Abstract
Vaccines have been valuable tools in the prevention of infectious diseases, and the rapid development of new vectors against constantly mutating foreign antigens in viruses such as influenza has become a regular, seasonal exercise. Harnessing the immune response against self-antigens is not necessarily analogous or as achievable by iterative processes, and since the desired outcome includes leaving the targeted organism intact, requires some precision engineering. In vaccine-based treatment of autoimmunity and cancer, the proper selection of antigens and generation of the desired antigen-specific therapeutic immunity has been challenging. Both cases involve a threshold of existing, undesired immunity that must be overcome, and despite considerable academic and industry efforts, this challenge has proven to be largely refractory to vaccine approaches leveraging enhanced vectors, adjuvants, and administration strategies. There are in silico approaches in development for predicting the immunogenicity of self-antigen epitopes, which are being validated slowly. One simple approach showing promise is the functional screening of self-antigen epitopes for selective Th1 antitumor immunogenicity, or inversely, selective Th2 immunogenicity for treatment of autoimmune inflammation. The approach reveals the importance of confirming both Th1 and Th2 components of a vaccine immunogen; the two can confound one another if not parsed but may be used individually to modulate antigen-specific inflammation in autoimmune disease or cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
I. Choosing antigens
Cancer
The identification of tumor antigens, such as HER2 [1], has catalyzed the development of biotechnologies, such as custom-designed antibodies, which when administered can recruit endogenous immune cells to tumors to destroy or suppress them. Indeed, trastuzumab has become the biggest-selling drug in breast cancer therapy [2] despite being relevant only for tumors that overexpress HER2 and despite the presence of HER2 in other tissues such as cardiac and vascular endothelial [3] that create toxic liabilities for the drug. Next-generation payload-bearing antibodies, dually specific antibodies, targeted fusion proteins, and antibody fragments populate an expanding compendium of antigen-targeting biotechnologies. Another approach taken against HER2 was to develop vaccines that could stimulate HER2-specific immunity by introducing it to the immune system in contexts that encourage an inflammatory (Th1) response. In this general strategy, antigens whose expression is highly restricted to tumors are desirable; cancer-testis (CT) antigens with obscure functions like MAGE-A3 and NY-ESO-1 were targeted, as were overexpressed proteins like HER-2, MUC-1, and CEA, whose biologic functions were better understood and expression patterns also restricted [4–6]. In most vaccine approaches, there has been little ability to select antigens for their biological relevance to cancer other than expression pattern and therefore a theoretical lack of ability to target key, truncal, or essentially functioning cells within a tumor. Moreover, the simple approach of introducing the full-length antigen accompanied by adjuvants or immune-skewing moieties in the vector, in attempt to break tolerance against these self-antigens, has produced inconsistent results for reasons discussed below.
In general, however, the purpose of a vaccine is often to turn a “cold” tumor “hot,” i.e., generate inflammatory T-cell immunity (Th1) against tumor antigens with enough potency to overcome either the absence of a T-cell immune response to the tumor or a preexisting tolerant response, which tends to be immune-suppressing (Th2). Successful vaccination would be reflected in the infiltration of tumors by lymphocytes (TIL) with predominantly Th1 rather than Th2 or regulatory T-cells (Treg), which correlate to positive and poor outcomes, respectively [7] across solid tumor types.
Autoimmune
In autoimmune disease, the problem of identifying good vaccine antigens requires a different perspective; the task is more akin to putting out a fire that has already started by identifying its source of fuel. Furthermore, unlike cancer, symptoms of autoimmune disease can result from generalized inflammation, bystander effects, and the irretrievable loss of target tissues; as such, these diseases can have elusive pathogeneses. An important caveat is that the T-cellular immune system is predisposed to putting out fires: Th2 immunity suppresses Th1, which is discussed below as a fundamental concern in vaccine strategies employing full-length antigens. Autoimmune diseases like multiple sclerosis, rheumatoid arthritis, and type I diabetes have well-established anatomical/tissue targets with disease-associated proteins; these include proteins enriched in the myelin sheath of neurons, such as myelin basic protein (MBP), myelin oligodendrocyte glycoprotein (MOG) [8]; abundant in the joints, such as type II collagen, aggrecan, vimentin, and fibrinogen [9]; and expressed in pancreatic beta cells, such as GAD65 [10], respectively. In this context, the goal of a vaccine would be to provide anti-inflammatory or suppressive T-cell immunity against a pathogenic antigen responsible for initiation of the existing autoimmune response. Here, issues arise as to which antigens are pathogenic, which are immunogenic due to epitope-spreading, and how important it is to discriminate between them for the purpose of avoiding off-target effects. For some autoimmune diseases, such as neuromyelitis optica, a pathogenic antigen, aquaporin-4, has been identified and attempts to target it are underway [11]. Unlike with cancer vaccines, the goal is not to generate new tissue-destructive immunity but to repress it in a targeted manner.
In autoimmune disease drug development, recapitulation of disease in testable models may be more complex than in cancer, and preclinical efficacy can be difficult to measure with reproducibility. This is important because of the therapeutic objective, which is to reduce inflammation that may have spread to multiple antigens by stimulating anti-inflammatory immunity against single, high-confidence antigens. It is not clear that the antigens useful for disease modeling are also the relevant therapeutic targets [12, 13]. In drug development for multiple sclerosis, for example, inferences are often made in animal models of experimental autoimmune encephalomyelitis (EAE) by vaccinating with antigens like MBP or MOG, or even simple homogenate of spinal cord in combination with complex adjuvant mixtures that stimulate strong non-specific inflammation. Each model produces symptoms that differ among rodent species and in responsiveness to experimental therapies, and likely in the immune threshold that must be overcome by the treatment being tested. These caveats notwithstanding, the key issue of therapeutic immunogenicity is analogous to the Th1 objective in cancer: there is a need for the ability to generate Th2 immunity with potency, durability, and safety against the antigens crucial for disease pathogenesis.
