Abstract
Herpes simplex virus (HSV)-1 keratitis (HSK) is a sight-threatening ocular infection with worldwide occurrence. A prompt laboratory diagnosis is often very useful. The purpose of this study was to evaluate molecular methods as rapid diagnostic tools compared with cell culture of HSK. Corneal scrapings from patients with clinically suspected HSK were tested by direct immunofluorescence assay (IFA) for HSV-1 antigen and by polymerase chain reaction (PCR) for HSV-1 DNA, and an attempt for viral isolation was performed on Vero cell line culture. Positive samples by cell culture were 20.8%, whereas PCR was positive in 29.2%, and IFA was positive in 33.3%. IFA had better sensitivity (80%) and negative predictive value (81.8%) than PCR (70% and 76.9%, respectively); however, PCR had better specificity (71.4%) and positive predictive value (63.6%). This indicates that a combination of cell culture, IFA and PCR constitutes the best set of tools for diagnosis of clinically suspected cases of HSK. Documented infection can be further assessed by cell-culture technique or PCR depending laboratory availability.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Herpes simplex virus (HSV)-1 keratitis (HSK) is a sight-threatening ocular infection and occurs worldwide, causing several complications and increasing the risk of severe corneal thinning and perforation [1]. For these reasons, a prompt laboratory diagnosis is often very useful [2].
Although virus isolation is considered a standard procedure for diagnosing viral infection, this procedure is relatively time-consuming, requiring 7 to 28 days depending on the availability of viable infectious material, which usually needs to be transferred to a special virology laboratory for processing [3].
Rapid diagnostic tests that allow results within hours have been reported in the past two decades [4, 5]. These tests have been based on immunologic detection of HSV-1 antigen [5, 6] or viral DNA by polymerase chain reaction (PCR) [7, 8].
A simple and rapid PCR procedure was developed for simultaneous detection and typing of herpes simplex virus (HSV)-1 and -2 [9]. It is possible to detect and type HSV using two primers pairs in a simultaneous double-PCR reaction, in which the type of HSV present is determined according to the band pattern obtained by agarose gel electrophoresis [9]. The advent of real-time PCR protocols now enables rapid turnaround with minimal “hands-on” time [10].
The purpose of this study was to evaluate the efficacy of rapid direct antigen detection of HSV-1 by direct immunofluorescence assay (IFA) and PCR for HSV-1 DNA compared with Vero cell line culture for the diagnosis of HSK.
Patients and Methods
Patient selection
Forty-eight patients shown to have a clinically typical dendritic epithelial defect in the cornea with underlying subendothelial infiltrate pathognomonic of viral keratitis comprised the study group. Informed written consent was obtained from all participants, and the Ethics Committee of Mansoura University Hospital approved the study.
Sample processing
Using standard techniques and with the patient having received topical anesthesia, three corneal scrapings were collected under slit lamp magnification with a sterile blade (no. 15) on a Bard-Parker handle. Scraped material was smeared onto a glass slide for direct IFA, and another sample was placed in 0.5 ml 10% phosphate buffer solution (pH 7.2) and stored at –70°C until processed for PCR. Scrapings for viral culture were transported on Dulbeccos’s minimal essential medium (pH 7.4; 200 IU/ml penicillin G, 200 μg/ml streptomycin sulfate, and 5 μg/ml amphotericin B).
Direct IFA
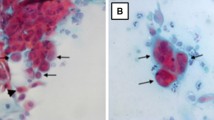
IFA was done by ACHERP kit (Vircell, Granada, Spain). Smears were fixed in cold acetone and reacted with monoclonal fluorescein isothiocyanate–labeled anti–HSV-1 specific for glycoprotein of herpes simplex capeside. The results were assessed under a reflected light fluorescence microscope at 250 × with wavelength of 450 nm. Positive staining for HSV was represented and qualified by the presence of ≥1 epithelial cells exhibiting specific bright apple-green fluorescence. A presumptive negative result was indicated by the absence of fluorescence in a minimum sampling of 20 basal cells.
