosphate, pH 6.0, 4 × 10-5% biotin, and 1% [wt/vol] methanol) to induce expression for 48 h at 30°C and 250 rpm. Individual Pichia isolates of amyC and amyD in the pPICZB and pPICZalphaA were also spotted on plates containing methanol induction media containing 0.25% soluble starch and 1.5% agar. The agar plates were incubated at 30°C for 96 h then stained with KI/I2 reagent, 0.5 M KI/ 1 mM I2, then stored at 4°C for 24 h.
Transcriptional Analysis of amyC and amyD
Total RNA was isolated from mycelia collected from 50-mL shake flask cultures grown for 24 h, 30°C, 250 rpm on RZ1 minimal media containing one of the following energy sources: glucose, glycerol, maltose, fructose arabinose, cellobiose, soluble starch, pullulan, or xylose. The mycelia were disrupted using glass beads [11] and the total RNA purified using the RNeasy Total RNA kit (Qiagen, Valencia, CA) followed by DNase treatment with Turbo DNA-free (Ambion, Austin, TX) for 30 min. RNA quality and quantity was confirmed using the 2100 Bioanalyzer Lab-on-Chip system (Agilent Technologies, Wilmington, DE). Reverse transcription (RT) was performed using the SuperScript III first-strand synthesis system (Invitrogen) and primed using the reverse primers listed above to obtain the amyC and amyD cDNA. Total RNA, 500 ng, of similar quality isolated from the respective cultures was used as template. The first strand synthesis reaction was carried out at 50°C for 50 min. Upon completion of the RT reaction, 1 μL of RNaseH was added to the reaction and incubated for 20 min at 37°C. The first strand cDNA was then amplified using EconoTaq polymerase (Lucigen, Madison, WI) with 5 μL of the RT reaction as template, and the forward and reverse primers listed above to amplify the full-length amyC and amyD cDNA. The RT-control reactions were set up in a similar manner, except no reverse transcriptase was added to the RT reaction. PCR reactions were performed using the following cycling conditions: 95°C; 30 s, 45°C annealing amyC; 46°C, amyD; 30 s, and 72°C extension; 1 min 45 s for 30 cycles.
Results and Discussion
Characterization of the Putative Glucoamylase Genes
Two hypothetical proteins with sequence similarity to Family 15 glycoside hydrolases were detected in a search of the Rhizopus genome database [9]. The two genes, amyC and amyD, encoding the hypothetical glucoamylases are 1495 bp and 1471 bp, respectively, with each gene predicted to have two introns located in the same position and spliced in an identical manner [9]. Further sequencing of amyC and amyD cDNA clones constructed and isolated from RNA of R. oryzae 99-880 cultured with soluble starch as the energy source, confirmed the presence and splicing of the two introns. The introns in amyC and amyD are located in different positions in comparison to the previously isolated Rhizopus amy genes. The putative genes do not possess an N-terminal SBD found in the previously isolated amyA gene and in this regard, are similar to the recently isolated amyB gene from R. oryzae NRRL 395 [8]. The amyC gene encodes a 459 amino acid protein with a putative 21 amino acid signal sequence for a mature peptide of 438 amino acids and a predicted molecular weight of 49.7 kD (Fig. 1). The amyD gene encodes a 453 amino acid protein, but is predicted to have a 24 amino acid signal peptide by the SignalP 3.0 program [3], resulting in a mature peptide of 429 amino acids and a molecular weight of 48.8 kD (Fig. 1). The amyC and amyD predicted proteins share 67% identity by Lippman Pearson methods [6], but are only 46% and 48% identical, respectively, to the amyB gene and the catalytic region of amyA gene from R. oryzae NRRL 395 (data not shown). While the previously identified amy genes are highly homologous, it is not unprecedented for fungal species to have divergent sequences as the gla genes of Aspergillus oryzae share only 52% identity [4]. Despite the divergence among the three glucoamylase genes lacking the N-terminal starch binding domain and amyA from Rhizopus, they all share extensive homology with respect to the signature sequences of Family 15 glycoside hydrolases (Fig. 1). The putative AmyC protein contains the conserved residues reportedly required for enzymatic activity. However, the AmyD protein, while containing the conserved tryptophan and consecutive glutamic acid residues, contains an aspartic acid residue in the active site rather than the conserved asparagine residue (Fig. 1).
Alignment of Rhizopus oryzae 99-880 deduced glucoamylase proteins. The upper sequence is the deduced amyC amino acid sequence and the lower is the amyD deduced amino acid sequence. The outlined residues denote conserved regions of family 15 glycoside hydrolase family members. The asterisks highlight residues required for enzymatic activity. The + symbol indicates the first amino acid of the expected mature peptide.
Expression of amy Genes in Pichia
To determine whether the amyC and amyD genes coded for active enzymes, we cloned the cDNA of the two genes using amyC and amyD specific primer sets. The PCR amplified 1377 bp amyC cDNA and 1359 bp amyD cDNA were cloned into Pichia expression vector PICZB for internal expression of the enzymes and transformed into Pichia strain X-33. After 72 h of methanol induction, cells were harvested and crude cell extracts of amyC and amyD as well as the culture media of amyD were tested for glucoamylase activity. Although activity was detected in the crude cell extracts of amyC and amyD transformants, no glucoamylase activity was detectable in the culture media of amyC or amyD transformants (data not shown). Although Pichia does recognize the secretion signal of the previously isolated amy genes from Rhizopus [7, 8], the significantly different signal sequence of amyC and amyD is apparently not recognized by Pichia. Alternatively, it is possible the signal sequence acts to direct these two enzymes to an internal organelle in Rhizopus, rather than outside the cell.