II. Attempts and failures, the problem
As an ongoing concern for the FDA when evaluating new vaccines against self-antigens, autoimmune toxicity is perhaps foremost but has been minimized by large retrospective studies showing the approach to be safe in general [14]. Vaccine approaches against cancer have expanded both in terms of the antigens targeted and the vectors and adjuvants developed in conjunction and have shown hints of efficacy in late-stage studies [15–17]. However, new vector and adjuvant systems have not improved on the inconsistent performance of these vaccines [18–20]. This is due, at least in part, to fundamental aspects of the vaccine antigens that have been overlooked.
Early efforts to develop vaccine approaches targeting self-antigens were made with heavy emphasis on the vector and context around the antigen-presenting event by antigen-presenting cells (APCs). This is particularly true in industry development efforts where a short list of single antigens have been, and indeed continue to be, fitted to myriad delivery platforms. Key examples that span an array of vectors/adjuvants include HER-2, MUC1, and MAGE-A3, which have all been in clinical trials in multiple forms with multiple companies, none of which have resulted in a successful vaccine product. In many cases, preclinical data indicating that a Th1 response to the full-length antigen could be generated was obtained, and the vaccines were carried forth into late-stage clinical development where they failed to demonstrate consistent, meaningful efficacy [21–25]. As product development efforts, these studies were not designed to identify or troubleshoot potentially fundamental, basic scientific issues with the technical approach. A prevailing concept that may contribute to slow progress in vaccine development is that there are no essential sequence-specific or other intrinsic properties that determine the immunogenicity of epitopes. That is, the concept that epitope immunogenicity is entirely manipulable with adjuvants or other technology, and basic epitope features do not commit the T-cell irreversibly to a specific immune phenotype when they are presented. However, basic principles of vaccine antigen-stimulated immunogenicity that undermine this assumption have been made in recent translational academic work and create new opportunities for innovation in immunotherapy.
As a prototypical cancer-testes antigen, NY-ESO-1 has been studied by academic groups extensively, and fundamental principles that govern the immunogenicity of vaccine constructs derived from it seem to reveal a likely source of problems encountered in late-stage clinical studies of cancer vaccines. Specifically, multiple predicted MHCII epitopes in NY-ESO-1 have been identified, tested, and found to have mixed immunogenicity, generating opposed or mixed immune responses of Th1 and/or Th2 due to promiscuity of MHC binding [26–28]. Such epitopes would be expected to produce confusing results if used in a vaccine study without somehow tracking the HLA types of patients. More direct evidence of the futility that inadequately characterized antigens can produce are studies demonstrating that there are multiple Treg-inducing peptide epitopes in the NY-ESO-1 sequence, conserved among patients, which suppress Th1 immunity (CTL) in a full-length vaccine, or even exacerbate disease [29–32].
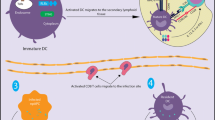
These observations support the existence of a fundamental problem with using full-length antigen or an imprecisely selected epitope in a vaccine for either cancer or autoimmune disease: mixed, confounding immunogenicity. The problem is most evident in the context of a cancer vaccine, wherein Th2 immunity suppresses the desired Th1 arising from a full-length vaccine antigen, illustrated in Fig. 1 (default Th2 responses to full-length antigens: Th2 epitopes in the full-length protein vaccine elicit preferentially suppressive immunity in the tumor microenvironment, abrogating inflammatory immunity produced by rarely presented Th1 epitopes). Historically, cancer vaccines have delivered full-length antigens or imprudently selected portions of the antigen sequence as the basic immunogenic component, relying on vector or adjuvant technologies to skew the immunogenicity towards Th1. The problems associated with full-length NY-ESO-1 have been associated with other vaccine antigens in other platforms as well [33, 34].
The importance of Th1, specifically CD4+ Th1 immunity derived from vaccines, is further exemplified in the personalized, self-escalating immunity it can generate. Removing Th2 epitopes and selecting for Th1-only MHCII/CD4+ T-cell epitopes can result in a construct that makes unfettered Th1 immunity in a vaccine, as illustrated in Fig. 2 (selection of Th1-only epitopes/removal of Th2-only epitopes produces unfettered Th1: Th1 epitope vaccine elicits CD4+ T-cell-mediated inflammatory type 1-immunity only, reversing the immune-suppressive cytokine environment, recruiting CD8 killer T-cells and escalating the response via epitope spreading). Furthermore, in contrast to MHCI epitope vaccine-stimulated CD8+ killer T-cells, which execute a simple cytotoxic attack and are subsequently dispatched, CD4+ T-cells coordinate escalation of IFNγ production and APC activity in the tumor microenvironment, which can lead to cross-priming, tumor cell lysis, new antigen presentation, and development of new immunity reflected in epitope spreading. This is a consequence of sufficient vaccine-stimulated immune activation in the tumor microenvironment to generate expanded antitumor immunity to other tumor antigens, custom-made by the patient’s immune system, and correlates to positive prognosis in patients treated with cancer vaccines [35–40]. Indeed, it resembles autoimmunity directed at the tumor.
