Abstract
A crude biosurfactant solution was produced by Pseudomonas aeruginosa growing on agroindustrial wastes as the substrate and used to study its effect on hydrocarbon biodegradation by the indigenous soil microflora under laboratory conditions. Two concentrations were studied at first and 1 mg of biosurfactant/g of soil showed to be the most efficient for the total petroleum hydrocarbon reduction, which reached 85% at the first 20 days in soil microcosms. Respirometric and microbial analyses showed that the biosurfactant added did not have toxic effects over the microbial population. The use of a biosurfactant for bioremediation has been limited because of its high cost production. Biosurfactants produced from cost-free by-products combines waste minimization with economic potential bioremediation process.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Petroleum hydrocarbons release is a widespread cause of environment contamination and its consequences for the living organisms is a problem of a great magnitude. It is estimated that ∼0.08–0.4% of the total worldwide production of petroleum eventually reaches the oceans [3]. Several oil spill accidents in recent years have resulted in significant contamination of the environment. In Brazil, many accidents involving petroleum hydrocarbons, such as gasoline and fuel oil has caused serious environmental problems. In 1998, 1200 m3 of fuel oil were released because of pipeline corrosion in Cubatão/SP. The corrosion of pipelines was also responsible for 1300 m3 of fuel oil discharged in Baía de Guanabara, Rio de Janeiro, which had been strongly contaminated before by other oil spills.
Such incidents have intensified attempts to develop procedures and technologies for combating oil pollution in the environment [3]. Soil that is accidentally contaminated with petroleum hydrocarbons can be remediated by physical, chemical, or biological methods. However, new trends in soil and water restoration avoid introducing synthetic chemicals. Among the remediation techniques available for contaminated sites, in situ bioremediation is regarded as environmentally friendly because it preserves the soil structure, requires little energy input, and involves the complete destruction or immobilization of the contaminants rather than their transfer from one environment compartment to another, as occurs in physical–chemical treatments [9, 15]. Biological remediation is economically and politically attractive and has shown promising results in the treatment of soil contaminated with organic compounds, particularly with petroleum hydrocarbons. However, the limiting step of hydrocarbon contaminants biodegradation is that they are poorly accessible to bacteria because of their low aqueous solubility [7].
Of the possible technological solutions to limited pollutant bioavailability, a promising one is the use of surfactants to mobilize the pollutant, because they increase the solubility of hydrocarbons by forming micelles. However, negative effects might also occur—for example, because these products might be toxic or because of their preferential biodegradation. Furthermore, surfactants might reduce the attachment of cells to substrates that are present as a separate phase, which can decrease degradation rates if attachment is needed for uptake [13]. The use of biologically produced surfactants, called biosurfactants, might overcome this problem, because they do not have harmful effects on the environment. Biosurfactants occur naturally in soil and the use of these products in bioremediation process might be more acceptable from a social point of view. In comparison with synthetic surfactants, a lower toxicity might be expected from most biosurfactants. A range of different biosurfactants have been studied for their use in hydrocarbon biodegration. Rhamnolipids produced by Pseudomonas aeruginosa have been one of the most widely studied biosurfactants. Several types of rhamnolipid produced by different P. aeruginosa strains have been characterized [1, 4]. Economy, however, is often the bottleneck for biotechnological products, especially in the case of biosurfactants. The success of biosurfactant production depends on the development of a less expensive process and the use of low-cost raw material, which accounts for 10–30% of the overall cost [10].
The aim of this work was to evaluate the effect of the addition of a nonsterile crude biosurfactant solution, produced at a low-cost from vegetable oil refinery waste, on petroleum-contaminated soil on a laboratory scale.
