Abstract
Systemic sclerosis (SSc) is a multisystem connective tissue disorder featured by vascular injury and fibrosis of the skin and various internal organs with autoimmune background. Although the pathogenesis of SSc still remains elusive, it is generally accepted that initial vascular injury due to autoimmunity and/or environmental factors causes structural and functional abnormalities of vasculature which eventually result in the constitutive activation of fibroblasts in various organs. Structural alterations consist of destructive vasculopathy (loss of small vessels) and proliferative obliterative vasculopathy (occlusion of arterioles and small arteries with fibro-proliferative change) caused by impaired compensatory vasculogenesis and angiogenesis. Impaired function of SSc vasculature includes the altered expression of cell adhesion molecules predominantly inducing Th2 and Th17 cell infiltration, endothelial dysfunction primarily due to the low availability of nitric oxide, the activated endothelial-to-mesenchymal transition leading to fibro-proliferative vascular change and tissue fibrosis, and the impaired coagulation/fibrinolysis system promoting the formation of intravascular fibrin deposits. Recent new insights into the therapeutic mechanisms of intravenous cyclophosphamide pulse and bosentan and the establishment of a new SSc animal model (Klf5 +/−;Fli1 +/− mice) provide us useful clues to further understand the development of vascular alterations characteristic of SSc. This article overviewed the present understanding of the pathogenesis of SSc vasculopathy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Systemic sclerosis (SSc) is a multisystem chronic connective tissue disease characterized by aberrant immune activation, vasculopathy, and resultant fibrosis of the skin and various internal organs with unknown etiology [1, 2]. Several independent case-control and genome-wide association studies have identified SSc susceptibility loci in the HLA region and non-HLA immune regulatory and inflammatory genes [3], suggesting that the immune and inflammatory system is a central mediator of this disease. Consistent with this idea, autoantibodies generally appear prior to the earliest dermal pathological changes, such as alterations of vascular structure and function clinically presented as nailfold capillary abnormalities and Raynaud’s phenomenon [4, 5]. Following and/or in parallel with these vascular changes, inflammatory mononuclear cells infiltrate into perivascular regions [6]. These vascular and inflammatory reactions finally result in the transition of interstitial fibroblasts originating from multiple sources, such as resident fibroblasts, bone marrow-derived progenitors, and epithelial- and endothelial-to-mesenchymal transition, to myofibroblasts producing excessive amount of extracellular matrix (ECM) proteins, which is a final consequence of the sequential pathological events of SSc [7].
Structural abnormality of SSc vasculature, which is believed to be caused by abnormal neovascularization and vascular remodeling due to the impairment of compensatory vasculogenesis and angiogenesis [8–10], is classified into two categories, which are destructive vasculopathy and proliferative obliterative vasculopathy. Destructive vasculopathy is characterized by progressive loss of capillaries, leading to tissue hypoxia and dermal fibroblast activation. On the other hand, proliferative obliterative vasculopathy is featured by proliferation of vascular cells, including endothelial cells and pericytes (PCs)/vascular smooth muscle cells (vSMCs), leading to the occlusion of arterioles and small arteries with fibro-proliferative change. Proliferative obliterative vasculopathy gradually and subclinically progresses along with disease duration and eventually becomes clinically evident as pulmonary arterial hypertension (PAH), digital ulcers, and scleroderma renal crisis [11]. All the vascular changes occur in almost all of SSc patients with variable degrees of severity.
In parallel with the progression of structural vascular changes, functional alteration of vascular cells induces endothelial dysfunction, impaired coagulation/fibrinolysis system, and aberrant expression of soluble factors and cell adhesion molecules leading to the pathological inflammation [12–14]. In combination with the intrinsic mechanism driving dermal fibroblast activation, such as autocrine TGF-β signaling [15] and the up-regulated expression of Toll-like receptor 4 [16], impaired vascular cell function eventually results in the initiation and maintenance of dermal fibroblast activation in SSc.
Thus, vasculopathy is a key pathological event inducing clinical symptoms associated with structural vascular changes and connecting inflammation and immune abnormalities with fibroblast activation in SSc. In this review article, the mechanisms underlying structural and functional abnormalities of SSc vasculature are overviewed.
Initial vascular injury in SSc
It is generally accepted that SSc is caused by a complex interplay between genetic factors and environmental influences [3]. This scenario is also applicable to initial vascular changes. Some environmental factors may injure endothelial cells, triggering aberrant inflammation and vascular remodeling leading to the development of SSc in individuals highly predisposed by genetic and epigenetic factors. Supporting this idea, the injection of bleomycin (BLM), a potential environmental factor of human SSc [3], enhances the induction of an SSc-like phenotype at the molecular level in endothelial cells of mice haploinsufficient for Fli1 gene which is epigenetically suppressed in the skin of SSc patients [14, 17].