Thus, Treg- and Th2-stimulating sequences in full-length or incompletely phenotyped vaccine antigens work to handicap the desired antitumor Th1 immunity. Importantly, even selecting a known, predicted MHCII-binding neoepitope from a tumor antigen does not presage effective immunogenicity, as demonstrated most dramatically with a neoepitope vaccine developed for glioblastoma, directed against the EGFRvIII point mutant. In phase 2 studies, this vaccine did not demonstrate generation of an effective T-cell response, and the phase 3 study was discontinued for futility [18]. This result suggests that simple novelty of epitope sequence does not confer particular immunogenicity to the vaccine construct, even if it is already predicted to bind MHCII. We hypothesized that the essential phenotype-determining signals may be specifically encoded in the epitope sequences themselves, and that careful dissection of the immunogenicity of individual epitopes in cancer antigens could reveal candidates that generate selective Th1 immunity and could be developed as active principle ingredients for broadly efficacious vaccines. Further, there may be a yin-yang relationship between Th2 and Th1 epitopes that generates a net “default” response of tolerance to a self-antigen and could serve as a buffer or threshold for breaking tolerance to the whole antigen.
New methods
This hypothesis led to a significant modification of our approach to epitope identification. First, we identify putative class II epitopes via a multialgorithm approach to ensure responsiveness across diverse HLA alleles [41]. Secondly, we perform population-based screening of predicted epitopes to determine potential sequences that may elicit Th2/Treg. IGFBP-2 had been defined as a tumor antigen in ovarian cancer [42]. We screened predicted epitopes in ELISPOT evaluating IFN-g and IL-10 responses in 40 individuals with class II alleles that were representative of the North American population [43]. We found that there were sequences of IGFBP-2 that, across all individuals studied, elicited primarily IFN-g or IL-10 responses. T-cell lines derived from the IFN-g-inducing peptides showed little to no evidence of type II cytokine secretion while similar T-cells specific for IL-10-inducing peptides showed little type I cytokine secretion. The expanded T-cells secreting type II cytokines in response to IGFBP-2 were not Treg but conventional Th2. However, it is known that vaccine-induced Th2 can have the same immunosuppressive effects as Treg [44]. The IFN-g-inducing sequences clustered in the N-terminus domain while the Th2-inducing peptides were found in the C-terminus domain. Mice immunized with the N-terminus vaccine (p1-163) developed significant levels of antigen-specific type I T-cells with little to no type II cytokine secretion. Mice immunized with the C-terminus (p164-328) construct developed T-cells that selectively elicited type II cytokine secretion. Immunization with the N-terminus vaccine significantly inhibited the growth of an implanted syngeneic epithelial tumor, while immunization with the C-terminus had no effect on tumor growth. Of note, when equal concentrations of the two vaccines were admixed and then used to immunize, the Th2-inducing vaccine completely abrogated the antitumor efficacy of the Th1-inducing vaccine. The Th2 generated are of a higher functional avidity than the Th1 cells elicited, thus may compete more effectively for antigen/MHC complexes at the site of the tumor. Thus, Th1-selective vaccines may allow unfettered expansion of both Th1 and CTL without the dampening effects of natural epitopes found within self-proteins associated with immune regulation.
Serendipitously, once the principle of splicing-out Th2 epitopes for unfettered Th1 immunogenicity is recognized, the selection of antigens becomes far more rewarding. One is not limited to seeking target antigens with expression restricted somehow to tumors or whose functions are unknown. Furthermore, one need not restrict oneself to “neo” epitopes that arise due to somatic mutations in each individual’s tumor. One can instead focus on antigens that (1) have a key role in tumorigenesis, (2) are overexpressed in tumors, and (3) whose overexpression is linked to poor prognoses. Genomic amplifications are perhaps more common in solid tumors than are any particular mutations; the resulting overexpressed proteins, therefore, make good targets for cancer immunotherapy. Key advantages to this strategy are that overexpressed tumor antigens are often conserved across species in function and are cellular “drivers,” making them more likely to be truncal to a tumor. Furthermore, these driver genes are often highly homologous across species in sequence, yielding convenient cross-reactivity of vaccine epitopes among humans and model animals. It is specifically the overexpression of a tumor antigen at high levels that generates vaccine targets, in the form of a “neo-repertoire” of subdominant epitopes that are presented and generate Th1 immunity [45, 46] targetable specifically to the tumor.
Applications in autoimmunity
Immune approaches to treatment of autoimmune disease are often less focused on the molecular features of self-antigens than are cutting-edge approaches to cancer. The most commonly used therapies sequester T-cells from their target tissues (antibodies like natalizumab; sphongosine-1-phosphate receptor agonists like fingolimod), destroy immune cells (antibodies like rituximab, drugs like cyclophosphamide), suppress immune cells (antibodies like abatacept), or suppress inflammatory cytokines or their receptors directly (antibodies like daclizumab and adalimumab; fusion proteins like etanercept). These approaches are effective but suppress immune responses broadly and leave patients susceptible to infections and some cancers. Even the most “targeted” of these approaches, focused on a single selected antigen, are so targeted by virtue of that antigen’s tissue or cellular expression pattern, rather than by leveraging molecular features of the antigen to modulate immunity against it per se. The concept of an epitope-based approach for enhancing tolerance to a self-antigen may not make as much intuitive sense as it does in cancer, where “skewing” the default immunity away from tolerance makes the concept seem compulsory.
Most antigen-focused research in autoimmune disease has been directed at identifying key, pathogenic antigens as the penultimate step to formulation of a tolerance-inducing therapeutic. Perhaps analogous to the “neoepitope” in tumor immunology, posttranslational modification of arginine residues in proteins yielding substitution with citrulline (“citrullination”) can be immunogenic. For example, biomarkers/diagnostics for rheumatoid arthritis (RA) include the presence of anticitrullinated protein antibodies (APCAs) in patients that accumulate in the joints and bind synovial proteins [47– 49] (such as citrullinated filaggrin, fibrinogen, vimentin, and collagen type II [50, 51]. The concept is that these new citrulline-based residues generate new T-cell epitopes that bind HLA-DR alleles with differing, perhaps higher affinity and lead to the generation of a tolerance-breaking and inflammatory immune T-cell response to the antigen. Furthermore, there is an association of increased presence of citrullinated proteins with autoimmune tissue damage and low rates of remission.
