Abstract
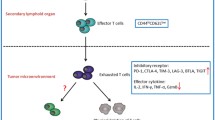
CD8+ T cells are important for the protective immunity against intracellular pathogens and tumor. In the case of chronic infection or cancer, CD8+ T cells are exposed to persistent antigen and/or inflammatory signals. This excessive amount of signals often leads CD8+ T cells to gradual deterioration of T cell function, a state called “exhaustion.” Exhausted T cells are characterized by progressive loss of effector functions (cytokine production and killing function), expression of multiple inhibitory receptors (such as PD-1 and LAG3), dysregulated metabolism, poor memory recall response, and homeostatic proliferation. These altered functions are closely related with altered transcriptional program and epigenetic landscape that clearly distinguish exhausted T cells from normal effector and memory T cells. T cell exhaustion is often associated with inefficient control of persisting infections and cancers, but re-invigoration of exhausted T cells with inhibitory receptor blockade can promote improved immunity and disease outcome. Accumulating evidences support the therapeutic potential of targeting exhausted T cells. However, exhausted T cells comprise heterogenous cell population with distinct responsiveness to intervention. Understanding molecular mechanism of T cell exhaustion is essential to establish rational immunotherapeutic interventions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Naive T cells get activated and differentiated into effector cells in 7~10 days during acute infection or vaccinations. To obtain appropriate differentiation program, naive T cells integrate signals from antigen (signal 1), co-stimulation (signal 2), and inflammation (signal 3) during priming and initial activation. This effector T cell differentiation from a naïve T cell is accompanied by robust cell proliferation, transcriptional, epigenetic, and metabolic global reprogramming, and the acquisition of cardinal features of effector T cells such as effector function, altered tissue homing, and a dramatic numerical expansion [1]. In the subsequent contraction phase, the majority of expanded effector cells die but a small fraction of effector cells persists and develops into memory T cells [1]. Memory T cells downregulate the activation program of effector cells and disarm effector molecules but keep the ability to rapidly re-activate effector functions upon re-encounter with the same antigen. During this transition, memory T cells also change homing and distribution capacity. Additionally, memory T cells develop a key characteristic of antigen-independent self-renewal, a type of stem cell-like slow proliferation driven by IL-7 and IL-15. Based on these tissue distribution preference and capability of slow proliferation in response to homeostatic cytokines, memory T cells are classified as central memory (TCM), effector memory (TEM), resident memory (TRM), and memory stem cells (TSCM) [2]. Overall, a key aspect of the development of highly functional, persisting memory T cells is that after the peak of effector expansion, this memory T cell differentiation program occurs in the absence of ongoing antigen stimulation and high levels of persisting inflammation.
On the other hand, during chronic infections or cancer where antigen and/or inflammation persist, the program of memory T cell differentiation is dramatically changed [2]. In normal setting, memory T cells undergo a transition to quiescence but still preserve potential effector capacity after effector phase. However, during chronic infection or cancer, antigen-specific T cells show progressive loss of effector functions, altered metabolism, and a unique transcriptional and epigenetic program that is characterized by an absence of a signature of quiescence [2, 3]. One of the major characteristics of exhaustion is co-expression of high levels of multiple inhibitory receptors, including PD-1 (CD279), cytotoxic T lymphocyte antigen-4 (CTLA-4, CD152), lymphocyte-activation gene 3 (LAG3), T cell immunoglobulin domain and mucin domain 3 (Tim-3), CD244/2B4, CD160, T cell immunoreceptor with Ig and ITIM domain (TIGIT), and others [2, 4]. Other changes are lack of antigen-independent homeostasis; altered transcriptional program including the distinct use of key transcription factors; and changes in homing and migration, signaling and cytokine and chemokine receptor expression, and metabolism [2, 5,6,7,8,9]. Development of T cell exhaustion is tightly associated with prolonged exposure to antigen and inflammation. Altered T cell function, differentiation, and maintenance together prevent optimal control of chronic infections and cancer. The discovery that blockade of the PD-1 pathway could partially reverse exhaustion and lead to reduced viral or tumor load was a significant breakthrough in the field [2, 10,11,12,13,14]. These data in animal models and clinical trials highlighted the idea that exhausted T cells were not terminally dysfunctional or irreversible, but at least a subset of exhausted T cells could be re-invigorated, with implications for the treatment of diseases including chronic infections and cancer. In this review, characteristic features of altered functionality, inhibitory receptors and negative regulatory pathways, and altered transcriptional control of exhausted CD8 T cells will be described.
Overview of T cell exhaustion
CD8 T cells play a pivotal role in clearing intracellular pathogens and tumors [1]. However, high and sustained antigen and inflammatory stimulation during chronic infection and tumors can lead to altered CD8 T cell differentiation or exhaustion. T cell exhaustion, first described in chronic virus infection in mice [15], and was defined as the persistence of antigen-specific T cells that lacked or possessed poor effector functions. These studies were followed rapidly by the realization that CD8 T cell exhaustion also occurred in humans during HIV, HCV, HBV, HTLV-1, and other infections as well as cancer [2, 16,17,18,19]. Although the severity of T cell dysfunction can differ for specific pathogens, the general principle originally defined during lymphocytic choriomeningitis virus (LCMV) infection in mice appears to apply in a wide variety of infectious and cancer settings. Importantly, this state of T cell differentiation prevents optimal control of infections and tumors and a better understanding of the molecular mechanism of exhaustion should lead to new clinical opportunities [17].
T cell exhaustion usually manifests in progressive and hierarchical loss of effector functions during persistent infection [2, 17]. Typically, functions such as IL-2 production and cytokine polyfunctionality, as well as high proliferative capacity are lost early, followed by defects in production of TNF, IFN-γ, and chemokines as well as degranulation capacity. Ultimately, virus-specific T cells can be physically deleted. Exhausted T cells express inhibitory receptors including PD-1, LAG-3, Tim-3, 2B4/CD244, CD160, TIGIT, and others that have a major role in regulating T cell function. The demonstration that blocking the PD-1 inhibitory receptor in vivo revigorated exhausted T cell responses and enhanced viral control was a critical advance in this field [10]. These studies demonstrated a novel concept that T cell exhaustion was reversible, rather than a, terminal or irreversible differentiation state. Moreover, these observations have become the foundation for remarkable clinical trials blocking the PD-1 pathway in human cancer and chronic infections that have resulted in impressive clinical response rates, sometimes in patients who have failed other immunotherapies [11, 13]. The immunological effects of these human treatments remain to be fully defined, but the emerging results support the notion that reversal of T cell exhaustion in humans is the causative mechanism for the profound antitumor effect seen in many patients receiving PD-1 pathway blocking reagents. In addition to loss of effector function and negative regulation by inhibitory receptors, considerable evidence such as comparative transcription analysis of functional memory versus exhausted CD4 and CD8 T cells indicates that exhausted CD8 T cells have a unique molecular signature distinct from naïve, effector, and memory T cells [3, 20, 21]. Thus, while loss of function is one of the key defining features of T cell exhaustion, recent work has also highlighted several other defining aspects of T cell exhaustion including sustained expression of inhibitory receptors, altered memory, and a unique pattern of transcriptional control [2].
Persisting antigen signaling drives T cell exhaustion
While there are clearly contributions from a variety of pathways one key feature appears to be the chronic (and likely continuous rather than intermittent) exposure to antigen. Additional factors including lack of CD4+ T cell help [22] and perhaps signals from inhibitory receptors [23] also likely contribute. Early studies in the chronic LCMV model demonstrated that the severity of exhaustion (and deletion) of antigen-specific T cells correlated to antigen abundance [24]. The importance of the level of antigen persistence exhaustion was also confirmed in other murine models and HIV-1 infection [2, 25]. Thus, the level and duration of chronic antigen stimulation appears to be a key event leading to exhaustion and correlating with the severity of dysfunction during chronic infection.