Material and Methods
Biosurfactant Production
A rhamnolipid biosurfactant was produced from P. aeruginosa LBI, isolated from petroleum-contaminated soil and maintained on nutrient agar slants. A 2% (v/v) cell suspension in 0.9% saline from an overnight culture on tryptcase soy agar was used to inoculate a mineral salt medium [4] The carbon source used at 2.5% (v/v) was soapstock (Cargill, Mairinque, Brazil). The final pH of the medium was adjusted to 6.8. Rhamnolipids were produced in a 5-L bioreactor, in a 60-h culture at 30°C, with an aeration rate of 2 L air/min, with a working volume of 3 L. The culture was stirred at 800 rpm. The rhamnolipid solution was obtained after the culture broth centrifugation for cell separation. Rhamnolipids were quantified from the supernatant as the rhamnose concentration, using rhamnose as a standard [6]. The rhamnolipid concentration was calculated as described elsewhere [5]. For the biosurfactant characterization, a pure extract of rhamnolipids, was recovered from the cell-free culture broth, as described by Reiling et al. [14]. To identify the chemical structure of the product, an aliquot of the purified extract was analyzed by high performance liquid chromatography-electro spray-mass spectroscopy (HPLC-ES-MS) at a –35 V, using a Waters 2690 Separation Module (Waters, Midford, MA, USA) as described by Benincasa et al. [4]. The analysis of the rhamnolipids showed a mixture of six homologues, with the pseudomolecular ions being between m/z 435 and 703. The homologues were found at the following concentrations: R2C10C10: 28,9%; R1C10C10: 23.5%; R2C10C12: 11.3%; R2C10C12:1: 23%; R1C10C12:1: 7.9%; R1C10C12: 5.5%. The use of liquid chromatograbhy (LC)-MS to the rhamnolipids mixture enables compounds to be identified and chromatographically unresolved pairs of congeners to be quantified.
Soil Samples
Soil was obtained from a petroleum-waste-contaminated site near Ribeirão Preto/SP, Brazil. The soil was composed by 8% clay, 62% silt, and 30% sand. The total organic matter was 16 g/kg and the soil density was 0.092 g/mL. The soil pH was 7.7 and the water content was 16.3%.
Biodegradation Experiments
The experiments were carried out in soil slurries and in soil microcosms. For both, soil samples were dried at room temperature, thoroughly homogenized, and sieved (<2 mm). The biosurfactant solutions used in all bioremediation experiments were composed by a nonsterile fermentation cell-free broth at a given concentration. The broth analysis showed that nitrogen was totally depleted and the carbon source was 98% consumed during the fermentation process.
Soil bioslurries
For the soil slurries (bioslurries), 50 g of soil were resuspended in 200 mL of distilled water (SA) in 400-mL aluminum recipients, which were maintained at room temperature and shaken periodically to provide proper aeration. The slurries were supplemented with two concentrations of biosurfactant (BS) solutions: 1 mg BS/g of soil and 3 mg BS/g of soil for the treatment BS1 and BS2, respectively. The slurries samples were taken after vigorous agitation and extracted for total petroleum hydrocarbons (TPH) determinations. For the abiotic losses determination, a sterile soil (autoclaved at 121°C and 1 atm for 30 min) was used (SE).
Soil microcosms
Microcosms were prepared in cardboard boxes covered with aluminum and then with plastic, each containing 300 g of soil. The water content was adjusted for 60% of the field hold capacity, with distilled water (MA) or with a biosurfactant solution with a concentration of 1 mg BS/ g of soil (MBS). This moisture content has been used in several bioremediation studies [11, 15]. The water content was adjusted periodically. The abiotic losses were determined with sterile soil.
Respirometric activity
In order to evaluate the potential metabolic activity and the toxic effects of the bisurfactant solution over the indigenous micro-organisms, respirometric activity was measured. The experiment was conducted with 20 g of soil, with the water content adjusted to 60% of the field holding capacity with distilled water (RA) or with a biosurfactant solution with a concentration of 1 mgBS/g soil (RBS). The amount of CO2 produced by each microcosm was determined as described by Sabaté et al. [15]. Sterile soil was used for abiotic losses determination (RE).