The therapeutic approach that leverages citrullinated antigens includes the attempted induction of tolerance to these antigens via chronic, low-level exposure to the citrullinated epitopes by administering them as peptide injections. In one study, a multi-epitope peptide composed of citrullinated collagen, filaggrin, and beta-fibrinogen peptides confirmed to bind ACPAs was shown to reduce symptoms in an experimental autoimmune arthritis model, and interestingly, to result in upregulation of Tregs, reduction in IL-17+ CD4 T-cells, and increased T-cell apoptosis [52]. These effects were presumed to be due to direct induction of a T-cell response by APC-mediated uptake and processing and presentation of the epitopes embedded in the peptide, although no attempt was made to characterize specifically the immunogenicity of these peptide epitopes.
Efforts to generate antigen-specific tolerance in RA have also been made in clinical studies using autologous dendritic cells (DCs), differentiated ex vivo by various protocols to a basal tolerogenic phenotype and then imbued with antigen specificity. In this case, citrullinated antigen peptides are pulsed into the DCs, and the immunogenicity of the response is controlled directly by the DC vehicle. In a phase I first-in-human study, HLA-DRB1-restricted RA patients treated with corresponding peptide-pulsed DC vaccine Rheumavax exhibited cellular immune responses to the vaccine that suggest a reduction in T-effector (Teff) cell population and increase in the ratio of Treg to Teff. Here again, the epitope sequence is not leveraged specifically for immunogenic properties [53].
Nanoparticles loaded with tolerance-inducing chemicals also show promise as vehicles for delivery of antigenic peptides, including proinsulin in preclinical models of type 1 diabetes, where they appear to induce tolerogenic DCs [54]. These approaches stay one degree of separation away from the event of epitope presentation to the T-cell receptor, where the first phenotype-determining signal to the T-cell occurs. The reliance on technology platforms to manipulate the immune response to an antigen irrespective of possibly unknown determinants in the antigens themselves may simply reflect the scarcity of other crucial tools.
As a more fundamental approach, there have been efforts to make peptide vaccines for multiple sclerosis (MS) by selecting possibly immunogenic sections of associated antigens like myelin basic protein (MBP), and MOG. ATX-MS-1467 is an interesting pool of MHCII peptide epitopes derived from MBP which bind directly to MHCII on mature DCs without intracellular processing [55]. Termed “apitopes”, for “antigen-processing independent epitopes,” these peptides are selected CD4+ T-cell epitopes and mimic the naturally processed epitopes [56, 57]. Dissimilar from a vaccine, ATX-MS-1467 is delivered as soluble peptides that bind to MHCII directly, inhibit both Th1 and Th2 responses, and produce a negative feedback response that includes IL-10 secretion [58]. In a small phase 1 study, investigators observed that treatment with ATX-MS-1467 was safe and led to a temporary reduction in T-cell proliferation and increase in IL-10 production in response to MBP 1 month after treatment, suggesting some tolerogenic efficacy. ATX-MS-1467 is typically compared to glatiramer acetate (GA), an immune “decoy” for MBP composed of a random polymer of its constituent amino acids, and an approved injectable drug for treatment of MS. GA is marketed as the injectable drug Copaxone, which typically generates in excess of $1 billion in sales and now has generic competition on the market.
In the realm of bona fide vaccines, there are few efforts that have reached the clinic. A full-length MBP antigen DNA vaccine for therapy of MS was taken through phase I and II studies in relapsing-remitting patients and showed (1) reduction of antigen-specific autoantibodies, (2) reduction in myelin-reactive IFNg-producing T-cells, and (3) some improvements in radiologically imaged lesions. However, these effects did not correspond to dose escalation and did not meet primary endpoints [59, 60]. The vaccine was given intramuscularly either alone or with atorvastatin as an adjuvant-like Th2-stimulating immunomodulator; atorvastatin had no added effect on endpoints in the study. The lack of well-characterized adjuvant/co-modulator and intramuscular injection route may have contributed to lack of immunogenic potency with this vaccine, and there remains a paucity of targeted antigens for MS that may be related to methods of immunogenic epitope selection.
Even in autoimmune diseases like neuromyelitis optica, ostensibly a singly aquaporin 4-pathogenic disease, there do not appear to be off-the-shelf vaccines in active development. In general, antigen-specific approaches in autoimmune disease seem to be lagging behind cancer in terms of industry clinical studies. This may be because some autoimmune diseases can be managed chronically, and non-specific cytotoxic or immune-suppressive therapies such as anti-B cell antibodies, cytotoxic drugs, or various cytokine-targeted biotherapeutics can be effective and administered somewhat infrequently.
The identification of Th2-selective epitopes from tumor antigens, which suppress the inflammatory immunity desired for cancer therapy, suggests that Th2 epitopes from pathogenic antigens in autoimmune disease could be used for anti-inflammatory therapy in this context.
Basic approaches to finding epitopes/Th1 vs Th2
The prediction of avid MHC binding has been used for a long time to identify potentially important vaccine and other immunogenic epitopes, often in combination with other identifying information such as homology to foreign species or common mutations. Sequence homology and in silico prediction algorithms have been used as an “immunoinformatic” approach to troubleshoot large peptide sequences for unwanted immunogenicity in vaccine designs or recombinant protein constructs, with “deimmunization” [61–64]. As the compendium of well-characterized self-antigen epitopes grows, there may be homology-based relationships uncovered that reveal sequence features linked to inherent Th1 vs Th2 or other immunogenic properties to epitopes.