Indeed, downstream of TCR signaling, NFAT, and Sprouty-2 (SPRY2) has been implicated in T cell exhaustion. Impaired NFAT nuclear translocation results in split exhaustion of virus-specific CD8+ T cell functions during chronic viral infection [26]. Sprouty-2, a negative regulator of the MAPK/ERK pathway, is upregulated by strong TCR signals and regulated T cell polyfunctionality [27]. Inhibition of Sprouty-2 in HIV-specific T cells increased polyfunctionality independently of PD-1, suggesting a central role for ongoing direct attenuation of T cell signaling in exhausted T cells. However, chronic antigen stimulation also leads to sustained expression of PD-1 through NFATc1 [28] and it is likely that PD-1 further modulates the level of TCR signaling [29]. Together, persistent antigen stimulation (signal 1) is key factor that initiates and leads T cell exhaustion and correlate with the severity of dysfunction during chronic infection.
Inhibitory signals in T cell exhaustion
Negative regulatory pathways that are responsible for T cell exhaustion can be classified in three major categories: (1) cell surface inhibitory receptors, (2) soluble factors and environmental factors, and (3) immunoregulatory cell populations.
Inhibitory receptors and co-stimulatory pathways
Inhibitory and co-stimulatory receptors play critical roles in adaptive immune cell responses [30]. Inhibitory receptors are critical negative regulatory pathways that function to control autoreactivity and immunopathology [4, 31]. Although inhibitory receptors are transiently expressed in functional effector T cells during activation, higher and sustained expression of inhibitory receptors is a central feature of the T cell exhaustion. The PD-1:PD-L1/L2 inhibitory pathway is the best studied inhibitory receptor pathway in T cell exhaustion [30, 32, 33]. The observation that blocking the PD-1 pathway re-invigorates exhausted CD8+ T cell responses during chronic LCMV infection and enhances viral control indicates that T cell exhaustion is under active control by inhibitory receptors such as PD-1 [10]. This observation originally from LCMV infection was rapidly confirmed in HIV infection [18, 19, 34], and PD-1 pathway signals are now known have a major role in negatively regulating immunity during a wide variety of human chronic infections and cancer [30, 32, 33]. Indeed, with the FDA approval of PD-1 pathway inhibitors for treatment of human cancer, the importance of the PD-1 pathway and reversal of T cell exhaustion for treatment of human disease cannot be understated.
Despite the promise of clinical targeting of the PD-1 pathway, the molecular mechanisms by which this inhibitory receptor controls T cell exhaustion remain poorly understood. There are several mechanisms by which inhibitory receptors such as PD-1 might regulate T cell function [32]. (1) Ectodomain competition: inhibitory receptors sequestrate target receptors/ligands and/or prevent the optimal formation of microclusters and lipid rafts (CTLA4 [35]), (2) modulation of intracellular mediators: local and transient intracellular attenuation of positive signals from activating receptors such as T cell receptors and co-stimulatory receptors [36], PD-1 [37] and Tim3 [38], (3) Induction of inhibitory genes: some inhibitory receptors upregulate expression of genes that inhibit T cell function. (4) Alteration in T cell motility [39]: PD-1 decreases exhausted T cell motility. The PD-1 intracellular domain contains an immunotyrosine inhibitory motif (ITIM) and an immunotyrosine switch motif (ITSM) [40]. Current evidence suggests an role for the ITSM in recruiting SHP1 and/or SHP2 [37, 41] in the ability of PD-1 signals to attenuate TCR signaling in vitro. The role of the ITIM in PD-1 function remains poorly understood. Other evidence suggests a role for PD-1 signals in modulating PI3K, AKT, and Ras pathways [36, 42] and also link PD-1 to control of cell cycle progression [43]. Notably, nearly all our information about how PD-1 controls T cell signaling is derived from in vitro studies of acutely activated T cells. In vivo studies of the role of PD-1 during acute T cell activation and expansion suggest a possible role for PD-1 signals in arresting T cell migration [29], which could have important implications for viral control. Finally, there is some evidence that signals from PD-1 may, in fact, induce expression of genes such as the transcription factor BATF that could negatively regulate gene expression in some settings [23]. Nevertheless, despite this elegant work, how these observations relate to exhausted T cells exposed to chronic stimulation through the TCR remains unclear.
Indeed, PD-1 expression is rapidly upregulated upon T cell activation and expression of PD-1 may persist at moderate levels in humans. For example, in healthy adult humans, many functional effector memory cells express PD-1 [44, 45], indicating that PD-1 expression alone is not a unique feature of exhausted T cells. However, during chronic infections, PD-1 expression can be substantially higher than observed on functional effector or memory CD8+ T cells [19, 46]. During chronic infection sustained upregulation of PD-1 and the functional inactivation of virus-specific T cells during is dependent upon continued epitope recognition [47], although examples exist of residual PD-1 expression even after removal of persisting antigen signaling [48, 49]. This latter observation may relate to epigenetic changes in the control of expression of the Pdcd1 gene encoding PD-1 [50].
In addition to PD-1, exhausted T cells express an extensive suite of other cell surface inhibitory molecules. Exhausted T cells co-express PD-1 together with LAG-3, CD244 (2B4), CD160, TIM-3, CTLA-4, and many other inhibitory receptors [25]. Typically, the higher the number of inhibitory receptors co-expressed by exhausted T cells the more severe the exhaustion. Indeed, while individual expression of PD-1 or other inhibitor receptors is not indicative of exhaustion, co-expression of multiple inhibitory receptors is a key-defining feature of exhaustion. These co-expression patterns are highly functionally relevant since simultaneous blockade of multiple inhibitory receptor pathways results in synergistic reversal of T cell exhaustion. This concept was first demonstrated for PD-1 and LAG-3 [25] in LCMV and then other infections [51] or cancer [52, 53], but also for many other combinations of inhibitory receptors including PD-1 and CTLA-4 [54, 55] and PD-1 and TIM-3 [56,57,58,59]. Again, these observations from mice are translating to humans and making significant clinical impact in the last decade. PD-1 and CTLA-4 blockade in human melanoma patients demonstrated impressive tumor control [60], and other combination inhibitory receptor clinical trials in multiple settings are underway. It is noteworthy that PD-1 pathway blockade is typically included as one arm of these combination therapies consistent with the central role of this inhibitory receptor in T cell exhaustion. Overall, these data on inhibitory receptor co-regulation of T cell exhaustion suggest that these pathways are non-redundant in how they control T cell function and differentiation in chronic infection and cancer. These molecules come from diverse structural families, bind ligands with distinct expression patterns and properties and possess impressively different intracellular signaling domains. Thus, substantial potential may exist to tailor or tune the type and magnitude of re-invigoration of exhausted T cell responses by appropriate co-blockade approaches. Indeed, an extensive set of additional potential blockade targets exist and are currently being explored for combination therapies to reverse T cell exhaustion [32].
Although inhibitory receptors draw considerable attention in cell-to-cell interaction mechanism of exhaustion, it has become clear that co-stimulatory receptors, which normally play a positive role in acute infection, are also involved for T cell exhaustion [4]. For example, desensitization of co-stimulatory pathway signaling through the loss of adaptor molecules can serve as a mechanism of T cell dysfunction during chronic infection. TNFR-associated factor 1 (TRAF1), a signaling adaptor of TNF superfamily, is downregulated in dysfunctional T cells in progressor of HIV and chronic phase of LCMV Cl-13 infection [61]. Adoptive transfer of TRAF1 expressing, but not TRAF1 deficiency, CD8+ T cell enhanced viral control of Cl-13 infection, indicating the essential role of co-stimulatory pathway for T cell exhaustion. Co-stimulatory pathways can also regulate T cell exhaustion in an indirect fashion. CD27 signaling on CD4+ T cells enhances TNF and IFN-γ secretion, which can lead to destruction of splenic architecture and immunodeficiency [62]. CD40 agonistic antibody can rescue PD-1 mediated CD8+ T cell exhaustion perhaps due to myeloid DC activation [63]. In pancreatic cancer, such CD40 targeting may overcome a substantial T cell extrinsic barrier leading to enhanced T cell responses and better tumor control [64]. It has also been possible to exploit this concept of agonizing a positive co-stimulatory pathway while blocking an inhibitory pathway. For example, combined blockade of and PD-1 and treatment with an agonistic antibody to 4-1BB dramatically improved exhausted CD8 T cell function and viral control [65]. In addition, PD-1 pathway blockade has been combined with other “positive” regulators of immune responses including therapeutic vaccination [66], delivery of IL-2 [67], or regulatory T cells (Treg) depletion [68]. Since combined therapy of blocking antibody to inhibitory receptors and agonistic antibody to co-stimulatory receptor showed synergistic effect, detailed mechanism of co-stimulatory pathway in T cell exhaustion would be of great interest.