As well as environmental factors, the contribution of aberrant immune system to initial vascular injury has been reported in γδT cells and B cells. In the early stage of the disease, the perivascular inflammatory cells are composed mainly of CD3+ T cells including CD4+ T cells, CD8+ T cells, and γδT cells [18, 19]. In SSc patients, the majority of γδT cells in the skin, peripheral circulation, and bronchoalveolar lavage fluid are positive for Vδ1, which is in contrast to circulating γδT cells of healthy controls primarily expressing Vδ2 [19]. Furthermore, SSc Vδ1+ γδT cells in peripheral circulation mostly express CD49d, the activation marker mediating the adherence to endothelial cells through the interaction with vascular cell adhesion molecule-1 (VCAM-1) [19]. Taken it into account that peripheral γδT cells derived from early dcSSc patients preferentially bind to endothelial cells and induce cell damage in vitro [20], γδT cells may be involved in an immune-mediated damage of the endothelium in the initial stage of SSc.
Another mediator of initial endothelial cell damage is anti-endothelial cell antibodies (AECAs) produced by aberrantly activated B cells. AECAs recognize heterogeneous antigens on endothelial cells and are detectable in 44 to 84% of SSc patients [21–23]. AECAs induce apoptosis of endothelial cells through antibody-dependent natural killer cell cytotoxicity via the Fas pathway, but not via the perforin/granzyme pathway [24]. Since AECAs-dependent induction of apoptosis is seen in human dermal microvascular endothelial cells, but not in human umbilical vein endothelial cells [24], these antibodies may partially explain the reason why endothelial cell damage preferentially occurs in microvasculature in early SSc. In addition to cytotoxic AECAs, ~30% of SSc patients have agonistic anti-intercellular adhesion molecule-1 (ICAM-1) antibodies which promote reactive oxygen species production and VCAM-1 expression in human umbilical vein endothelial cells (19). Given that reactive oxygen species induces apoptosis of endothelial cells and proliferation of vSMCs, anti-ICAM-1 antibodies may contribute to the loss of small vessels (destructive vasculopathy) and the fibro-proliferative remodeling of arterioles and small arteries (proliferative obliterative vasculopathy). Also, anti-ICAM-1 antibodies are likely to facilitate the infiltration of inflammatory cells through the up-regulated expression of VCAM-1 on post-capillary venules. Importantly, a wealth of evidence in clinical studies has demonstrated that the presence of AECAs is related to the severity of vascular and fibrotic symptoms in SSc patients [25], suggesting the pathological significance of these antibodies.
Thus, these clinical and experimental data suggest the contribution of innate and adaptive immunity to the initial vascular injury of SSc (Fig. 1).
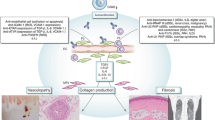
The potential mechanism of SSc vasculopathy leading to tissue fibrosis. Initial vascular injury is potentially caused by environmental factors and autoimmune attacks of anti-endothelial cell antibodies (AECAs) and γδT cells. Vascular injury subsequently results in structural and functional abnormalities characteristic of SSc. Structural abnormalities are classified into destructive vasculopathy and proliferative obliterative vasculopathy which are due to impaired compensatory vasculogenesis and angiogenesis. Functional abnormalities include the excessive production of reactive oxygen species (ROS), the altered expression of cell adhesion molecules inducing the infiltration of Th2 and Th17 cells, mast cells and macrophages, endothelial dysfunction primarily due to the low availability of nitric oxide (NO), the activated endothelial-to-mesenchymal transition (EndoMT) leading to fibro-proliferative vascular change and tissue fibrosis, and the impaired coagulation/fibrinolysis system promoting the formation of intravascular fibrin deposits. These vascular changes eventually induce fibroblast activation mediating the initiation and maintenance of tissue fibrosis in SSc. ICAM-1 intercellular cell adhesion molecule-1, GlyCAM-1 glycosylation-dependent cell adhesion molecule-1, vWF von Willebrand factor, TGF-β transforming growth factor-β, CTGF connective tissue growth factor, PDGF platelet-derived growth factor, ECs endothelial cells, vSMCs vascular smooth muscle cells, PAH pulmonary arterial hypertension, DUs digital ulcers, SRC scleroderma renal crisis
Impaired neovascularization and vascular remodeling in SSc
The histological features of SSc vasculopathy include the decrease in the number of small vessels, dilation of capillaries, and stenosis of arterioles and small arteries. Although the detailed molecular mechanism causing these vascular changes still remains largely elusive, the impairment of neovascularization and vascular remodeling following vascular injury has been believed to be involved. The processes of neovascularization and vascular remodeling consist of vasculogenesis and angiogenesis. Accumulating evidence suggests that both of vasculogenesis and angiogenesis are uniquely impaired in SSc [8–10].