The most modern and data-driven approaches to self-antigen vaccine development have recognized that simple delivery of a full-length recombinant protein corresponding to a tumor-associated marker is not an effective means of vaccination. The vast majority of new vaccine platforms have incorporated heavily engineered vectors that carry intrinsic danger signals (listeria, oncolytic viruses), complex prime-boost strategies, approaches to deliver antigens directly to APCs, autologous cellular vehicles, or approaches to “personalize” a vaccine to actual peptides found in the tumor. In fact, “personalization” of vaccine by targeting neoepitopes, which may be immunogenic in addition to being restricted to the individual’s tumor, is generating enormous new enthusiasm for tumor vaccine development. It is not clear that a neoepitope has intrinsically greater efficacy in a vaccine than do Th1 epitope discovered a priori from an antigen overexpressed in cancer, and the balance between the value and cost of personalization may make it less clear that the approach will be feasible from a practical standpoint.
In therapy of autoimmune disease, awareness of the need for antigen-specific treatments is escalating because of the dangers and inadequacies of non-specific, systemic suppression of inflammatory immunity. Entire classes of drugs designed to treat underlying disease rather than just the inflammation (e.g., disease-modifying antirheumatic drugs or DMARDs) have been defined but even targeted biologics have potentially deadly side effects because they target cell types responsible for immunity broadly. The ability to target specific antigens playing an instigating role in the establishment of chronic inflammation by leveraging the right, Th2-selective epitopes in a vaccine would provide an unprecedented degree of specificity and safety, and furthermore, possibly even prevent disease.
Regardless, it is clear that the balance of Th1-/Th2-selective immunogenicity of self-antigen epitopes must be fully characterized when targeting an antigen for a specific immune application, and novel methods to do so may accelerate the development of effective vaccines for the treatment and prevention of cancer and autoimmune diseases.
References
Disis ML, Calenoff E, McLaughlin G, Murphy AE, Chen W, Groner B, Jeschke M, Lydon N, McGlynn E, Livingston RB et al (1994) Jan 1 Existent T-cell and antibody immunity to HER-2/neu protein in patients with breast cancer. Cancer Res 54(1):16–20
Adler MJ, Dimitrov DS (2012) Therapeutic antibodies against cancer. Hematol Oncol Clin North Am 26(3):447–481 vii
Aghajanian H, Cho YK, Manderfield LJ, Herling MR, Gupta M, Ho VC, Li L, Degenhardt K, Aharonov A, Tzahor E, Epstein JA (2016) Jun 30 Coronary vasculature patterning requires a novel endothelial ErbB2 holoreceptor. Nat Commun 7:12038
Melief CJ, van Hall T, Arens R, Ossendorp F, van der Burg SH (2015) Therapeutic cancer vaccines. J Clin Invest 125(9):3401–3412
Gjerstorff MF, Andersen MH, Ditzel HJ (2015) Jun 30 Oncogenic cancer/testis antigens: prime candidates for immunotherapy. Oncotarget 6(18):15772–15787
Yang B, Jeang J, Yang A, Wu TC, Hung CF (2014) DNA vaccine for cancer immunotherapy. Hum Vaccin Immunother 10(11):3153–3164
Fridman WH, Pagès F, Sautès-Fridman C, Galon J (2012) Mar 15 The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer 12(4):298–306
Bernard CC, de Rosbo NK (1991) Immunopathological recognition of autoantigens in multiple sclerosis. Acta Neurol (Napoli) 13(2):171–178
Rosenthal KS, Mikecz K, Steiner HL 3rd, Glant TT, Finnegan A, Carambula RE (2015) Zimmerman DH. Rheumatoid arthritis vaccine therapies: perspectives and lessons from therapeutic ligand epitope antigen presentation system vaccines for models of rheumatoid arthritis. Expert Rev Vaccines 14(6):891–908
Reijonen H, Kwok WW, Nepom GT (2003) Detection of CD4+ autoreactive T cells in T1D using HLA class II tetramers. Ann N Y Acad Sci 1005:82–87
Lennon VA, Kryzer TJ, Pittock SJ, Verkman AS, Hinson SR (2005) Aug 15 IgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel. J Exp Med 202(4):473–477
Behan PO, Chaudhuri A (2014) EAE is not a useful model for demyelinating disease. Mult Scler Relat Disord 3(5):565–574
Ben-Nun A, Kaushansky N, Kawakami N, Krishnamoorthy G, Berer K, Liblau R, Hohlfeld R, Wekerle H (2014) From classic to spontaneous and humanized models of multiple sclerosis: impact on understanding pathogenesis and drug development. J Autoimmun 54:33–50
Rahma OE, Gammoh E, Simon RM, Khleif SN (2014) Sep 15 Is the “3 + 3” dose-escalation phase I clinical trial design suitable for therapeutic cancer vaccine development? A recommendation for alternative design. Clin Cancer Res 20(18):4758–4767