Cell culture
For cell culture, scrapings were inoculated into monolayered Vero cell lines, [11] which were previously cultivated on 24-well plates, and examined under inverted microscope for the presence of cytopathic effects typical for HSV-1 by Giemsa stain [11]. The cytopathic effect begins as clusters of enlarged, rounded refractive cells and spreads to involve the entire monolayer, usually within 48 hours [12]. The results were confirmed by IFA and enzyme immunoassay (EIA) assays for HSV-1 antigen (home made).
PCR
For PCR, DNA was extracted from the corneal scrapings with the commercially available Qiagen kit (GmbH-Hilden). Primers were designed to bracket a well-conserved region in the DNA polymerase gene. Primer for HSV-P1 (5′-GTGGTGGACTTTGCCAGCC TGTACCC-3′) was used to amplify HSV-1 [13].
Each reaction was performed in a 0.6-ml tube (PRE 050; Diamed) in a total volume of 50 μl overlaid with 50 μl of mineral oil. Each reaction contained 5 μl 10 × Cetus buffer II (Perkin-Elmer), 5 μl 25 mM MgCl2, 5 μl deoxynucleoside triphosphate mixture (each deoxynucleoside triphosphate at a concentration of 2 mM; Pharmacia), 2.5 μl dimethyl sulfoxide (Sigma), 37.5 pmol of each primer, 0.5 μl (2.5 U) Amplitaq Gold (Perkin-Elmer), and 40 μl molecular-grade double-distilled water. The master mixture was then divided into aliquots and placed into tubes, to which 10 μl template DNA dissolved in molecular-grade water was added. Sterile distilled water was used as negative control. PCR was performed on a Stratagene Robocycler 40 instrument.
With the HSV-P1 primer pair, the cycling parameters were initial preincubation at 95°C for 12 minutes; then 3 cycles consisting of 95°C for 1 minute, 60°C for 1 minute, and 72°C for 1 minute; then 37 cycles of 95°C for 1 minute, 55°C for 45 seconds, and 72°C for 1 minute; and then a final incubation at 72°C for 3 minutes. A 10-μl volume of each reaction mixture was subjected to electrophoresis. The amplicon (150 bp) for HSV-1 was resolved on a 1.5% agarose gel and visualized using ethidium bromide (0.5 μg/ml) under ultraviolet illumination.
The laboratory staff performing the tests were blinded to the clinical diagnosis. Laboratory-confirmed keratitis was defined as any culture-positive specimen or any specimen that had at least two positive nonculture tests; if either of these was present, a patient was considered infected. For example, a patient who was positive by both PCR and IFA but negative by culture would be classified as infected.
Results
Forty-eight patients shown to have a clinically typical dendritic epithelial defect in the cornea with underlying subendothelial infiltrate pathognomonic of viral keratitis comprised the study group. They were 30 men and 18 women, and the mean age was 49.2 years (SD 17.5).
Samples were corneal scrapings studied for the presence of HSV-1 by direct IFA, PCR, and viral isolation by cell culture. In our study, the positive cases for HSV-1 found by cell culture or by ≥2 laboratory tests were 20 of 48 (41.7%). The positive cases by cell culture were 10 of 48 (20.8%). Both direct IFA and PCR accurately detected more positive cases (33.3% and 29.2%, respectively) than cell-culture technique (Fig. 1). Fig. 2 demonstrates positive IFA for HSV-1 antigen in corneal scrapings, and Fig. 3 demonstrates positive PCR bands for HSV-1 DNA.
IFA showed higher sensitivity (80%) and negative predictive value (81.8%) than PCR (70% and 76.9%, respectively), however, PCR had better specificity (71.4%) and positive predictive value (63.6%) (Table 1). IFA required approximately 2 hours for staining; PCR required approximately 4 hours to perform; and cell culture required approximately 1 week to reach completion.