Impaired vasculogenesis in SSc
The term “vasculogenesis” represents the de novo differentiation of mature endothelial cells through the recruitment and differentiation of endothelial progenitor cells (EPCs). Vasculogenesis is classified into two distinct categories, which are embryonic vasculogenesis and postnatal vasculogenesis. The initial stage of embryonic vasculogenesis is the formation of a primitive network of tubules known as a capillary or vascular plexus occurring concurrent with the induction of definitive hematopoiesis, both of which are derived from primitive endothelial and hematopoietic cells [26], suggesting that hematopoietic lineage gives rise to EPCs. Postnatal vasculogenesis is the physiological and pathological vascular remodeling by bone marrow-derived EPCs which occurs throughout adult life [27]. In response to vascular injury, EPCs are mobilized from bone marrow into the circulation, recruited to the vascular lesions and differentiate into a variety of mature cell types, including endothelial cells, PCs/vSMCs, fibroblasts, and macrophages, according to their origin and the local environment [28]. The decreased number, dysfunction, and/or impaired recruitment of circulating EPCs are the potential factors underlying inadequate vascular repair due to defective vasculogenesis.
Circulating EPCs are strictly divided into two types of cells, which are endothelial colony-forming cells (ECFCs) and pro-angiogenic hematopoietic cells (PHCs). ECFCs are thought to be “true endothelial progenitor cells” because of their abilities to expand clonogenically in vitro and to form vessels in vivo [29]. ECFCs are included in the population of CD34+CD31+CD133−CD45−CD14− circulating cells [30], but the exact precursors of those cells have not been identified so far. On the other hand, PHCs are further divided into at least two distinct groups of cells, which are CD14+ monocytic PHCs (mPHCs) and CD14- non-monocytic PHCs (non-mPHCs). PHCs are originally identified as EPCs, but the currently accepted notion is that PHCs differentiates into pro-angiogenic support cells, but not into endothelial cells [31].
Although the evaluation of ECFCs in terms of function and number is difficult due to the small number of those cells in the peripheral circulation, the studies on the number and property of PHCs have been reported. In SSc patients, the number of non-mPHCs is greatly reduced compared with healthy controls [9]. On the contrary, the larger quantity of mPHCs is present, and those cells tend to differentiate into fibroblast-like cells in SSc patients [32]. Therefore, it is speculated that vascular injury preferentially mobilizes mPHCs differentiating primarily into fibroblast-like cells in SSc, leading to the loss of neovascularization (destructive vasculopathy) and the development of fibro-proliferative vascular change (proliferative obliterative vasculopathy) characteristic of SSc (Fig. 1) [33].
The altered expression of molecules regulating the recruitment of EPCs is also reported in SSc. For example, CCN1, a secreted cysteine-rich heparin-binding protein [34], binds as a soluble factor to EPCs through integrin αVβ3 and αMβ2, which aids in their attachment and transmigration into the area of neovascularization [35]. CCN1 also induces the release of various chemokines, cytokines, growth factors, and proteolytic enzymes from EPCs, resulting in subsequent proliferation of endothelial cells and angiogenesis [35]. In SSc patients, the expression of CCN1 is markedly decreased in dermal small vessels and decreased serum CCN1 levels correlate with the current and past history of digital ulcers [36]. Therefore, the decrease in endothelial CCN1 expression may be associated with the impaired recruitment of EPCs to injured vascular areas, contributing to defective vasculogenesis, but the research on EPC recruitment is still limited in SSc.
Aberrantly activated pro-angiogenic gene program in SSc endothelial cells
Angiogenesis is the formation of new blood vessels that sprout from pre-existing vessels by a process involving the proliferation and migration of mature endothelial cells. Reflecting impaired angiogenesis, serum levels of various angiogenic/angiostatic factors are largely altered in SSc patients. So far, most of studies have revealed that pro-angiogenic factors are predominant throughout the disease course, especially in the active stage of the disease, suggesting the constitutive pro-angiogenic status in SSc. For instance, serum levels of vascular endothelial growth factor are elevated in SSc patients, especially in its earliest disease stage [37]. Furthermore, serum levels of angiopoietin-2, which reflect a pro-angiogenic status, positively correlate with disease activity and severity of SSc [38]. The constitutive pro-angiogenic status of SSc vasculature is also proven by the studies on the phenotype of PCs. In small vasculature of SSc lesional skin, PCs express Rgs5, a marker of PCs with pro-angiogenic phenotype, at higher levels and α-smooth muscle actin, a marker of PCs with angiostatic phenotype, at lower levels [39, 40]. Furthermore, SSc endothelial cells express low levels of VE-cadherin, platelet endothelial cell adhesion molecule-1, platelet-derived growth factor (PDGF)-B, S1p1, cathepsin V, and CCN1 (vascular stabilization-related factors) and elevated levels of MMP-9, cathepsin B, and lipocalin-2 (pro-angiogenic factors) [36, 40–43]. Given that endothelial cell-specific Fli1 knockout mice, which recapitulate the pro-angiogenic vascular property of SSc at the molecular level [40], exhibit leaky small vessels, pro-angiogenic status may be related to the development of vascular fragility, such as nailfold bleeding, in SSc.