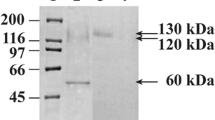
In an effort to increase enzyme production and to possibly overcome any potential toxic effects of the AmyC and AmyD proteins toward Pichia, the amyC and amyD cDNA was inserted into Pichia expression vector pPICZalpha A for secreted expression. The constructs did not contain the putative signal sequence to avoid potential negative interaction with the alpha mating factor signal sequence, which is in frame with the coding sequence of the two genes. As shown in Fig. 2, secreted Pichia-expressed AmyC (Fig. 2, 3–5) and AmyD (Fig. 2, 6–8) were able to degrade starch. The untransformed, as well as the vector-only transformant do not show any evidence of a starch activity (Fig. 2, 1–2). In addition, no starch-degrading activity is demonstrated with the amyC and amyD constructs designed for internal expression, confirming our earlier results that the amyC and amyD signal sequence does not result in secreted enzyme in the Pichia system (Fig. 2, 9–14). The amyD constructs appear to either produce more enzyme or a more active enzyme based on the radius of the halos produced on the starch plate (Fig. 2). However, based on the work presented here, we cannot make a definitive conclusion.
Transcriptional Analysis of amyC and amyD
To gain a clearer understanding of the regulation and potential clues to the purpose of the glucoamylase genes, RT-PCR was performed with total RNA isolated from R. oryzae 99-880 mycelia grown in shake flask cultures using various energy sources. Amplification product from both genes was detected when 99-880 was cultured in the presence of all nine carbon sources (Fig. 3). The product obtained in each reaction was of the anticipated size for processed transcript and is presumed to be from expression of amyC and amyD, because no amplification product was detectable in reactions without performing the RT reaction (Fig. 3).
amyC and amyD Reverse transcription–polymerase chain reaction assays using total RNA isolated from cultures grown on multiple carbon sources. Assays were performed as described in Materials and Methods and run on a 1% agarose gel. The abbreviations for the carbon sources used in the different cultures are as follows: Ara, arabinose; Cel, cellobiose; Fru, fructose; Glu, glucose; Gly, glycerol; Mal, maltose; Pul, pullulan; SS, soluble starch; and Xyl, xylose.
We have demonstrated that the hypothetical Family 15 glycoside hydrolase genes identified in the sequencing of the R. oryzae 99-880 do indeed encode for active glucoamylase genes. The amyC and amyD genes encode enzymes without an N-terminal SBD and may explain differences found in raw starch binding with commercial preparations of Rhizopus glucoamylase.
Literature Cited
Abe A, Sone T, Sujaya IN, et al. (2003) rDNA ITS sequence of Rhizopus oryzae: its application to classification and identification of lactic acid producers. Biosci Biotechnol Biochem 67:1725–1731
Ashikari T, Nakamura N, Tanaka Y, et al. (1986) Rhizopus raw starch degrading glucoamylase: its cloning and expression in yeast. Agric Biol Chem 50:957–964
Bendtsen JD, Nielsen H, vonHeijne G, et al. (2004) Improved prediction signal peptides: SignalP 3.0. J Mol Biol 340:783–795
Hata Y, Ishida H, Ichikawa E, et al. (1998) Nucleotide sequence of an alternative glucoamylase-encoding gene (glaB) expressed in solid-state culture of Aspergillus oryzae. Gene 207:127–134
Ishida H, Hata Y, Ichikawa E, et al. (1998) Regulation of the glucoamylase-encoding gene (glaB), expressed in solid-state culture (Koji) of Aspergillus oryzae. J Ferm Bioeng 86:301–307
Lipman DJ, Pearson WR (1985) Rapid and sensitive protein similarity searches. Science 227:1435–1441
Liu SH, Chou WI, Sheu CC, Chang MDT (2005) Improved secretory production of glucoamylase in Pichia pastoris by combination of genetic manipulations. Biochem Biophys Res Commun 326:817–824
Mertens JA, Skory CD (2007) Isolation and characterization of a second glucoamylase gene without a starch binding domain from Rhizopus oryzae. Enzyme Microbiol Tech 40:874–880
Rhizopus oryzae Sequencing Project. Broad Institute of Harvard and MIT (http://www.broad.mit.edu). Accessed August 2006
Saito K, Saito A, Ohnishi M, Oda Y (2004) Genetic diversity in Rhizopus oryzae strains as revealed by the sequence of lactate dehydrogenase genes. Arch Microbiol 182:30–36
Skory CD (2002) Homologous recombination and double strand break repair in the transformation of Rhizopus oryzae. Mol Genet Genomics 268:397–406
Takahashi T, Tsuchida Y, Irie M (1978) Purification and some properties of three forms of glucoamylase from a Rhizopus species. J Biochem 84:1183–1194
Takahashi T, Tsuchida Y, Irie M (1982) Isolation of two inactive fragments of Rhizopus sp. glucoamylase: relationship among three forms of the enzyme and the isolated fragments. J Biochem 92:1623–1633
Yu R, Hang YD (1991) Purification and characterization of a glucoamylase from Rhizopus oryzae. Food Chem 40:301–308
Author information
Authors and Affiliations
Corresponding author
Additional information
The mention of firm names or trade products does not imply that they are endorsed or recommended by the U.S. Department of Agriculture over other firms or similar products not mentioned.
Rights and permissions
About this article
Cite this article
Mertens, J.A., Skory, C.D. Isolation and Characterization of Two Genes That Encode Active Glucoamylase Without a Starch Binding Domain from Rhizopus oryzae . Curr Microbiol 54, 462–466 (2007). https://doi.org/10.1007/s00284-006-0655-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-006-0655-8