Discussion
Herpetic ocular disease is a major cause of blindness. Rapid and accurate diagnosis is essential for prompt and proper treatment [7]. This study was undertaken to find an optimum combination of laboratory tests that would help to establish rapid diagnosis of suspected HSK. For better resolution of this issue, ≥2 positive laboratory tests (IFA and PCR) were chosen for diagnosis of HSK.
In our study, positive cases by cell culture were 20.8% and by direct IFA were 33.3%. Similar rates were obtained by Pramod et al. (1998) [6]: Virus-specific antigen was detected by indirect IFA in 22 of 70 (31.4%) cases, and only 20% [14] of the cases had positive viral isolation. In contrast, lower rates for cell-culture positivity (12.1%) were reported in cases prediagnosed as herpetic keratitis [14].
Cell-culture isolation requires viable organisms necessitating special transport media and prompt transport of specimens between patient and laboratory. This process is difficult and time consuming. In contrast, antigen-detection techniques circumvent the requirements for preservation of infectivity required for culture, but they can produce false-negative and -positive results [15].
In our study, PCR accurately detected more positive cases (29.2%) than cell-culture technique. Khodadoost et al. [8] reported that PCR for HSV DNA was identified in 88% (23 of 25) of suspected herpetic keratitis patients, whereas viral isolation rate of detection was 12% (3 of 25).
It appears that both direct IFA and PCR are rapid tools for detection of HSV-1 in corneal scrapings. Both tests, in conjunction with cell culture, can serve as supplemental methods for diagnosis of HSK to diagnose all possible suspected cases.
In the present work, the positive cases for HSV-1 found by ≥2 laboratory tests were 41.7%. The reported rates of HSV-1 infection in keratitis ranged widely, from 78.6% [16] to 23% [17] and 15% [18]. This difference could be attributed to the difference in the “gold-standard” technique used as a basis for the diagnosis.
The results of our study confirmed that direct IFA has better sensitivity and negative predictive value than PCR. The reported range of sensitivity for direct IFA diagnosis of HSV-1 was 77% to 86% [19, 20]. Thus, this test can be used as screening tool for HSV-1. It is a rapid and sensitive technique for demonstrating HSV-1 antigen in corneal scrapings [6].
Nevertheless, were 2 or more tests, such as PCR, necessary to confirm the diagnosis? The present study confirmed good specificity of PCR. A similar range of specificity–65% to 87%–has been reported by Kowalski et al. (1993) [21]. The sensitivity of PCR in our study, which was lower than that found by Farhatullah et al. [16], could be attributed to sample variation, with the absence of DNA for six cases accounting for <100% sensitivity.
From this study, we can conclude that the combination of cell culture, IFA, and PCR constitutes the best set of laboratory tests for diagnosis of clinically suspected cases of HSK. IFA rapid and simple and can be used as primary screening tool. Documented infection can be further assessed by cell-culture technique or PCR depending on laboratory availability.
Literature Cited
Butler TK, Spencer NA, Chan CC, Singh Gilhortra J, McClella K (2005) Infective keratitis in older patients: A 4 year review, 1998–2002. Br J Ophthalmol 89:591–596
Athmanathan S, Bandlapally SR, Rao GN (2001) Collection of corneal impression cytology directly on a sterile glass slide for the detection of viral antigen an inexpensive and simple technique for the diagnosis of HSV epithelial keratitis—A pilot study. BMC Ophthalmol. 1:3–10
Madhavan HN, Priya K, Anand AR, Therese KL (1999). Detection of herpes simplex (HSV) genome using polymerase chain reaction (PCR) in clinical samples comparison of PCR with standard laboratory methods for detection of HSV. J Clin Virol 14:145–151