The impaired interaction between endothelial cells and PCs/vSMCs, which is characterized by the decreased expression of α-smooth muscle actin in PCs/vSMCs, also seems to be involved in the development of telangiectasia and PAH in SSc. It is well known that vascular complications of Rendu-Osler-Weber disease, including telangiectasia on fingers, palms, lips, and tongue and PAH, are quite similar to those of SSc. Rendu-Osler-Weber disease is caused by the genetic mutation of ENG and ALK1 genes [44], both of which encode the molecules regulating the interaction between endothelial cells and PCs/vSMCs; therefore, loss of the interaction between those cells due to the constitutive pro-angiogenic state may contribute to the development of telangiectasia and PAH as well as vascular disintegrity in SSc.
The role of vascular activation in tissue fibrosis of SSc
The induction of Th2 and Th17 cell infiltration by SSc vasculopathy
Endothelial cell damage generally modifies the expression profiles of cell adhesion molecules, chemokines, cytokines, and growth factors, activates inflammatory cells, and promotes their infiltration into the perivascular areas. The intensity and property of inflammation are largely regulated by cell adhesion molecules expressing on inflammatory cells and endothelial cells.
In the peripheral blood mononuclear cells from SSc patients, the expression of a cluster of gene-encoding molecules targeting those cells to the endothelium is elevated [45]. These molecules include L-selectin and integrin α6, key leukocyte adhesion molecules involved in initial tethering to the endothelial cells, and selectin-P ligand-regulating leukocyte rolling on endothelial cells and T cell skin homing, suggesting the high ability of SSc inflammatory cells to infiltrate into injured tissues.
The following association of cell adhesion molecules expressing on endothelial cells, including ICAM-1, glycosylation-dependent cell adhesion molecule-1 (GlyCAM-1, a physiologic ligand for L-selectin), E-selectin, and P-selectin, with the pathological tissue fibrosis has been well studied in BLM-treated mice: (i) BLM-induced dermal fibrosis is augmented in Selp −/− and Sele −/− mice, while suppressed in Icam1 −/− and Sell −/− mice, (ii) the number of mast cells is increased in Selp −/− and Sele −/− mice, while decreased in Icam1 −/− and Sell −/− mice, (iii) the number of macrophages is decreased in Icam1 −/− and Sell −/− mice, and (iv) L-selectin and ICAM-1 regulate Th2 and Th17 cell accumulation, while P-selectin and E-selectin regulate Th1 cell infiltration [46]. The lesional skin of early dcSSc patients highly expresses all of these cell adhesion molecules, especially ICAM-1 and GlyCAM-1 predominating over E-selectin and P-selectin [14]. Therefore, SSc endothelial cells theoretically possess the property to promote the infiltration of Th2 and Th17 cells, mast cells, and macrophages (Fig. 1).
A series of studies have revealed the increased infiltration of macrophages and mast cells and the Th2/Th17 skewed immune polarization during the early and progressive stage of SSc [47–50]. In particular, the dynamics of Th1/Th2 paradigm has been well studied in association with SSc disease stage. In the early stage of dcSSc with progressive skin sclerosis, serum IL-6 and IL-10 levels are significantly elevated while decreased to normal levels in the late stage of dcSSc characterized by the improvement of skin sclerosis [51]. IL-4 keeps normal levels in the early stage of dcSSc but is decreased along with the resolution of skin sclerosis. In contrast, serum IL-12 levels are decreased in the early stage of dcSSc, then gradually increase in parallel with disease duration, and finally reach significantly higher levels than normal controls in the late stage of dcSSc [49]. Of note, maximal serum IL-12 levels throughout the disease course inversely correlate with mortality of dcSSc patients. Thus, immune polarization generally shifts from Th2 to Th1 in parallel with disease duration in SSc, while the sustained Th2 immune polarization closely associates with exacerbation of the disease. As for Th17 cytokines, the expression levels of IL-17A, but not IL-17 F, are increased in the lesional skin of early dcSSc [52]. Furthermore, the percentage of circulating Th17 cells and IL-17 production are elevated in peripheral blood mononuclear cells of SSc patients compared with those of healthy controls, and the number of Th17 cells correlates with disease activity [50]. Thus, the predominant infiltration of Th2/Th17 cells into the lesional skin of SSc patients is coordinately regulated by Th2/Th17 skewed immune polarization and the altered expression of cell adhesion molecules on endothelial cells.