Soluble pathways and environmental factors
A second class of signals that regulates T cell exhaustion is from soluble molecules. Broadly, three distinct classes of such soluble mediators can be discussed including immunosuppressive cytokines such as IL-10 and TGF-β, inflammatory cytokines such as type I IFN and common-γ chain cytokines (such as IL-2, IL-7, and IL-21).
IL-10
The IL-10/IL-10R pathway has received considerable attention for its role in T cell exhaustion [17]. Blockade of IL-10 restores T cell function and improves viral control during chronic viral infections, indicating that IL-10 facilitates T cell exhaustion [69, 70]. Studies of LCMV infection in mice and HIV in humans demonstrated that during chronic infection, IL-10 can be secreted from many cell types including dendritic cells, monocytes, and/or CD4+ T cells [71,72,73], though the important or most relevant source of this cytokine remains a matter of debate. Simultaneous blockade of IL-10 and PD-1 axis significantly enhances T cell response and viral control when compared with either blockade alone, indicating that the immunosuppressive mechanism of IL-10 in T cell exhaustion is mechanistically distinct from PD-1 [74]. Interestingly, however, some evidence suggests a connection between the PD-1 pathway and IL-10 production through induction of IL-10 by monocytes following PD-L1 ligation [72]. Despite the clear evidence that IL-10 contributes to exhaustion, the molecular events downstream of IL-10 signaling (presumably via STAT3) that shape T cell exhaustion remain to be more precisely defined.
TGF-β
Another suppressive cytokine implicated T cell exhaustion is transforming growth factor-β (TGF-β). Earlier studies indicated that phosphorylation of Smad2 (indicator of TGF-β signaling) in CD8+ T cells was increased during chronic infection compared with acute infection, and inhibiting TGF-β signaling in CD8+ T cells using dominant negative receptor improved the function of exhausted cells [75]. However, studies using systemic administration of TGF-β inhibitor/blocking antibody in mice found little benefit of these treatments [76, 77]. While it is difficult to directly compare the genetic approach to antibody- and inhibitor-based strategies, these observations warrant perhaps further evaluation of this immunoregulatory pathway in T cell exhaustion.
IFN-α/β
Type I interferons (IFN-α/β) are critical inflammatory cytokines that have essential antiviral effects in the early stages of infection. In most cases, in the absence of IFN-α/β signaling, acute viral control is dramatically compromised. Moreover, IFN-α/β signals can provide a critical “signal 3” for proper activation and differentiation of CD8+ T cells following priming [78]. In addition to these critical innate antiviral effects, however, recent studies demonstrated a surprising and crucial role for chronic type I interferon signaling in promoting immune suppression and lymphoid tissue destruction. Surprisingly, blockade of this pathway reversed and/or prevented T cell exhaustion [79, 80]. This effect appeared to operate via CD4+ T cells, though the precise mechanism remains to be defined. For example, IFN-α/β can induce several immunoregulatory pathways including IL-10, PD-L1, and indoleamine dioxygenase (IDO) (ref). It is also interesting that the signal 3 cytokines IL-12 and type I IFN differentially program CD8+ T cells for PD-1 re-expression levels and tumor control in a cancer re-challenge model [81], suggesting an important role for inflammatory signals in perhaps modulating the availability of other immunoregulatory pathways that influence T cell exhaustion. Indeed, exposure to chronic inflammation in the absence of TCR signaling can dramatically skew the pattern of memory CD8+ T cell differentiation, although overt exhaustion does not occur without persisting TCR signaling [82].
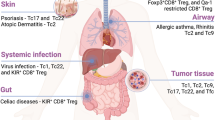
In addition to important roles for inflammatory pathways mentioned above, anatomical factors such as cellular and tissue tropism, lymphoid architecture integrity could also influence the severity of exhaustion. Given that exhausted T cells express altered pattern of trafficking and adhesion molecules [20] and expression levels of inhibitory receptors are different among organs [83], spatial and temporal regulation of T cell exhaustion should be examined in future study. Destruction of tissue architecture and fibrosis of secondary lymphoid organs have been reported in both mice and human chronic infection [84,85,86]. As an orchestrated immune cell trafficking and fine cell-to-cell interaction is critical for optimal immune response, a major goal is to determine how these anatomical features influence T cell exhaustion.
Regulatory subsets
Immune system has multiple subsets, and interactions among these subsets are essential to maximize immune responses against infection and cancer. For optimal responses, CD8+ T cells require optimal antigen presentation from professional APC, help from CD4+ T cells, and intact tissue trafficking ability to increase immune cell interaction. These events must be orchestrated in a setting that prevents excessive immune-mediated tissue damage and also appropriately shuts off T cell responses when necessary. Thus, suppressive and/or regulatory cell populations such as Treg and altered APC may contribute to CD8+ T cell exhaustion. DCs can be direct targets of viruses, and dysfunction of DCs for cytokine production and cross-presentation has been reported in some chronic infections [87]. In addition, persistent inflammation associated with chronic infections and cancer as well as viral targeting of hematopoietic progenitors can alter DC maturation and differentiation at multiple levels, generating suppressive subsets such as immunoregulatory APC and myeloid-derived suppressor cells (MDSCs), which can inhibitor T cell function and/or promote exhaustion [88,89,90]. Both immunoregulatory APC and MDSCs have been described in cancer and are thought to negatively regulate T cell responses. Analogous populations have recently been shown to promote T cell exhaustion in murine LCMV Cl-13 infection [91, 92].
Loss of CD4+ T cell activity in many settings can underlie or contribute to defective CD8+ T cell responses. In HIV infection, CD4+ T cells are direct targets of infection and loss of CD4+ T cells is associated with increased exhaustion of CD8+ T cells. While CD4+ T cells can clearly become exhausted, the impact of changes in the CD4+ response for CD8+ T cell exhaustion may be highly relevant. In the absence of IL-21 signaling, for example, CD8+ T cell exhaustion is substantially worse during chronic LCMV infection [93,94,95] with supporting observations in HIV infection [96, 97]. Since CD4+ T cells are the likely source of IL-21 signals, these observations suggest a key role for CD4 help to CD8+ T cells to avoid severe exhaustion via IL-21. Furthermore, there is evidence that activated NK cells have an immunoregulatory role during chronic viral infection perhaps by directly eliminating CD4+ T cells [98,99,100]. Foxp3+ CD4+ Treg are well known to influence immune responses during many infection and cancer [101, 102]. Although it is relatively clear that Treg have suppressive roles for T cell response in acute infection and acute phase of chronic infection (which can enhance T cell exhaustion as a result of high pathogen burden) [68], and frequency of Treg is increased in some human chronic HIV and HCV infection, it is still unclear whether Treg directly facilitate T cell exhaustion. As a source of IL-10, TGF-β or perhaps other suppressive cytokines (e.g., IL-35), one can envision such a scenario. However, precisely how Treg affect developing T cell exhaustion remains to be more completely defined. Nevertheless, Treg are an important therapeutic target since their deletion or modulation can often unleash effective antitumor or antipathogen responses [68].
Transcriptional changes in T cell exhaustion
How T cell exhaustion is transcriptionally programed? Recent studies have applied genomics approached to investigating the transcriptional circuitry that underlies development of T cell exhaustion. Exhausted CD4+ and CD8+ T cells have a transcriptional profile profoundly different from memory CD4+ and CD8+ T cells, respectively, including major changes in the expression of inhibitory and co-stimulatory receptors, transcription factors, signaling molecules, cytokine and chemokine receptors, and genes involved in metabolism [20, 21]. In addition, a major feature of CD8 T cell exhaustion is the absence of key CD8+ T cell memory-associated modules of gene expression including specific coordinated gene sets associated quiescence [3]. Thus, in addition to phenotyping, fate-mapping, and functional analysis, genomic studies also support the concept that exhausted T cells represent a unique state of T cell differentiation.