Microbial count
Bacteria cell number was estimated by plate count according to Gallego et al. [8], using a rich complex medium for growing heterotrophic bacteria and a synthetic one, impregnated with 0.2% of diesel, for the hydrocarbon degraders counting.
Contaminant determination
The TPH from 10 g of bioslurries and microcosms samples were Soxhlet extracted with n-hexane for 6 h with a 125-mL extraction flask. The bioslurries samples were vacuum filtered before extraction. The extracts were evaporated and the amount of recovered hydrocarbons was gravimetrically measured.
Results and Discussion
To demonstrate that a bioremediation technology is potentially useful, it is important that the ability to enhance the rate of hydrocarbon biodegradation be demonstrated under controlled conditions. The biodegradation potential can be evaluated using reactors or recipients with soil resuspended in aqueous solution (5–15%), called bioslurries, that offer some advantages over the studies that reproduce the natural conditions. With efficient agitation and aeration, the substrate bioavailability is enhanced and these experiments’ duration is reduced. The contamination reduction on these “treatability assays” is higher compared to solid-phase recipients or reactors because of the increased solid–liquid mass transfer [2]. The microbial population and the TPH were evaluated on the studied slurries before the treatment and after 80 days. Taking into account the abiotic losses, such as volatilization, verified in the slurries composed by sterile soil (SE), which corresponded to 42% of the total hydrocarbon content, the TPH reduction in the bioslurries BS1 was 97%, whereas in BS2, it was 81%, versus 62% in the bioslurry composed with water (SA). The microbial counts of the population in bioslurries with and without biosurfactant addition showed that the ratio of hydrocarbon degraders/heterotrophic bacteria increased with the addition of biosurfactant, but the viable micro-organisms decreased, which might be associated with the easily biodegradable carbon source reduction in the environment during the process. The micro-organism number depletion coincides with the TPH consumption presented earlier. The increase on heterotrophic counts is probably due to the metabolic compound release by the partial TPH biodegradation by the hydrocarbon degrader population and that would become the substrate for the heterotrophic bacteria. The persistence of the TPH in the soil even with an existing specialized population demonstrates that the pollutant was not available to the endogenous micro-organisms. The remarkable increase in the bioslurries reached in the presence of a biosurfactant indicated that sorption of TPH to soil was the limiting factor controlling the biodegradation of contaminants. The higher water-to-solid ratio and the efficient mixing in the system, together with the biosurfactant addition, increase the hydrocarbon dissolution rate, which is in accordance with the literature [2].
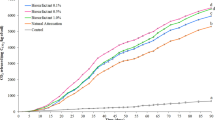
The most important effect of surfactants on the interactions between soil and pollutant is the stimulation of the mass transport of the pollutant from the soil to the aqueous phase, thus promoting the uptake by the micro-organisms. Nevertheless, the effects of these compounds on pollutant bioavailability are so complex that they range from stimulation to inhibition of desorption and biodegradation of polluting compounds [17]. Previous studies of the physicochemical properties of the rhamnolipidic biosurfactant produced by P. aeruginosa LBI demonstrated its potential use in the bioremediation process, but it was also demonstrated that these compounds have good antimicrobial activity against different micro-organism species [4], making it necessary to study the inhibition effect over soil indigenous populations. From the respirometric experiment it was possible to obtain information about the microbial metabolic activity during petroleum hydrocarbons biodegradation and about biosurfactant toxicity to the endogenous population. The evolution of the cumulative CO2 in the different microcosms is shown on Figure 1. Abiotic losses were measured and deduced from the values presented. The results indicated a greater increase in the respiratory activity when the biosurfactant solution was added, which can be associated with the increase in the carbon source (hydrocarbons) availability. The decrease of CO2 production at the time might be attributed to the exhaustion of the biodegradable hydrocarbons [2], which were rapidly used by the microbial metabolism, that entered a death phase because of the energy source absence. These results coincide with the microbial enumeration shown earlier.