Cell adhesion also plays a pivotal role in the activation of SSc fibroblasts. In the early stage of SSc, the majority of infiltrating T cells express activation markers, such as CD69 [6]. Since CD69 regulates cell contact interaction between T cells and other cells, including fibroblasts, activated T cells may affect the activation status of SSc dermal fibroblasts. Importantly, SSc dermal fibroblasts exhibit unique responses to activated T cells in vitro. In normal dermal fibroblasts, collagen production is suppressed by Th1 cells through membrane-associated IFN-γ [53] and by Th2 cells through membrane-associated TNF-α [54], which overcome a pro-fibrotic effect of IL-4. In SSc dermal fibroblasts, by contrast, increased collagen synthesis is resistant to Th1 cell- and Th2 cell-mediated suppression, especially to the latter [53, 54]. Considering Th2 skewed immune polarization during the early active stage of dcSSc, unresponsiveness to Th2 cell-mediated suppression may contribute to fibroblast activation in early stage of dcSSc. In addition to direct interaction, cytokines secreted by activated T cells are involved in the regulation of ECM metabolism by fibroblasts [55]. It has been suggested that ECM deposition is decreased by Th1 cytokines, including IFN-γ, whereas increased by Th2 cytokines, including IL-4, IL-6, IL-10, and IL-13 [55]. Furthermore, IL-17A suppresses collagen production in normal dermal fibroblasts while not in SSc dermal fibroblasts at least partly due to the decrease in IL-17 receptor type A expression [52]. Therefore, SSc dermal fibroblasts possess a unique phenotype to selectively respond to pro-fibrotic stimulation from activated T cells.
Thus, the dynamics of Th1/Th2/Th17 paradigm throughout the disease course and the altered response of SSc dermal fibroblasts to activated T helper cells explain a part of the fibrotic process of SSc. SSc vasculopathy contributes to this process by controlling the infiltration of T helper cells through the altered expression of cell adhesion molecules. Supporting this idea, serum levels of cell adhesion molecules correlate with the severity of tissue fibrosis and/or vascular complications in SSc patients [56–60].
Endothelial-to-mesenchymal transition in SSc
The hallmark of SSc vasculopathy is vascular disintegrity with constitutively activated pro-angiogenic gene program at the molecular level [40]. According to the experimental data on animal models of fibrotic disorders, such as diabetic nephropathy, cardiac fibrosis, and BLM-induced dermal and pulmonary fibrosis [14, 61–64], vascular activation may be associated with the induction of endothelial-to-mesenchymal transition (EndoMT). Since EndoMT provides mesenchymal cells which potentially participate in fibro-proliferative vasculopathy and tissue fibrosis, EndoMT is believed to be involved in the pathological process of SSc (Fig. 1) [65]. The molecular process underlying EndoMT has been well studied, and the most important signaling molecules identified so far include PKC-δ and c-Abl [66]. The blockade of either of these two molecules is enough to inhibit EndoMT. Importantly, there have been a couple of case reports showing the dramatic improvement of dermal fibrosis and PAH in SSc patients treated with imatinib mesylate [67, 68], an inhibitor of c-Abl, PDGF receptor and c-kit tyrosine kinases. Although imatinib mesylate exerts its beneficial effect on pulmonary arterial pressure primarily through the inhibition of PDGF receptor tyrosine kinase [69], the inhibition of EndoMT by acting on c-Abl tyrosine kinase may be also involved. Although at the time of writing imatinib mesylate is not approved for PAH due to the high frequency of serious adverse events, these clinical data suggest the potential of c-Abl tyrosine kinase as a therapeutic target of SSc vasculopathy.
Endothelial dysfunction in SSc
Another hallmark of functional abnormalities related to SSc vasculopathy is impaired endothelial function. Raynaud’s phenomenon is the most common manifestation of the SSc-related endothelial dysfunction characterized by exaggerated but reversible vasospasm in response to cold exposure, stress, or emotional upset. The vasodilatory response to blood flow-associated shear stress is a useful parameter to quantify endothelial function. Flow-mediated dilation (FMD) of brachial artery is a validated non-invasive physiological measure widely used as a research tool to evaluate endothelial function [70]. The increased shear stress stimulates endothelial cells to release several vasodilators, primarily nitric oxide (NO) [71]; therefore, the resulting augmentation of arterial diameter estimates the endothelium-dependent vasodilatation reflecting the bioavailability of NO. Since calcification and fibrosis reduce vascular compliance and subsequently affect NO signaling by limiting vascular stretch [72, 73], vascular stiffness itself is also an important factor determining FMD. In SSc patients, several studies have demonstrated the reduction of FMD values [74–81]. More importantly, the inverse correlation of FMD values with pulmonary arterial pressure and the association of decreased FMD values with the presence of PAH and digital ulcers have been reported [12, 79]. Furthermore, FMD values are already decreased in SSc patients with early nailfold capillary changes and further reduced in parallel with the progression of microvascular damages [78].