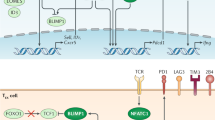
These genomic studies raise questions about how key genes and molecules identified regulate the development and differentiation of exhausted T cells. Although considerable progress has been made to define centrally important transcription factors, a lineage-specific transcription factor for exhausted T cells has not yet been identified. However, the transcription factors Tbet, Eomes, Blimp1, NFAT, BATF, IRF4, von Hippel-Lindau disease tumor suppressor (VHL), FOXO1, FOXP1, and TCF1 have been shown to be involved in T cell exhaustion [2, 5, 6, 23, 26, 103,104,105,106]. Interestingly, despite the absence of an obvious unique transcription factor associated with exhaustion, a key concept that has emerged is that several of these transcription factors function in an exhaustion-specific manner in exhausted CD8 T cells [5, 6, 23, 107]. In other words, while the transcription factors can play roles in other T cell populations, the expression pattern, genes controlled, and manner in which key transcription factors operate in exhausted T cells is, in some cases, highly divergent from the function of these transcription factors elsewhere. For example, while T-bet is expressed by, and plays a functional role in the formation of terminally differentiated CD8 T cell populations in acute infection [1, 108], T-bet controls the population of non-terminal progenitor cells with the exhausted T cell pool [6]. Similarly, Eomes is involved in central memory T cells following acute infection including playing a role in being essential for central memory CD8 T cell quiescence and homeostatic turnover [109, 110], but during chronic infection, Eomes controls the formation of terminally differentiated exhausted T cells highly enriched in peripheral tissues [6]. This distinct context-specific re-use of transcription factors was initially revealed using transcriptional network analysis that revealed differential transcriptional connections for specific transcription factors with genes they control in memory versus exhaustion [3]. Together, these studies suggest that differentiation to T cell exhaustion is governed by multiple transcription factors and context-specific combination of these transcription factors might play a critical role. In addition to transcription factors, it has recently been shown that microRNA (e.g., miR-150, miR-155) play as a key regulator of development or maintenance of exhausted T cells [111].
Another important mechanism that regulates transcriptional program is epigenetic modification. As the epigonome can influence a cell differentiation through modification of transcriptional program, understanding of global epigenetic landscape of exhausted T cell appears to be one of the next fundamental steps in the field [2]. However, only limited information has been established in terms of epigenetic regulation of T cell exhaustion [8, 9, 112]. An earlier study revealed that DNA methylation status of Pdcd1 (encoding PD-1) locus in the antigen-specific T cells is different among acute (effector and memory) and chronic (exhaustion) infection. During acute infection, Pdcd1 promotor regions were largely demethylated in the effector phase and then remethylated as T cells differentiate into memory cells. On the other hand, the Pdcd1 locus were demethylated in exhausted T cells, and this demethylation was not changed even when antigen removed and PD1 expression on exhausted T cells decreased [50]. Similarly, reinvigorated CD8+ T cells by PD-L1 blockade have a distinct epigenetic profile when compared with memory T cells that was minimally remodeled after PD-L1 blockade [9]. Thus, these epigenetic analysis also supports the idea that exhausted T cells are distinct lineage. Intervention to overcome this epigenetic fate inflexibility will be the next main target [8]. Future studies should be directed to context-dependent molecular interaction among multiple transcription factors and interaction of transcription factors and epigenetic DNA modifications, which enable context-dependent regulation of T cell exhaustion.
Conclusion
Although recent studies have provided significant advance in our understanding of T cell exhaustion, our understanding of T cell exhaustion and how to most effectively reverse this state remains incomplete. In addition, most of the research of T cell exhaustion has been utilized mouse LCMV and tumor model, and our understanding of T cell exhaustion in human chronic infection and cancer is still limited. Future mechanistic and clinical studies are needed to develop the next generation of immune based interventions for chronic infection and cancer.
References
Kaech SM, Cui W (2012) Transcriptional control of effector and memory CD8+ T cell differentiation. Nat Rev Immunol 12(11):749–761. https://doi.org/10.1038/nri3307
Wherry EJ, Kurachi M (2015) Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol 15(8):486–499. https://doi.org/10.1038/nri3862
Doering TA, Crawford A, Angelosanto JM, Paley MA, Ziegler CG, Wherry EJ (2012) Network analysis reveals centrally connected genes and pathways involved in CD8+ T cell exhaustion versus memory. Immunity 37(6):1130–1144. https://doi.org/10.1016/j.immuni.2012.08.021
Attanasio J, Wherry EJ (2016) Costimulatory and coinhibitory receptor pathways in infectious disease. Immunity 44(5):1052–1068. https://doi.org/10.1016/j.immuni.2016.04.022
Kao C, Oestreich KJ, Paley MA, Crawford A, Angelosanto JM, Ali M-AA, Intlekofer AM, Boss JM, Reiner SL, Weinmann AS, Wherry EJ (2011) Transcription factor T-bet represses expression of the inhibitory receptor PD-1 and sustains virus-specific CD8(+) T cell responses during chronic infection. Nat Immunol 12(7):663–671. https://doi.org/10.1038/ni.2046
Paley MA, Kroy DC, Odorizzi PM, Johnnidis JB, Dolfi DV, Barnett BE, Bikoff EK, Robertson EJ, Lauer GM, Reiner SL, Wherry EJ (2012) Progenitor and terminal subsets of CD8+ T cells cooperate to contain chronic viral infection. Science 338(6111):1220–1225. https://doi.org/10.1126/science.1229620
Bengsch B, Johnson AL, Kurachi M, Odorizzi PM, Pauken KE, Attanasio J, Stelekati E, McLane LM, Paley MA, Delgoffe GM, Wherry EJ (2016) Bioenergetic insufficiencies due to metabolic alterations regulated by the inhibitory receptor PD-1 are an early driver of CD8(+) T cell exhaustion. Immunity 45(2):358–373. https://doi.org/10.1016/j.immuni.2016.07.008
Sen DR, Kaminski J, Barnitz RA, Kurachi M, Gerdemann U, Yates KB, Tsao HW, Godec J, LaFleur MW, Brown FD, Tonnerre P, Chung RT, Tully DC, Allen TM, Frahm N, Lauer GM, Wherry EJ, Yosef N, Haining WN (2016) The epigenetic landscape of T cell exhaustion. Science 354(6316):1165–1169. https://doi.org/10.1126/science.aae0491
Pauken KE, Sammons MA, Odorizzi PM, Manne S, Godec J, Khan O, Drake AM, Chen Z, Sen DR, Kurachi M, Barnitz RA, Bartman C, Bengsch B, Huang AC, Schenkel JM, Vahedi G, Haining WN, Berger SL, Wherry EJ (2016) Epigenetic stability of exhausted T cells limits durability of reinvigoration by PD-1 blockade. Science 354(6316):1160–1165. https://doi.org/10.1126/science.aaf2807
Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R (2006) Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 439(7077):682–687. https://doi.org/10.1038/nature04444
Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M (2012) Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 366(26):2443–2454. https://doi.org/10.1056/NEJMoa1200690
Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, Wolchok JD, Hersey P, Joseph RW, Weber JS, Dronca R, Gangadhar TC, Patnaik A, Zarour H, Joshua AM, Gergich K, Elassaiss-Schaap J, Algazi A, Mateus C, Boasberg P, Tumeh PC, Chmielowski B, Ebbinghaus SW, Li XN, Kang SP, Ribas A (2013) Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med 369(2):134–144. https://doi.org/10.1056/NEJMoa1305133
Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, Pitot HC, Hamid O, Bhatia S, Martins R, Eaton K, Chen S, Salay TM, Alaparthy S, Grosso JF, Korman AJ, Parker SM, Agrawal S, Goldberg SM, Pardoll DM, Gupta A, Wigginton JM (2012) Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 366(26):2455–2465. https://doi.org/10.1056/NEJMoa1200694
Gardiner D, Lalezari J, Lawitz E, DiMicco M, Ghalib R, Reddy KR, Chang KM, Sulkowski M, Marro SO, Anderson J, He B, Kansra V, McPhee F, Wind-Rotolo M, Grasela D, Selby M, Korman AJ, Lowy I (2013) A randomized, double-blind, placebo-controlled assessment of BMS-936558, a fully human monoclonal antibody to programmed death-1 (PD-1), in patients with chronic hepatitis C virus infection. PLoS One 8(5):e63818. https://doi.org/10.1371/journal.pone.0063818
Gallimore A, Glithero A, Godkin A, Tissot AC, Pluckthun A, Elliott T, Hengartner H, Zinkernagel R (1998) Induction and exhaustion of lymphocytic choriomeningitis virus-specific cytotoxic T lymphocytes visualized using soluble tetrameric major histocompatibility complex class I-peptide complexes. J Exp Med 187(9):1383–1393
Lee PP, Yee C, Savage PA, Fong L, Brockstedt D, Weber JS, Johnson D, Swetter S, Thompson J, Greenberg PD, Roederer M, Davis MM (1999) Characterization of circulating T cells specific for tumor-associated antigens in melanoma patients. Nat Med 5(6):677–685. https://doi.org/10.1038/9525
Wherry EJ (2011) T cell exhaustion. Nat Immunol 131(6):492–499. https://doi.org/10.1038/ni.2035
Trautmann L, Janbazian L, Chomont N, Said EA, Gimmig S, Bessette B, Boulassel M-R, Delwart E, Sepulveda H, Balderas RS, Routy J-P, Haddad EK, Sekaly R-P (2006) Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med 12(10):1198–1202. https://doi.org/10.1038/nm1482
Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, DePierres C, Mncube Z, Duraiswamy J, Zhu B, Eichbaum Q, Altfeld M, Wherry EJ, Coovadia HM, Goulder PJ, Klenerman P, Ahmed R, Freeman GJ, Walker BD (2006) PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature 443(7109):350–354. https://doi.org/10.1038/nature05115
Wherry EJ, Ha S-J, Kaech SM, Haining WN, Sarkar S, Kalia V, Subramaniam S, Blattman JN, Barber DL, Ahmed R (2007) Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity 27(4):670–684. https://doi.org/10.1016/j.immuni.2007.09.006
Crawford A, Angelosanto JM, Kao C, Doering TA, Odorizzi PM, Barnett BE, Wherry EJ (2014) Molecular and transcriptional basis of CD4(+) T cell dysfunction during chronic infection. Immunity 40(2):289–302. https://doi.org/10.1016/j.immuni.2014.01.005
Matloubian M, Concepcion RJ, Ahmed R (1994) CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection. J Virol 68(12):8056–8063
Quigley M, Pereyra F, Nilsson B, Porichis F, Fonseca C, Eichbaum Q, Julg B, Jesneck JL, Brosnahan K, Imam S, Russell K, Toth I, Piechocka-Trocha A, Dolfi D, Angelosanto J, Crawford A, Shin H, Kwon DS, Zupkosky J, Francisco L, Freeman GJ, Wherry EJ, Kaufmann DE, Walker BD, Ebert B, Haining WN (2010) Transcriptional analysis of HIV-specific CD8+ T cells shows that PD-1 inhibits T cell function by upregulating BATF. Nat Med 16(10):1147–1151. https://doi.org/10.1038/nm.2232
Wherry EJ, Blattman JN, Murali-Krishna K, van der Most R, Ahmed R (2003) Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J Virol 77(8):4911–4927
Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, Betts MR, Freeman GJ, Vignali DAA, Wherry EJ (2009) Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol 10(1):29–37. https://doi.org/10.1038/ni.1679
Agnellini P, Wolint P, Rehr M, Cahenzli J, Karrer U, Oxenius A (2007) Impaired NFAT nuclear translocation results in split exhaustion of virus-specific CD8+ T cell functions during chronic viral infection. Proc Natl Acad Sci U S A 104(11):4565–4570. https://doi.org/10.1073/pnas.0610335104
Chiu YL, Shan L, Huang H, Haupt C, Bessell C, Canaday DH, Zhang H, Ho YC, Powell JD, Oelke M, Margolick JB, Blankson JN, Griffin DE, Schneck JP (2013) Sprouty-2 regulates HIV-specific T cell polyfunctionality. J Clin Invest 124:198–208. https://doi.org/10.1172/jci70510
Oestreich KJ, Yoon H, Ahmed R, Boss JM (2008) NFATc1 regulates PD-1 expression upon T cell activation. J Immunol 181(7):4832–4839
Honda T, Egen JG, Lammermann T, Kastenmuller W, Torabi-Parizi P, Germain RN (2014) Tuning of antigen sensitivity by T cell receptor-dependent negative feedback controls T cell effector function in inflamed tissues. Immunity 40(2):235–247. https://doi.org/10.1016/j.immuni.2013.11.017
Okazaki T, Chikuma S, Iwai Y, Fagarasan S, Honjo T (2013) A rheostat for immune responses: the unique properties of PD-1 and their advantages for clinical application. Nat Immunol 14(12):1212–1218. https://doi.org/10.1038/ni.2762
Sharpe AH, Wherry EJ, Ahmed R, Freeman GJ (2007) The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat Immunol 8(3):239–245. https://doi.org/10.1038/ni1443
Odorizzi PM, Wherry EJ (2012) Inhibitory receptors on lymphocytes: insights from infections. J Immunol 188(7):2957–2965. https://doi.org/10.4049/jimmunol.1100038
Araki K, Youngblood B, Ahmed R (2014) Programmed cell death 1-directed immunotherapy for enhancing T-cell function. Cold Spring Harb Symp Quant Biol 78:239–247. https://doi.org/10.1101/sqb.78.019869
Petrovas C, Casazza JP, Brenchley JM, Price DA, Gostick E, Adams WC, Precopio ML, Schacker T, Roederer M, Douek DC, Koup RA (2006) PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J Exp Med 203(10):2281–2292. https://doi.org/10.1084/jem.20061496
Pentcheva-Hoang T, Egen JG, Wojnoonski K, Allison JP (2004) B7-1 and B7-2 selectively recruit CTLA-4 and CD28 to the immunological synapse. Immunity 21(3):401–413. https://doi.org/10.1016/j.immuni.2004.06.017
Parry RV, Chemnitz JM, Frauwirth KA, Lanfranco AR, Braunstein I, Kobayashi SV, Linsley PS, Thompson CB, Riley JL (2005) CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol 25(21):9543–9553. https://doi.org/10.1128/MCB.25.21.9543-9553.2005