Small EJ, Schellhammer PF, Higano CS, Redfern CH, Nemunaitis JJ, Valone FH, Verjee SS, Jones LA, Hershberg RM (2006) Jul 1 Placebo-controlled phase III trial of immunologic therapy with sipuleucel-T (APC8015) in patients with metastatic, asymptomatic hormone refractory prostate cancer. J Clin Oncol 24(19):3089–3094
Le DT, Wang-Gillam A, Picozzi V, Greten TF, Crocenzi T, Springett G, Morse M, Zeh H, Cohen D, Fine RL, Onners B, Uram JN, Laheru DA, Lutz ER, Solt S, Murphy AL, Skoble J, Lemmens E, Grous J, Dubensky T Jr, Brockstedt DG, Jaffee EM (2015) Apr 20 Safety and survival with GVAX pancreas prime and listeria monocytogenes-expressing mesothelin (CRS-207) boost vaccines for metastatic pancreatic cancer. J Clin Oncol 33(12):1325–1333
Trimble CL, Morrow MP, Kraynyak KA, Shen X, Dallas M, Yan J, Edwards L, Parker RL, Denny L, Giffear M, Brown AS, Marcozzi-Pierce K, Shah D, Slager AM, Sylvester AJ, Khan A, Broderick KE, Juba RJ, Herring TA, Boyer J, Lee J, Sardesai NY, Weiner DB, Bagarazzi ML (2015) Nov 21 Safety, efficacy, and immunogenicity of VGX-3100, a therapeutic synthetic DNA vaccine targeting human papillomavirus 16 and 18 E6 and E7 proteins for cervical intraepithelial neoplasia 2/3: a randomised, double-blind, placebo-controlled phase 2b trial. Lancet 386(10008):2078–2088
Schuster J, Lai RK, Recht LD, Reardon DA, Paleologos NA, Groves MD, Mrugala MM, Jensen R, Baehring JM, Sloan A, Archer GE, Bigner DD, Cruickshank S, Green JA, Keler T, Davis TA, Heimberger AB, Sampson JH (2015) A phase II, multicenter trial of rindopepimut (CDX-110) in newly diagnosed glioblastoma: the ACT III study. Neuro-Oncology 17(6):854–861
Coveler AL, Rossi GR, Vahanian NN, Link C, Chiorean EG (2016) Algenpantucel-L immunotherapy in pancreatic adenocarcinoma. Immunotherapy 8(2):117–125
http://investors.aduro.com/phoenix.zhtml?c=242043&p=irol-newsArticle&ID=2168543
Hamilton E, Blackwell K, Hobeika AC, Clay TM, Broadwater G, Ren XR, Chen W, Castro H, Lehmann F, Spector N, Wei J, Osada T, Lyerly HK, Morse MA (2012) Feb 10 Phase 1 clinical trial of HER2-specific immunotherapy with concomitant HER2 kinase inhibition [corrected]. J Transl Med 10:28
Schneble EJ, Berry JS, Trappey FA, Clifton GT, Ponniah S, Mittendorf E, Peoples GE (2014) The HER2 peptide nelipepimut-S (E75) vaccine (NeuVax™) in breast cancer patients at risk for recurrence: correlation of immunologic data with clinical response. Immunotherapy 6(5):519–531
Vansteenkiste JF, Cho BC, Vanakesa T, De Pas T, Zielinski M, Kim MS, Jassem J, Yoshimura M, Dahabreh J, Nakayama H, Havel L, Kondo H, Mitsudomi T, Zarogoulidis K, Gladkov OA, Udud K, Tada H, Hoffman H, Bugge A, Taylor P, Gonzalez EE, Liao ML, He J, Pujol JL, Louahed J, Debois M, Brichard V, Debruyne C, Therasse P, Altorki N (2016) Efficacy of the MAGE-A3 cancer immunotherapeutic as adjuvant therapy in patients with resected MAGE-A3-positive non-small-cell lung cancer (MAGRIT): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 17(6):822–835
Wurz GT, Kao CJ, Wolf M, DeGregorio MW (2014) Tecemotide: an antigen-specific cancer immunotherapy. Hum Vaccin Immunother 10(11):3383–3393
Goldman B, DeFrancesco L (2009) The cancer vaccine roller coaster. Nat Biotechnol 27(2):129–139
Qian F, Gnjatic S, Jäger E, Santiago D, Jungbluth A, Grande C, Schneider S, Keitz B, Driscoll D, Ritter G, Lele S, Sood A, Old LJ, Odunsi K (2004) Nov 3 Th1/Th2 CD4+ T cell responses against NY-ESO-1 in HLA-DPB1*0401/0402 patients with epithelial ovarian cancer. Cancer Immun 4:12
Zarour HM, Maillere B, Brusic V, Coval K, Williams E, Pouvelle-Moratille S, Castelli F, Land S, Bennouna J, Logan T, Kirkwood JM (2002) Jan 1 NY-ESO-1 119-143 is a promiscuous major histocompatibility complex class II T-helper epitope recognized by Th1- and Th2-type tumor-reactive CD4+ T cells. Cancer Res 62(1):213–218
Mandic M, Castelli F, Janjic B, Almunia C, Andrade P, Gillet D, Brusic V, Kirkwood JM, Maillere B, Zarour HM (2005) Feb 1 One NY-ESO-1-derived epitope that promiscuously binds to multiple HLA-DR and HLA-DP4 molecules and stimulates autologous CD4+ T cells from patients with NY-ESO-1-expressing melanoma. J Immunol 174(3):1751–1759
Tsuji K, Hamada T, Uenaka A, Wada H, Sato E, Isobe M, Asagoe K, Yamasaki O, Shiku H, Ritter G, Murphy R, Hoffman EW, Old LJ, Nakayama E, Iwatsuki K (2008) Induction of immune response against NY-ESO-1 by CHP-NY-ESO-1 vaccination and immune regulation in a melanoma patient. Cancer Immunol Immunother 57(10):1429–1437