Given that FMD reflects the degree of shear stress-induced NO release [71], the significant decrease of FMD values in SSc patients indicates the impaired NO production from SSc endothelial cells. There are a couple of possible mechanisms responsible for this observation. First, the expression levels of NO synthase (NOS), including endothelial NOS (eNOS) and inducible NOS (iNOS), are altered in SSc endothelial cells, namely eNOS levels are decreased, while iNOS levels are increased, potentially affecting the NO production in those cells [82]. Second, sera from SSc patients attenuate the activity of NOS without affecting the expression levels of eNOS and iNOS in cultured human dermal microvascular endothelial cells [83]. Finally, the activity of NOS is decreased in endothelial cells exposed to pulse perfusion when cultured in less distensible conduits [72, 73]. Therefore, it is likely that NOS production is decreased in SSc endothelial cells due to the suppression of NOS activity and the decreased compliance of vasculature with fibro-proliferative change [84].
In aggregate, the decrease in endothelial NO production is a characteristic feature of SSc vasculopathy, which is largely related to the dysfunction of SSc endothelial cells (Fig. 1). This notion is important to consider the administration of drugs acting on NO signaling pathways, such as phosphodiesterase type 5 inhibitors and a stimulator of soluble guanylate cyclase, in SSc patients.
Impaired coagulation/fibrinolysis system in SSc
As described above, endothelial injury induces inflammation and aberrant vascular remodeling leading to fibroblast activation. In addition to these events, vascular injury also activates platelets and subsequently promotes the formation of intravascular fibrin deposits leading to luminal narrowing and vessel obstruction. An increased production of vasoconstrictors such as endothelin, an underproduction of vasodilators such as prostacyclin and NO, and the increased expression of von Willebrand factor may contribute to hypercoagulation status in SSc [85–87]. Furthermore, integrin αIIb and glycoprotein Ibβ, receptors for von Willebrand factor expressing on platelets, are abnormally up-regulated in SSc [45]. Although the altered balance of coagulation/fibrinolysis still leaves a room for debate, it is widely accepted that the coagulation system is activated while fibrinolytic activity is impaired in SSc. A variable degree of luminal thrombosis following vascular injury may contribute to impaired peripheral circulation, the induction of inflammation, and the subsequent activation of vascular cells and fibroblasts (Fig. 1) [88–90]. Based on this idea, anticoagulant and antiplatelet agents have been used for the treatment of SSc.
Animal models of SSc vasculopathy
In SSc, the understanding of the molecular mechanism and the development of the therapeutic strategy have been hindered largely due to the lack of animal models recapitulating the three cardinal features which are immune abnormalities, vasculopathy, and tissue fibrosis characteristic of this disease. Under this situation, a new genetic animal model of SSc, double heterozygous mice for Klf5 and Fli1 genes, has been established. This new SSc model is novel in that immune abnormalities, vasculopathy, and tissue fibrosis spontaneously and sequentially occur in this order, which is reminiscent of the natural disease course of SSc [91]. Given that KLF5 and FLI1 genes are epigenetically suppressed in SSc dermal fibroblasts [17, 91], this animal model supports the notion that environmental factors play a pivotal role in the development of SSc in individuals highly predisposed by genetic factors [3].
In Klf5 +/−;Fli1 +/− mice, the number of dermal and pulmonary blood vessels is decreased along with aging, accompanied by decreased blood flow and tissue hypoxia. Furthermore, structural abnormalities of vasculature such as stenosis of arterioles and bushy capillaries appear in the skin. Moreover, pulmonary vascular changes corresponding to PAH and pulmonary veno-occlusive disease are also evident [91]. Therefore, Klf5 +/−;Fli1 +/− mice develop both destructive vasculopathy and proliferative obliterative vasculopathy characteristic of SSc. Given that vascular network alterations of Klf5 +/− mice and Fli1 +/− mice are normal and modest, respectively, simultaneous haploinsufficiency is required for the development of far more significant vascular abnormalities. Although the molecular mechanism underlying vasculopathy in Klf5 +/−;Fli1 +/− mice still remains unknown, Fli1 deficiency-dependent activation of pro-angiogenic signaling and Klf5 deficiency-mediated connective tissue growth factor (CTGF) induction may contribute to this process [40, 91]. Endothelial cell-specific Fli1 knockout mice mimic vascular disintegrity of SSc which is characterized by reduced interaction between endothelial cells and PCs/vSMCs due to the activation of pro-angiogenic gene program [40]. Periadventitial administration of recombinant CTGF induces neointimal thickening, enlargement of an external elastic lamina area, adventitial collagen accumulation, adventitial myofibroblast transformation, and proliferation of vascular wall cells [92]. Given that SSc is a multifactorial disease including heterogeneous disease subsets, it is speculated that the simultaneous deficiency of KLF5 and Fli1 genes contributes to the development of a certain subset of SSc.