Yokosuka T, Takamatsu M, Kobayashi-Imanishi W, Hashimoto-Tane A, Azuma M, Saito T (2012) Programmed cell death 1 forms negative costimulatory microclusters that directly inhibit T cell receptor signaling by recruiting phosphatase SHP2. J Exp Med 209(6):1201–1217. https://doi.org/10.1084/jem.20112741
Clayton KL, Haaland MS, Douglas-Vail MB, Mujib S, Chew GM, Ndhlovu LC, Ostrowski MA (2014) T cell Ig and mucin domain-containing protein 3 is recruited to the immune synapse, disrupts stable synapse formation, and associates with receptor phosphatases. J Immunol 192(2):782–791. https://doi.org/10.4049/jimmunol.1302663
Zinselmeyer BH, Heydari S, Sacristan C, Nayak D, Cammer M, Herz J, Cheng X, Davis SJ, Dustin ML, McGavern DB (2013) PD-1 promotes immune exhaustion by inducing antiviral T cell motility paralysis. J Exp Med 210(4):757–774. https://doi.org/10.1084/jem.20121416
Riley JL (2009) PD-1 signaling in primary T cells. Immunol Rev 229(1):114–125. https://doi.org/10.1111/j.1600-065X.2009.00767.x
Chemnitz JM, Parry RV, Nichols KE, June CH, Riley JL (2004) SHP-1 and SHP-2 associate with immunoreceptor tyrosine-based switch motif of programmed death 1 upon primary human T cell stimulation, but only receptor ligation prevents T cell activation. J Immunol 173(2):945–954
Patsoukis N, Brown J, Petkova V, Liu F, Li L, Boussiotis VA (2012) Selective effects of PD-1 on Akt and Ras pathways regulate molecular components of the cell cycle and inhibit T cell proliferation. Sci Signal 5(230):ra46. https://doi.org/10.1126/scisignal.2002796
Patsoukis N, Sari D, Boussiotis VA (2012) PD-1 inhibits T cell proliferation by upregulating p27 and p15 and suppressing Cdc25A. Cell Cycle (Georgetown, Tex) 11(23):4305–4309. https://doi.org/10.4161/cc.22135
Duraiswamy J, Ibegbu CC, Masopust D, Miller JD, Araki K, Doho GH, Tata P, Gupta S, Zilliox MJ, Nakaya HI, Pulendran B, Haining WN, Freeman GJ, Ahmed R (2011) Phenotype, function, and gene expression profiles of programmed death-1(hi) CD8 T cells in healthy human adults. J Immunol 186(7):4200–4212. https://doi.org/10.4049/jimmunol.1001783
Dolfi DV, Mansfield KD, Polley AM, Doyle SA, Freeman GJ, Pircher H, Schmader KE, Wherry EJ (2013) Increased T-bet is associated with senescence of influenza virus-specific CD8 T cells in aged humans. J Leukoc Biol 93(6):825–836. https://doi.org/10.1189/jlb.0912438
Blackburn SD, Shin H, Freeman GJ, Wherry EJ (2008) Selective expansion of a subset of exhausted CD8 T cells by anti-PD-L1 blockade. Proc Natl Acad Sci U S A 105(39):15016–15021. https://doi.org/10.1073/pnas.0801497105
Blattman JN, Wherry EJ, Ha SJ, van der Most RG, Ahmed R (2009) Impact of epitope escape on PD-1 expression and CD8 T-cell exhaustion during chronic infection. J Virol 83(9):4386–4394. https://doi.org/10.1128/JVI.02524-08
Angelosanto JM, Blackburn SD, Crawford A, Wherry EJ (2012) Progressive loss of memory T cell potential and commitment to exhaustion during chronic viral infection. J Virol 86(15):8161–8170. https://doi.org/10.1128/JVI.00889-12
Utzschneider DT, Legat A, Fuertes Marraco SA, Carrie L, Luescher I, Speiser DE, Zehn D (2013) T cells maintain an exhausted phenotype after antigen withdrawal and population reexpansion. Nat Immunol 14(6):603–610. https://doi.org/10.1038/ni.2606
Youngblood B, Oestreich KJ, Ha SJ, Duraiswamy J, Akondy RS, West EE, Wei Z, Lu P, Austin JW, Riley JL, Boss JM, Ahmed R (2011) Chronic virus infection enforces demethylation of the locus that encodes PD-1 in antigen-specific CD8(+) T cells. Immunity 35(3):400–412. https://doi.org/10.1016/j.immuni.2011.06.015
Butler NS, Moebius J, Pewe LL, Traore B, Doumbo OK, Tygrett LT, Waldschmidt TJ, Crompton PD, Harty JT (2011) Therapeutic blockade of PD-L1 and LAG-3 rapidly clears established blood-stage Plasmodium infection. Nat Immunol 13(2):188–195. https://doi.org/10.1038/ni.2180
Grosso JF, Goldberg MV, Getnet D, Bruno TC, Yen HR, Pyle KJ, Hipkiss E, Vignali DA, Pardoll DM, Drake CG (2009) Functionally distinct LAG-3 and PD-1 subsets on activated and chronically stimulated CD8 T cells. J Immunol 182(11):6659–6669. https://doi.org/10.4049/jimmunol.0804211
Matsuzaki J, Gnjatic S, Mhawech-Fauceglia P, Beck A, Miller A, Tsuji T, Eppolito C, Qian F, Lele S, Shrikant P, Old LJ, Odunsi K (2010) Tumor-infiltrating NY-ESO-1-specific CD8+ T cells are negatively regulated by LAG-3 and PD-1 in human ovarian cancer. Proc Natl Acad Sci U S A 107(17):7875–7880. https://doi.org/10.1073/pnas.1003345107
Kaufmann DE, Kavanagh DG, Pereyra F, Zaunders JJ, Mackey EW, Miura T, Palmer S, Brockman M, Rathod A, Piechocka-Trocha A, Baker B, Zhu B, Le Gall S, Waring MT, Ahern R, Moss K, Kelleher AD, Coffin JM, Freeman GJ, Rosenberg ES, Walker BD (2007) Upregulation of CTLA-4 by HIV-specific CD4+ T cells correlates with disease progression and defines a reversible immune dysfunction. Nat Immunol 8(11):1246–1254. https://doi.org/10.1038/ni1515
Nakamoto N, Cho H, Shaked A, Olthoff K, Valiga ME, Kaminski M, Gostick E, Price DA, Freeman GJ, Wherry EJ, Chang KM (2009) Synergistic reversal of intrahepatic HCV-specific CD8 T cell exhaustion by combined PD-1/CTLA-4 blockade. PLoS Pathog 5(2):e1000313. https://doi.org/10.1371/journal.ppat.1000313
Jin HT, Anderson AC, Tan WG, West EE, Ha SJ, Araki K, Freeman GJ, Kuchroo VK, Ahmed R (2010) Cooperation of Tim-3 and PD-1 in CD8 T-cell exhaustion during chronic viral infection. Proc Natl Acad Sci U S A 107(33):14733–14738. https://doi.org/10.1073/pnas.1009731107
Kassu A, Marcus RA, D'Souza MB, Kelly-McKnight EA, Golden-Mason L, Akkina R, Fontenot AP, Wilson CC, Palmer BE (2010) Regulation of virus-specific CD4+ T cell function by multiple costimulatory receptors during chronic HIV infection. J Immunol 185(5):3007–3018. https://doi.org/10.4049/jimmunol.1000156
Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC (2010) Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med 207(10):2187–2194. https://doi.org/10.1084/jem.20100643
Fourcade J, Sun Z, Benallaoua M, Guillaume P, Luescher IF, Sander C, Kirkwood JM, Kuchroo V, Zarour HM (2010) Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8+ T cell dysfunction in melanoma patients. J Exp Med 207(10):2175–2186. https://doi.org/10.1084/jem.20100637
Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon RA, Reed K, Burke MM, Caldwell A, Kronenberg SA, Agunwamba BU, Zhang X, Lowy I, Inzunza HD, Feely W, Horak CE, Hong Q, Korman AJ, Wigginton JM, Gupta A, Sznol M (2013) Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med 369(2):122–133. https://doi.org/10.1056/NEJMoa1302369
Wang C, McPherson AJ, Jones RB, Kawamura KS, Lin GH, Lang PA, Ambagala T, Pellegrini M, Calzascia T, Aidarus N, Elford AR, Yue FY, Kremmer E, Kovacs CM, Benko E, Tremblay C, Routy JP, Bernard NF, Ostrowski MA, Ohashi PS, Watts TH (2012) Loss of the signaling adaptor TRAF1 causes CD8+ T cell dysregulation during human and murine chronic infection. J Exp Med 209(1):77–91. https://doi.org/10.1084/jem.20110675
Matter M, Odermatt B, Yagita H, Nuoffer JM, Ochsenbein AF (2006) Elimination of chronic viral infection by blocking CD27 signaling. J Exp Med 203(9):2145–2155. https://doi.org/10.1084/jem.20060651