Kowalski RP, Gordon YJ (1989). Evaluation of immunologic tests for the detection of ocular herpes simplex virus. Ophthalmology 96:1583–1586
Thiel MA, Bossart W, Bernauer W (1997). Improved impression cytology techniques for the immunopathological diagnosis of superficial viral infections. Br J Ophthalmol 81:984–988
Pramod NP, Gopalakrishnan V, Mohan R, Chandriga S, Anadakannan K, Menon T, et al. (1998). Enhanced detection of herpes simplex virus from ocular specimens of herpetic keratitis patients. Indian J Pathol Microbiol 41:49–53
Pramod NP, Thyagarajan SP, Mohan KV, Anandakannan K (2000). Polymerase chain reaction in the diagnosis of herpetic keratitis: Experience in a developing country. Can J Ophthalmol 35:134–140
Khodadoost MA, Sabahi F, Behroz MJ, Roustai MH, Saderi H, Amini-Bavil-Olyaee S, et al. (2004). Study of a polymerase chain reaction based method for detection of herpes simplex virus 1 DNA among Iranian patients with ocular herpetic keratitis infection. Jpn J Ophthalmol 48:328–332
Lucotte G, Bathelier C, Lespiaux V, Bali C, Champenios T (1995). Detection and genotyping of herpes simplex virus types 1 and 2 by polymerase chain reaction. Mol Cell Probes 9:287–290
Whiley DM, Mackay IM, Syrmis MW, Witt MJ, Sloots TP (2004). Detection and differentiation of herpes simplex virus type 1 and 2 by a duplex light cycler PCR that incorporates an internal control PCR reaction. J Clin Virol 30:32–38
Schmidt NJ (1979). Cell culture techniques for diagnostic virology. In: Lennette EH, Schmidt NJ (eds) Diagnostic procedures for viral, richettsial and chlamydial infections. Washington, DC: American Public Health Association, pp 65–139
Landry ML, Hsuing GD (2001) Primary isolation of viruses. In: Specter S, Hodinka RL, Young SA (eds) Clinical virology manual, 3rd ed, ASM Press, Washington, pp 27–42
Johnson G, Nelson S, Petric M, Tellier R (2000). Comprehensive PCR-based assay for detection and species identification of human herpes viruses. J Clin Microb 38:3274–3279
Biriken D, Yildiz S, Ozkul A, Ozsan M (2004). Investigation of herpes simplex type 1 in the samples of patients with clinically prediagnosed as herpetic keratitis or keratoconjunctivitis. Mikrobiyol Bul 38:51–59
Elnifro EM, Cooper RJ, Klapper PE, et al. (1999). Diagnosis of viral and chlamydial keratoconjunctivitis : Which laboratory test? Br J Ophthalmol 83:622–627
Farhatullah S, Kaja S, Athmanathan S, Reddy SB, Sharma S (2004). Diagnosis of herpes simplex virus-1 keratitis using Giemsa stain, immunofluorescence assay and polymerase chain reaction assay on corneal scrapings. Br J Ophthalmol 88:142–144
Kodama T, Hayasaka S, Setogawa T (1992). Immunofluorescence staining and corneal sensitivity in patients suspected of having herpes simplex keratitis . Am J Ophthalmol 113:187–89
Kaye SB, Lynas C, Patterson A (2000). Human herpes viruses in the cornea. Br J Ophthalmol 84:563–571
Chan EL, Brandt K, Horsman GB (2001). Comparison of chemicon simulfluor direct fluorescent antibody staining with cell culture and shell vial direct immunoperoxidase staining for detection of herpes simplex virus and direct immunofluorescence staining for detection of varicella zoster virus. Clin Diagn Lab Immunol 8:909–912
Subhan S, Jose RJ, Duggirala A, et al. (2004). Diagnosis of herpes simplex virus-1 keratitis: Comparison of Giemsa, immunofluorescence assay and polymerase chain reaction. Curr Eye Res 29:209–113
Kowalski RP, Gordon YJ, Romanowski EG, et al. (1993) A comparison of enzyme immunoassay and polymerase chain reaction with the clinical examination for diagnosing ocular herpetic disease. Ophthalmology 100:530–534
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
El-Aal, A.M.A., Sayed, M.E., Mohammed, E. et al. Evaluation of Herpes Simplex Detection in Corneal Scrapings by Three Molecular Methods. Curr Microbiol 52, 379–382 (2006). https://doi.org/10.1007/s00284-005-0289-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-005-0289-2