The strategy for the treatment of SSc vasculopathy
As described above, impaired vascular remodeling is a critical step in the pathogenesis of SSc. Therefore, defective vasculogenesis and aberrantly activated angiogenesis are the major two targets for the treatment of SSc vasculopathy. A series of clinical and experimental data suggests that intravenous cyclophosphamide pulse (IVCY) and bosentan potentially reverse destructive vasculogenesis and aberrantly activated angiogenesis in SSc, respectively.
IVCY
The combination therapy of oral corticosteroid with IVCY is the first-line treatment against interstitial lung disease (ILD) associated with SSc (SSc-ILD) [93–95]. IVCY is used for SSc-ILD as an immunosuppressant because autoreactive immune responses seem to largely contribute to the development of alveolitis and resultant pulmonary fibrosis [96]. However, a new aspect of IVCY as a treatment targeting vascular complications of SSc has been revealed [97, 98]. IVCY attenuates clinical features associated with SSc vascular damage, such as nailfold capillary abnormalities and Raynaud’s phenomenon [99]. Therefore, it is speculated that IVCY improves skin sclerosis and ILD at least partially by targeting vasculopathy of these organs. Consistent with this notion, IVCY significantly increases the number of circulating CD34+CD133+VEGFR2+ EPCs (non-mPHCs) in SSc-ILD patients compared with baseline, although the degree of its beneficial effect on the number of non-mPHCs is different in each case. Importantly, IVCY decreases circulating vascular injury markers, such as plasma VEGF and E-selectin levels, in the responders (SSc-ILD patients with the increased number of non-mPHCs after IVCY), while not in the non-responders (SSc-ILD patients with no increase in the number of non-mPHCs after IVCY). Furthermore, all the patients with progressive ILD even after the treatment are reported to be the non-responders [100]. Although the detailed mechanism by which IVCY exerts its beneficial effect on SSc vasculopathy is still unknown, these clinical data suggest that IVCY improves SSc vasculopathy at least partially by promoting vascular repair through the induction of EPCs.
Bosentan
Endothelin 1 (ET-1) is a critical mediator involved in the developmental process of proliferative obliterative vasculopathy of SSc by inducing fibro-proliferative changes in the vessel walls. This notion is supported by the clinical observation that serum ET-1 levels are elevated in SSc patients with PAH and scleroderma renal crisis [86, 101]. Given that the presence of ulnar artery occlusion, a typical vascular change of proliferative obliterative vasculopathy, is closely related to new or recurrent onset of digital ulcers in SSc [102], ET-1 is also thought to be involved in the development of digital ulcers. Consistent with this notion, as shown in two high-quality randomized controlled trails, RAPIDS1 and RAPID2 studies, bosentan, a dual endothelin receptor antagonist, prevents the development of new digital ulcers in SSc without having a beneficial effect on healing of the pre-existing digital ulcers [103, 104]. Since the improvement of ulnar arterial stenosis and peripheral circulation in response to bosentan is reported [105], bosentan may elicit a significant preventive effect on digital ulcers through a potential reverse remodeling effect in addition to a potent vasodilatory effect.
On the other hand, ET-1 also plays a part in the pathologically activated angiogenesis. For instance, bosentan alleviates increased neovascularization and leaky vessels in cerebrovascular system of a type 2-diabetic animal model and inhibits tumor vascularization and bone metastasis in an animal model of breast carcinoma cell metastasis [106, 107]. This is also the case in SSc because the number of nailfold ramified capillaries, which reflect activated angiogenesis, is decreased after a one-year administration of bosentan [108]. As a result of its dual conflicting effects on SSc vasculopathy, such as the improvement of peripheral circulation and the inhibition of angiogenesis, bosentan seems not to have a beneficial impact on the pre-existing digital ulcers in SSc [103, 104]. However, the combination therapy of bosentan and iloprost, but not iloprost alone, increases the number of nailfold ramified capillaries [109], suggesting that the combination therapy, but neither bosentan nor iloprost alone, promotes angiogenesis. Given that the combination therapy of bosentan with prostanoids was not allowed in RAPIDS1 and RAPIDS2 studies, the combination therapy may show some beneficial effect on the healing of pre-existing digital ulcers by promoting angiogenesis in SSc.