Isogawa M, Chung J, Murata Y, Kakimi K, Chisari FV (2013) CD40 activation rescues antiviral CD8+ T cells from PD-1-mediated exhaustion. PLoS Pathog 9(7):e1003490. https://doi.org/10.1371/journal.ppat.1003490
Beatty GL, Chiorean EG, Fishman MP, Saboury B, Teitelbaum UR, Sun W, Huhn RD, Song W, Li D, Sharp LL, Torigian DA, O'Dwyer PJ, Vonderheide RH (2011) CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science 331(6024):1612–1616. https://doi.org/10.1126/science.1198443
Vezys V, Penaloza-MacMaster P, Barber DL, Ha SJ, Konieczny B, Freeman GJ, Mittler RS, Ahmed R (2011) 4-1BB signaling synergizes with programmed death ligand 1 blockade to augment CD8 T cell responses during chronic viral infection. J Immunol 187(4):1634–1642. https://doi.org/10.4049/jimmunol.1100077
Ha SJ, Mueller SN, Wherry EJ, Barber DL, Aubert RD, Sharpe AH, Freeman GJ, Ahmed R (2008) Enhancing therapeutic vaccination by blocking PD-1-mediated inhibitory signals during chronic infection. J Exp Med 205(3):543–555. https://doi.org/10.1084/jem.20071949
West EE, Jin HT, Rasheed AU, Penaloza-Macmaster P, Ha SJ, Tan WG, Youngblood B, Freeman GJ, Smith KA, Ahmed R (2013) PD-L1 blockade synergizes with IL-2 therapy in reinvigorating exhausted T cells. J Clin Invest 123(6):2604–2615. https://doi.org/10.1172/JCI67008
Penaloza-MacMaster P, Kamphorst AO, Wieland A, Araki K, Iyer SS, West EE, O'Mara L, Yang S, Konieczny BT, Sharpe AH, Freeman GJ, Rudensky AY, Ahmed R (2014) Interplay between regulatory T cells and PD-1 in modulating T cell exhaustion and viral control during chronic LCMV infection. J Exp Med 211(9):1905–1918. https://doi.org/10.1084/jem.20132577
Brooks DG, Trifilo MJ, Edelmann KH, Teyton L, McGavern DB, Oldstone MBA (2006) Interleukin-10 determines viral clearance or persistence in vivo. Nat Med 12(11):1301–1309. https://doi.org/10.1038/nm1492
Ejrnaes M, Filippi CM, Martinic MM, Ling EM, Togher LM, Crotty S, von Herrath MG (2006) Resolution of a chronic viral infection after interleukin-10 receptor blockade. J Exp Med 203(11):2461–2472. https://doi.org/10.1084/jem.20061462
Ng CT, Oldstone MB (2012) Infected CD8alpha-dendritic cells are the predominant source of IL-10 during establishment of persistent viral infection. Proc Natl Acad Sci U S A 109(35):14116–14121. https://doi.org/10.1073/pnas.1211910109
Said EA, Dupuy FP, Trautmann L, Zhang Y, Shi Y, El-Far M, Hill BJ, Noto A, Ancuta P, Peretz Y, Fonseca SG, Van Grevenynghe J, Boulassel MR, Bruneau J, Shoukry NH, Routy JP, Douek DC, Haddad EK, Sekaly RP (2010) Programmed death-1-induced interleukin-10 production by monocytes impairs CD4+ T cell activation during HIV infection. Nat Med 16(4):452–459. https://doi.org/10.1038/nm.2106
Richter K, Perriard G, Behrendt R, Schwendener RA, Sexl V, Dunn R, Kamanaka M, Flavell RA, Roers A, Oxenius A (2013) Macrophage and T cell produced IL-10 promotes viral chronicity. PLoS Pathog 9(11):e1003735. https://doi.org/10.1371/journal.ppat.1003735
Brooks DG, Ha SJ, Elsaesser H, Sharpe AH, Freeman GJ, Oldstone MB (2008) IL-10 and PD-L1 operate through distinct pathways to suppress T-cell activity during persistent viral infection. Proc Natl Acad Sci U S A 105(51):20428–20433. https://doi.org/10.1073/pnas.0811139106
Tinoco R, Alcalde V, Yang Y, Sauer K, Zuniga EI (2009) Cell-intrinsic transforming growth factor-beta signaling mediates virus-specific CD8+ T cell deletion and viral persistence in vivo. Immunity 31(1):145–157. https://doi.org/10.1016/j.immuni.2009.06.015
Garidou L, Heydari S, Gossa S, McGavern DB (2012) Therapeutic blockade of transforming growth factor beta fails to promote clearance of a persistent viral infection. J Virol 86(13):7060–7071. https://doi.org/10.1128/JVI.00164-12
Boettler T, Cheng Y, Ehrhardt K, von Herrath M (2012) TGF-beta blockade does not improve control of an established persistent viral infection. Viral Immunol 25(3):232–238. https://doi.org/10.1089/vim.2011.0079
Curtsinger JM, Mescher MF (2010) Inflammatory cytokines as a third signal for T cell activation. Curr Opin Immunol 22(3):333–340. https://doi.org/10.1016/j.coi.2010.02.013
Teijaro JR, Ng C, Lee AM, Sullivan BM, Sheehan KC, Welch M, Schreiber RD, de la Torre JC, Oldstone MB (2013) Persistent LCMV infection is controlled by blockade of type I interferon signaling. Science 340(6129):207–211. https://doi.org/10.1126/science.1235214
Wilson EB, Yamada DH, Elsaesser H, Herskovitz J, Deng J, Cheng G, Aronow BJ, Karp CL, Brooks DG (2013) Blockade of chronic type I interferon signaling to control persistent LCMV infection. Science 340(6129):202–207. https://doi.org/10.1126/science.1235208
Gerner MY, Heltemes-Harris LM, Fife BT, Mescher MF (2013) Cutting edge: IL-12 and type I IFN differentially program CD8 T cells for programmed death 1 re-expression levels and tumor control. J Immunol 191(3):1011–1015. https://doi.org/10.4049/jimmunol.1300652
Stelekati E, Shin H, Doering TA, Dolfi DV, Ziegler CG, Beiting DP, Dawson L, Liboon J, Wolski D, Ali MA, Katsikis PD, Shen H, Roos DS, Haining WN, Lauer GM, Wherry EJ (2014) Bystander chronic infection negatively impacts development of CD8(+) T cell memory. Immunity 40(5):801–813. https://doi.org/10.1016/j.immuni.2014.04.010
Blackburn SD, Crawford A, Shin H, Polley A, Freeman GJ, Wherry EJ (2010) Tissue-specific differences in PD-1 and PD-L1 expression during chronic viral infection: implications for CD8 T-cell exhaustion. J Virol 84(4):2078–2089. https://doi.org/10.1128/JVI.01579-09
Mueller SN, Matloubian M, Clemens DM, Sharpe AH, Freeman GJ, Gangappa S, Larsen CP, Ahmed R (2007) Viral targeting of fibroblastic reticular cells contributes to immunosuppression and persistence during chronic infection. Proc Natl Acad Sci U S A 104(39):15430–15435. https://doi.org/10.1073/pnas.0702579104
Schacker T (2008) The role of secondary lymphatic tissue in immune deficiency of HIV infection. Aids 22(Suppl 3):S13–S18. https://doi.org/10.1097/01.aids.0000327511.76126.b5
Zeng M, Smith AJ, Wietgrefe SW, Southern PJ, Schacker TW, Reilly CS, Estes JD, Burton GF, Silvestri G, Lifson JD, Carlis JV, Haase AT (2011) Cumulative mechanisms of lymphoid tissue fibrosis and T cell depletion in HIV-1 and SIV infections. J Clin Invest 121(3):998–1008. https://doi.org/10.1172/JCI45157
Ng CT, Snell LM, Brooks DG, Oldstone MB (2013) Networking at the level of host immunity: immune cell interactions during persistent viral infections. Cell Host Microbe 13(6):652–664. https://doi.org/10.1016/j.chom.2013.05.014
Sevilla N, McGavern DB, Teng C, Kunz S, Oldstone MBA (2004) Viral targeting of hematopoietic progenitors and inhibition of DC maturation as a dual strategy for immune subversion. J Clin Invest 113(5):737–745. https://doi.org/10.1172/JCI20243
King KY, Goodell MA (2011) Inflammatory modulation of HSCs: viewing the HSC as a foundation for the immune response. Nat Rev Immunol 11(10):685–692. https://doi.org/10.1038/nri3062