Comparing the two microcosms, it is possible to observe that the biosurfactant addition did not present any toxic effect for the microbial population, as would have been expected if a chemical surfactant were added. The respirometric test provided the confirmation that the microbial population was metabolically active. The bioslurries and respirometric experiments indicated that the studied soil was suitable for the biorremdiation process and that the biosurfactant concentration of 1 mg BS/g soil was the most efficient on TPH reduction. In this way, further biodegradation studies on microcosms were conducted with 1 mgBS/g of soil.
The TPH reduction during the 67 days of the microcosm experiment is represented in Figure 2. The values are arithmetic means for three replicates per treatment. The initial TPH concentration was 24,437 mg/kg. The observed TPH biodegradation followed a biphasic behavior. A fast biodegradation occurred in the first 30 days of incubation. The biosurfactant addition accelerated the first phase, decreasing the adaptapion period observed in the microcosm without the product. The MBS treatment reached 85% of the TPH biodegradation in the first 20 days, versus 67% in the MA treatment, corrensponding to a difference of 4000 mg/kg of hydrocarbon content between the two treatments. Hydrocarbon reduction reached 92% in 30 and 60 days for MBS and MA treatments, respectively. The observed biodegradation behavior, with an initial decrease of the hydrocarbons, followed by a period with no relevant reductions is knowed as “hockey stick” phenomenon. This dynamics is explained by the depletion of inorganic nutrients, the decrease in the microbial population, the lower bioavailability, the unfavorable physicochemical conditions, and the increase in residual contaminants recalcitrance [15]. It was also observed that, in addition to the biosurfactant addition, the adjustment of the water content to 60% of the field holding capacity and the periodical agitation, promoting the microcosm aeration, contributed to hydrocarbon biodegradation. The humidity correction and aeration are well-know bioremediation techniques that stimulate endigenous microbial population metabolic activities.
The microbial counts of heterotrophic and hydrocarbon degrader micro-organisms are shown on Figures 3a and 3b, respectively. The heterotrophic population remained approximately constant during almost all of incubation time, showing a decrease in the last 10 days, which might be related to the lack of organic and inorganic nutrients, because the hydrocarbon degrader population presented an evolution during the experiment. The biosurfactant addition had a stimulating effect on the specialized population, which is probably due the increase in the carbon source bioavailability. The hydrocarbon degrader population increase during the first 30 days of incubation coincided with the fastest decrease in hydrocarbon concentration. Similar results have been presented elsewhere [16]. Nevertheless, this population appears to be stable until the end of the experiment: the TPH does not follow a proportional profile. A reduction in the biodegradation rates might be a consequence of the increase in the concentration of compounds with high molecular weight and, therefore, of higher recalcitrance, as a function of the increase of lower-molecular-weight compounds. This could also explain the heterotrophic population reduction at the end of the experiment, which might be related to accessibility problems and/or metabolization of residual contaminants.
The positive effect of biosurfactant on hydrocarbon biodegradation in contaminated sites have been reported. Cubitto et al. [7] and Moran et al. [12] demonstrated that biosurfactant from Bacillus subtilis O9 stimulated the growth of the population involved in the crude oil degradation and accelerated the biodegradation of aliphatic hydrocarbons. On the other hand, the addition of a synthetic surfactant, Tween-80 on contaminated soil did not show a satisfactory effect when compared to nutrient addition [15].
Despite of the advantages of microbial-produced surfactants over the chemical ones for bioremediation and other diverse applications, its use has been limited because of its high cost. However, if the biosurfactant can be produced from cost-free by-products, such as the rhamnolipids produced by P. aeruginosa cultivated on vegetable oil refinery waste, the process would combine waste minimization with economical biosurfactant production.
Conclusions
Rhamnolipids produced by P. aeruginosa LBI growing on agroindustrial wastes as substrates, added as a crude solution, stimulate hydrocarbon biodegradation and did not cause any toxic effects to the indigenous micro-organisms. Of the two concentrations studied, 1 mg of biosurfactant/g of soil was shown to be the most efficient. Biosurfactant reduced the adaptation phase of microbial population, increasing the TPH reduction to 20% in the first 20 days in the microcosms experiment.