Gnjatic S, Altorki NK, Tang DN, Tu SM, Kundra V, Ritter G, Old LJ, Logothetis CJ, Sharma P (2009) Mar 15 NY-ESO-1 DNA vaccine induces T-cell responses that are suppressed by regulatory T cells. Clin Cancer Res 15(6):2130–2139
Ebert LM, MacRaild SE, Zanker D, Davis ID, Cebon J, Chen W (2012) A cancer vaccine induces expansion of NY-ESO-1-specific regulatory T cells in patients with advanced melanoma. PLoS One 7(10):e48424
Chakraborty NG, Li L, Sporn JR, Kurtzman SH, Ergin MT, Mukherji B (1999) May 1 Emergence of regulatory CD4+ T cell response to repetitive stimulation with antigen-presenting cells in vitro: implications in designing antigen-presenting cell-based tumor vaccines. J Immunol 162(9):5576–5583
Chakraborty NG, Chattopadhyay S, Mehrotra S, Chhabra A, Mukherji B (2004) Regulatory T-cell response and tumor vaccine-induced cytotoxic T lymphocytes in human melanoma. Hum Immunol 65(8):794–802
Disis ML (2011) Immunologic biomarkers as correlates of clinical response to cancer immunotherapy. Cancer Immunol Immunother 60(3):433–442
Ribas A, Timmerman JM, Butterfield LH, Economou JS (2003) Determinant spreading and tumor responses after peptide-based cancer immunotherapy. Trends Immunol 24(2):58–61
Disis ML, Wallace DR, Gooley TA, Dang Y, Slota M, Lu H, Coveler AL, Childs JS, Higgins DM, Fintak PA, Dela Rosa C, Tietje K, link J, Waisman J, Salazar LG (2009) Concurrent trastuzumab and HER2/neu-specific vaccination in patients with metastatic breast cancer. J Clin Oncol 27(28):4685–4692
Disis ML, Gooley TA, Rinn K, Davis D, Piepkorn M, Cheever MA, Knutson KL, Schiffman K (2002) Jun 1 Generation of T-cell immunity to the HER-2/neu protein after active immunization with HER-2/neu peptide-based vaccines. J Clin Oncol 20(11):2624–2632
GuhaThakurta D, Sheikh NA, Fan LQ, Kandadi H, Meagher TC, Hall SJ, Kantoff PW, Higano CS, Small EJ, Gardner TA, Bailey K, Vu T, DeVries T, Whitmore JB, Frohlich MW, Trager JB, Drake CG (2015) Aug 15 Humoral immune response against nontargeted tumor antigens after treatment with sipuleucel-T and its association with improved clinical outcome. Clin Cancer Res 21(16):3619–3630
Corbière V, Chapiro J, Stroobant V, Ma W, Lurquin C, Lethé B, van Baren N, Van den Eynde BJ, Boon T, Coulie PG (2011) Feb 15 Antigen spreading contributes to MAGE vaccination-induced regression of melanoma metastases. Cancer Res 71(4):1253–1262
Salazar LG, Fikes J, Southwood S, Ishioka G, Knutson KL, Gooley TA, Schiffman K, Disis ML (2003) Nov 15 Immunization of cancer patients with HER-2/neu-derived peptides demonstrating high-affinity binding to multiple class II alleles. Clin Cancer Res 9(15):5559–5565
Park KH, Gad E, Goodell V, Dang Y, Wild T, Higgins D, Fintak P, Childs J, Dela Rosa C, Disis ML (2008) Oct 15 Insulin-like growth factor-binding protein-2 is a target for the immunomodulation of breast cancer. Cancer Res 68(20):8400–8409
Cecil DL, Holt GE, Park KH, Gad E, Rastetter L, Childs J, Higgins D, Disis ML (2014) May 15 Elimination of IL-10-inducing T-helper epitopes from an IGFBP-2 vaccine ensures potent antitumor activity. Cancer Res 74(10):2710–2718
Guenova E, Watanabe R, Teague JE, Desimone JA, Jiang Y, Dowlatshahi M, Schlapbach C, Schaekel K, Rook AH, Tawa M, Fisher DC, Kupper TS, Clark RA (2013) Jul 15 TH2 cytokines from malignant cells suppress TH1 responses and enforce a global TH2 bias in leukemic cutaneous T-cell lymphoma. Clin Cancer Res 19(14):3755–3763
Milich DR, McLachlan A, Raney AK, Houghten R, Thornton GB, Maruyama T, Hughes JL, Jones JE (1991) May 15 Autoantibody production in hepatitis B e antigen transgenic mice elicited with a self T-cell peptide and inhibited with nonself peptides. Proc Natl Acad Sci U S A 88(10):4348–4352
Cibotti R, Kanellopoulos JM, Cabaniols JP, Halle-Panenko O, Kosmatopoulos K, Sercarz E, Kourilsky P (1992) Jan 1 Tolerance to a self-protein involves its immunodominant but does not involve its subdominant determinants. Proc Natl Acad Sci U S A 89(1):416–420
Cabaniols JP, Cibotti R, Kourilsky P, Kosmatopoulos K, Kanellopoulos JM (1994) Dose-dependent T cell tolerance to an immunodominant self peptide. Eur J Immunol 24(8):1743–1749
Nielen MM, van Schaardenburg D, Reesink HW, van de Stadt RJ, van der Horst-Bruinsma IE, de Koning MH, Habibuw MR, Vandenbroucke JP, Dijkmans BA (2004) Specific autoantibodies precede the symptoms of rheumatoid arthritis: a study of serial measurements in blood donors. Arthritis Rheum 50(2):380–386
Meyer O, Labarre C, Dougados M, Goupille P, Cantagrel A, Dubois A, Nicaise-Roland P, Sibilia J, Combe B (2003) Anticitrullinated protein/peptide antibody assays in early rheumatoid arthritis for predicting five year radiographic damage. Ann Rheum Dis 62(2):120–126