Goh C, Narayanan S, Hahn YS (2013) Myeloid-derived suppressor cells: the dark knight or the joker in viral infections? Immunol Rev 255(1):210–221. https://doi.org/10.1111/imr.12084
Wilson EB, Kidani Y, Elsaesser H, Barnard J, Raff L, Karp CL, Bensinger S, Brooks DG (2012) Emergence of distinct multiarmed immunoregulatory antigen-presenting cells during persistent viral infection. Cell Host Microbe 11(5):481–491. https://doi.org/10.1016/j.chom.2012.03.009
Norris BA, Uebelhoer LS, Nakaya HI, Price AA, Grakoui A, Pulendran B (2013) Chronic but not acute virus infection induces sustained expansion of myeloid suppressor cell numbers that inhibit viral-specific T cell immunity. Immunity 38(2):309–321. https://doi.org/10.1016/j.immuni.2012.10.022
Elsaesser H, Sauer K, Brooks DG (2009) IL-21 is required to control chronic viral infection. Science 324(5934):1569–1572. https://doi.org/10.1126/science.1174182
Frohlich A, Kisielow J, Schmitz I, Freigang S, Shamshiev AT, Weber J, Marsland BJ, Oxenius A, Kopf M (2009) IL-21R on T cells is critical for sustained functionality and control of chronic viral infection. Science 324(5934):1576–1580. https://doi.org/10.1126/science.1172815
Yi JS, Du M, Zajac AJ (2009) A vital role for interleukin-21 in the control of a chronic viral infection. Science 324(5934):1572–1576. https://doi.org/10.1126/science.1175194
Williams LD, Bansal A, Sabbaj S, Heath SL, Song W, Tang J, Zajac AJ, Goepfert PA (2011) Interleukin-21-producing HIV-1-specific CD8 T cells are preferentially seen in elite controllers. J Virol 85(5):2316–2324. https://doi.org/10.1128/JVI.01476-10
Chevalier MF, Julg B, Pyo A, Flanders M, Ranasinghe S, Soghoian DZ, Kwon DS, Rychert J, Lian J, Muller MI, Cutler S, McAndrew E, Jessen H, Pereyra F, Rosenberg ES, Altfeld M, Walker BD, Streeck H (2011) HIV-1-specific interleukin-21+ CD4+ T cell responses contribute to durable viral control through the modulation of HIV-specific CD8+ T cell function. J Virol 85(2):733–741. https://doi.org/10.1128/JVI.02030-10
Waggoner SN, Cornberg M, Selin LK, Welsh RM (2012) Natural killer cells act as rheostats modulating antiviral T cells. Nature 481(7381):394–398. https://doi.org/10.1038/nature10624
Lang PA, Lang KS, Xu HC, Grusdat M, Parish IA, Recher M, Elford AR, Dhanji S, Shaabani N, Tran CW, Dissanayake D, Rahbar R, Ghazarian M, Brustle A, Fine J, Chen P, Weaver CT, Klose C, Diefenbach A, Haussinger D, Carlyle JR, Kaech SM, Mak TW, Ohashi PS (2012) Natural killer cell activation enhances immune pathology and promotes chronic infection by limiting CD8+ T-cell immunity. Proc Natl Acad Sci U S A 109(4):1210–1215. https://doi.org/10.1073/pnas.1118834109
Cook KD, Whitmire JK (2013) The depletion of NK cells prevents T cell exhaustion to efficiently control disseminating virus infection. J Immunol 190(2):641–649. https://doi.org/10.4049/jimmunol.1202448
Belkaid Y, Tarbell K (2009) Regulatory T cells in the control of host-microorganism interactions (*). Annu Rev Immunol 27:551–589. https://doi.org/10.1146/annurev.immunol.021908.132723
Veiga-Parga T, Sehrawat S, Rouse BT (2013) Role of regulatory T cells during virus infection. Immunol Rev 255(1):182–196. https://doi.org/10.1111/imr.12085
Doedens AL, Phan AT, Stradner MH, Fujimoto JK, Nguyen JV, Yang E, Johnson RS, Goldrath AW (2013) Hypoxia-inducible factors enhance the effector responses of CD8(+) T cells to persistent antigen. Nat Immunol 14(11):1173–1182. https://doi.org/10.1038/ni.2714
Man K, Gabriel SS, Liao Y, Gloury R, Preston S, Henstridge DC, Pellegrini M, Zehn D, Berberich-Siebelt F, Febbraio MA, Shi W, Kallies A (2017) Transcription factor IRF4 promotes CD8(+) T cell exhaustion and limits the development of memory-like T cells during chronic infection. Immunity 47(6):1129–1141 e1125. https://doi.org/10.1016/j.immuni.2017.11.021
Utzschneider DT, Charmoy M, Chennupati V, Pousse L, Ferreira DP, Calderon-Copete S, Danilo M, Alfei F, Hofmann M, Wieland D, Pradervand S, Thimme R, Zehn D, Held W (2016) T cell factor 1-expressing memory-like CD8(+) T cells sustain the immune response to chronic viral infections. Immunity 45(2):415–427. https://doi.org/10.1016/j.immuni.2016.07.021
Im SJ, Hashimoto M, Gerner MY, Lee J, Kissick HT, Burger MC, Shan Q, Hale JS, Lee J, Nasti TH, Sharpe AH, Freeman GJ, Germain RN, Nakaya HI, Xue HH, Ahmed R (2016) Defining CD8+ T cells that provide the proliferative burst after PD-1 therapy. Nature 537(7620):417–421. https://doi.org/10.1038/nature19330
Kurachi M, Barnitz RA, Yosef N, Odorizzi PM, DiIorio MA, Lemieux ME, Yates K, Godec J, Klatt MG, Regev A, Wherry EJ, Haining WN (2014) The transcription factor BATF operates as an essential differentiation checkpoint in early effector CD8+ T cells. Nat Immunol 15(4):373–383. https://doi.org/10.1038/ni.2834
Intlekofer AM, Takemoto N, Kao C, Banerjee A, Schambach F, Northrop JK, Shen H, Wherry EJ, Reiner SL (2007) Requirement for T-bet in the aberrant differentiation of unhelped memory CD8+ T cells. J Exp Med 204(9):2015–2021. https://doi.org/10.1084/jem.20070841
Paley MA, Gordon SM, Bikoff EK, Robertson EJ, Wherry EJ, Reiner SL (2013) Technical advance: fluorescent reporter reveals insights into eomesodermin biology in cytotoxic lymphocytes. J Leukoc Biol 93(2):307–315. https://doi.org/10.1189/jlb.0812400
Banerjee A, Gordon SM, Intlekofer AM, Paley MA, Mooney EC, Lindsten T, Wherry EJ, Reiner SL (2010) Cutting edge: the transcription factor eomesodermin enables CD8+ T cells to compete for the memory cell niche. J Immunol 185(9):4988–4992. https://doi.org/10.4049/jimmunol.1002042
Stelekati E, Chen Z, Manne S, Kurachi M, Ali MA, Lewy K, Cai Z, Nzingha K, McLane LM, Hope JL, Fike AJ, Katsikis PD, Wherry EJ (2018) Long-term persistence of exhausted CD8 T cells in chronic infection is regulated by MicroRNA-155. Cell Rep 23(7):2142–2156. https://doi.org/10.1016/j.celrep.2018.04.038
Youngblood B, Noto A, Porichis F, Akondy RS, Ndhlovu ZM, Austin JW, Bordi R, Procopio FA, Miura T, Allen TM, Sidney J, Sette A, Walker BD, Ahmed R, Boss JM, Sekaly RP, Kaufmann DE (2013) Cutting edge: prolonged exposure to HIV reinforces a poised epigenetic program for PD-1 expression in virus-specific CD8 T cells. J Immunol 191(2):540–544. https://doi.org/10.4049/jimmunol.1203161
Acknowledgments
I would like to thank Junko Kurachi and John Wherry for technical assistance and helpful discussion. This work was supported by Chozen project (Kanazawa University).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that he has no conflict of interest.
Additional information
This article is a contribution to the special issue on The Pathogenicity of Acquired Immunity in Human Diseases - Guest Editor: Kiyoshi Hirahara
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kurachi, M. CD8+ T cell exhaustion. Semin Immunopathol 41, 327–337 (2019). https://doi.org/10.1007/s00281-019-00744-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00281-019-00744-5