Literature Cited
Abalos A, Pinazo A, Infante MR, Casals M, Garcia F, Manresa A (2001) Physicochemical and antimicrobial properties of new rhamnolipids produced by Pseudomonas at 10 from soy bean oil refinery wastes. Langmuir 17:1367–1371
Balba MT, Al-Awadhi N, Al-Daher R (1998) Bioremediation of oil-contaminated soil: microbiological methods for feasibility assessment and field evaluation. J Microbiol Methods 32:155–164
Banat IM, MAkkar RS, Cameotra SS (2000) Potential commercial application of microbial surfactants. Appl Microbiol Biotech 53:495–508
Benincasa M, Abalos A, Moraes IO, Manresa A (2004) Chemical structure, surface properties and biological activities of the biosurfactant produced by Pseudomonas aeruginosa LBI from soapstock. Antonie van Leeuw 85:1–8
Benincasa M, Contiero J, Manresa A, Moraes IO (2002) Rhamnolipid production by Pseudomonas aeruginosa LBI growing on soapstock as the sole carbon source. J Food Eng 54:283–288
Chandrasekaran EV, Bemiller JN (1980) Constituent analysis of glycosaminoglycans. In: Wrhiste L, Wolfrom ML (eds.). Methods in carbohydrate chemistry, vol III. Academic Press, New York, Pages 89–96
Cubitto MA, Morán AC, Commendatore M, et al. (2004) Effects of Bacillus subtilis O9 biosurfactant on the bioremediation of crude oil-polluted soils. Biodegradation 15:281–287
Gallego JLR, Loredo J, Llamas JF, et al. (2001) Bioremediation of diesel-contaminated soils: evaluation of potential in situ techniques by study of bacterial degradation. Biodegradation 12:325–335
HolledeR J, Althoff K, Mundt M, Dott W (2003) Assessing the microbial activity of soil samples, its nutrient limitation and toxic effects of contaminants using a simple respiration test Chemospehre 53(3):269–275
Makkar RS, Cameotra SS (2002) An update on use of unconventional substrates for biosurfactant production and thir new applications. Appl Microbiol Biotech 58:428–434
Margesin R, Schinner F (1997) Efficiency of indigenous and inoculatd cold-adaptede soil microorganisms for bidegradation of diesel oil in alpine soils. Appl Environ Microbiol 63:2660–2664
Morán AC, Commendatore M, Esteves JL, Siñeriz F (2000) Enhancement of hydrocarbon waste biodegradation by addition of a biosurfactant from Bacillus subtillis O9. Biodegradation 11:65–71
Noordman W, Janseen DB (2002) Rhamnolipid stimulates up-take of hydrophobic compounds by Pseudomonas aeruginosa. Appl Environ Microb 68:4502–4508
Reiling HEE, Thanei-Wyss U, Guerra-santos LH, et al. (1986) A pilot plant production of rhamnolipid biosurfactant by Pseudomonas aeruginosa. Appl Environ Microbiol 51:985–989
Sabaté J, Viñas M, Solanas AM (2004) Laboratory-scale bioremediation experiments on hydrocarbon-contaminated soils. Int Biodeter Biodegrad 54:19–25
Song HG, Wang X, Bartha R (1990) Bioremediation potential of terrestrial fuel spills. Appl Environ Microbiol 56:652–656
Volkering F, Breure AM, Rulkens WH (1998) Microbiological aspects of surfactant use for biological soil remediation. Biodegradation 8:401–417
Akcnowledgments
This work was supported by FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo) and IFS (International Foundation for Science– Sweeden). The soapstock was kindly donated by Cargill-Mairinque/SP, Brazil.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Benincasa, M. Rhamnolipid Produced from Agroindustrial Wastes Enhances Hydrocarbon Biodegradation in Contaminated Soil. Curr Microbiol 54, 445–449 (2007). https://doi.org/10.1007/s00284-006-0610-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-006-0610-8