Simon M, Girbal E, Sebbag M, Gomès-Daudrix V, Vincent C, Salama G, Serre G (1993) The cytokeratin filament-aggregating protein filaggrin is the target of the so-called “antikeratin antibodies,” autoantibodies specific for rheumatoid arthritis. J Clin Invest 92(3):1387–1393
Turunen S, Koivula MK, Melkko J, Alasaarela E, Lehenkari P, Risteli J (2013) Sep 23 Different amounts of protein-bound citrulline and homocitrulline in foot joint tissues of a patient with anti-citrullinated protein antibody positive erosive rheumatoid arthritis. J Transl Med 11:224
Gertel S, Serre G, Shoenfeld Y, Amital H (2015) Jun 15 Immune tolerance induction with multiepitope peptide derived from citrullinated autoantigens attenuates arthritis manifestations in adjuvant arthritis rats. J Immunol 194(12):5674–5680
Benham H, Nel HJ, Law SC, Mehdi AM, Street S, Ramnoruth N, Pahau H, Lee BT, Ng J, Brunck ME, Hyde C, Trouw LA, Dudek NL, Purcell AW, O’Sullivan BJ, Connolly JE, Paul SK, Lê Cao KA, Thomas R (2015) Citrullinated peptide dendritic cell immunotherapy in HLA risk genotype-positive rheumatoid arthritis patients. Sci Transl Med 7(290):290ra87
Yeste A, Takenaka MC, Mascanfroni ID, Nadeau M, Kenison JE, Patel B, Tukpah AM, Babon JA, DeNicola M, Kent SC, Pozo D, Quintana FJ (2016) Tolerogenic nanoparticles inhibit T cell-mediated autoimmunity through SOCS2. Sci Signal 9(433):ra61
Streeter HB, Rigden R, Martin KF, Scolding NJ, Wraith DC (2015) Mar 12 Preclinical development and first-in-human study of ATX-MS-1467 for immunotherapy of MS. Neurol Neuroimmunol Neuroinflamm 2(3):e93
Anderton SM, Viner NJ, Matharu P, Lowrey PA, Wraith DC (2002) Influence of a dominant cryptic epitope on autoimmune T cell tolerance. Nat Immunol 3(2):175–181
Santambrogio L, Sato AK, Fischer FR, Dorf ME, Stern LJ (1999) Dec 21 Abundant empty class II MHC molecules on the surface of immature dendritic cells. Proc Natl Acad Sci U S A 96(26):15050–15055
Burkhart C, Liu GY, Anderton SM, Metzler B, Wraith DC (1999) Peptide-induced T cell regulation of experimental autoimmune encephalomyelitis: a role for IL-10. Int Immunol 11(10):1625–1634
Gabrysová L, Nicolson KS, Streeter HB, Verhagen J, Sabatos-Peyton CA, Morgan DJ, Wraith DC (2009) Aug 3 Negative feedback control of the autoimmune response through antigen-induced differentiation of IL-10-secreting Th1 cells. J Exp Med 206(8):1755–1767
Bar-Or A, Vollmer T, Antel J, Arnold DL, Bodner CA, Campagnolo D, Gianettoni J, Jalili F, Kachuck N, Lapierre Y, Niino M, Oger J, Price M, Rhodes S, Robinson WH, Shi FD, Utz PJ, Valone F, Weiner L, Steinman L, Garren H (2007) Induction of antigen-specific tolerance in multiple sclerosis after immunization with DNA encoding myelin basic protein in a randomized, placebo-controlled phase 1/2 trial. Arch Neurol 64(10):1407–1415
Garren H, Robinson WH, Krasulová E, Havrdová E, Nadj C, Selmaj K, Losy J, Nadj I, Radue EW, Kidd BA, Gianettoni J, Tersini K, Utz PJ, Valone F (2008) Steinman L; BHT-3009 study group. Phase 2 trial of a DNA vaccine encoding myelin basic protein for multiple sclerosis. Ann Neurol 63(5):611–620
Liu R, Moise L, Tassone R, Gutierrez AH, Terry FE, Sangare K, Ardito MT, Martin WD, De Groot AS (2015) H7N9 T-cell epitopes that mimic human sequences are less immunogenic and may induce Treg-mediated tolerance. Hum Vaccin Immunother. 11(9):2241–2252
Cousens LP, Moise L, De Groot AS. Novel methods for addressing immunogenicity in therapeutic enzymes. In A. Rosenberg, B. Demeule (eds) Biobetters, AAPS Advances. American Association of Pharmaceutical Scientists 2015 in the Pharmaceutical Sciences Series 9
Losikoff PT, Mishra S, Terry F, Gutierrez A, Ardito MT, Fast L, Nevola M, Martin WD, Bailey-Kellogg C, De Groot AS, Gregory SH (2015) HCV epitope, homologous to multiple human protein sequences, induces a regulatory T cell response in infected patients. J Hepatol 62(1):48–55
De Groot AS, Moise L, Liu R, Gutierrez AH, Tassone R, Bailey-Kellogg C, Martin W (2014) Immune camouflage: relevance to vaccines and human immunology. Hum Vaccin Immunother 10(12):3570–3575
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is a contribution to the special issue on Cancer and Autoimmunity - Guest Editor: Mads Hald Andersen
Rights and permissions
About this article
Cite this article
Watt, W.C., Cecil, D.L. & Disis, M.L. Selection of epitopes from self-antigens for eliciting Th2 or Th1 activity in the treatment of autoimmune disease or cancer. Semin Immunopathol 39, 245–253 (2017). https://doi.org/10.1007/s00281-016-0596-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00281-016-0596-7