The beneficial effect of bosentan on SSc vasculopathy may be partially explained by its reversal effect on endothelial expression of Fli1, a potential predisposing factor of SSc. In endothelial cells, ET-1 stimulation sequentially activates c-Abl and PKC-δ [110]. Then, activated PKC-δ translocates into the nucleus and phosphorylates Fli1 at threonine 312 [111]. Phosphorylated Fli1 increases its affinity for p300/CREB-binding protein-associated factor possessing histone acetyltransferase activity, resulting in Fli1 acetylation at lysine 380. Acetylated Fli1 loses its DNA-binding ability and is rapidly degraded [112]. Since endothelial cells maintain homeostasis by autocrine ET-1 stimulation, blockade of autocrine ET-1 induces Fli1 up-regulation by increasing its protein stability [110]. In line with these in vitro data, bosentan reverses vascular disintegrity of endothelial cell-specific Fli1 knockout mice in parallel with the up-regulation of endothelial Fli1 expression [110], suggesting that bosentan potentially improves Fli1 deficiency-dependent vascular changes in vivo. Since Fli1 deficiency due to an epigenetic mechanism is potentially involved in the development of SSc vasculopathy [17, 113], the reversal of Fli1 expression may partially explain the beneficial effect of bosentan on SSc vasculopathy.
Conclusions
SSc vasculopathy is caused by the complex interaction of various pathological processes including autoimmune attacks, impaired compensatory vasculogenesis, and angiogenesis, EndoMT, endothelial dysfunction, and impaired coagulation/fibrinolysis system. The recent progresses in the research field of SSc vasculopathy, in particular the potential mechanism explaining the efficacy of IVCY and bosentan and the establishment of a new animal model, provide us useful clues to better understand its developmental process. Another promising therapy for SSc, B cell depletion by rituximab (a chimeric monoclonal antibody against CD20), has been reported to potentially improve skin sclerosis, ILD, and vasculopathy [114–118], supporting the concept of the sequential pathological process of this disease starting from immune activation and leading to vasculopathy and tissue fibrosis. In the near future, the emergence and evaluation of biologic therapies and small-molecule inhibitors of intracellular signal transduction pathways would facilitate the understanding of the pathogenesis of SSc as is the case with other autoimmune diseases.
References
Abraham DJ, Krieg T, Distler J, Distler O (2009) Overview of pathogenesis of systemic sclerosis. Rheumatology (Oxford) 48(Suppl 3):iii3–iii7
Asano Y (2010) Future treatments in systemic sclerosis. J Dermatol 37:54–70
Broen JC, Radstake TR, Rossato M (2014) The role of genetics and epigenetics in the pathogenesis of systemic sclerosis. Nat Rev Rheumatol 10:671–681
Prescott RJ, Freemont AJ, Jones CJ, Hoyland J, Fielding P (1992) Sequential dermal microvascular and perivascular changes in the development of scleroderma. J Pathol 166:255–263
Sgonc R, Gruschwitz MS, Dietrich H, Recheis H, Gershwin ME, Wick G (1996) Endothelial cell apoptosis is a primary pathogenetic event underlying skin lesions in avian and human scleroderma. J Clin Investig 98:785–792
Kalogerou A, Gelou E, Mountantonakis S, Settas L, Zafiriou E, Sakkas L (2005) Early T cell activation in the skin from patients with systemic sclerosis. Ann Rheum Dis 64:1233–1235
Ihn H (2005) Scleroderma, fibroblasts, signaling, and excessive extracellular matrix. Curr Rheumatol Rep 7:156–162
Distler JH, Gay S, Distler O (2006) Angiogenesis and vasculogenesis in systemic sclerosis. Rheumatology (Oxford) 45(Suppl 3):iii26–iii27
Kuwana M, Okazaki Y, Yasuoka H, Kawakami Y, Ikeda Y (2004) Defective vasculogenesis in systemic sclerosis. Lancet 364:603–610
Rabquer BJ, Koch AE (2012) Angiogenesis and vasculopathy in systemic sclerosis: evolving concepts. Curr Rheumatol Rep 14:56–63
Gabrielli A, Avvedimento EV, Krieg T (2009) Scleroderma. N Engl J Med 360:1989–2003
Takahashi T, Asano Y, Amiya E, Hatano M, Tamaki Z, Takata M et al (2014) Clinical correlation of brachial artery flow-mediated dilation in patients with systemic sclerosis. Mod Rheumatol 24:106–111
Cerinic MM, Valentini G, Sorano GG, D’Angelo S, Cuomo G, Fenu L et al (2003) Blood coagulation, fibrinolysis, and markers of endothelial dysfunction in systemic sclerosis. Semin Arthritis Rheum 32:285–295
Taniguchi T, Asano Y, Akamata K, Noda S, Takahashi T, Ichimura Y et al (2015) Fibrosis, vascular activation, and immune abnormalities resembling systemic sclerosis in bleomycin-treated Fli-1-haploinsufficient mice. Arthr Rheumatol 67:517–526
Ihn H (2008) Autocrine TGF-beta signaling in the pathogenesis of systemic sclerosis. J Dermatol Sci 49:103–113
Bhattacharyya S, Kelley K, Melichian DS, Tamaki Z, Fang F, Su Y et al (2013) Toll-like receptor 4 signaling augments transforming growth factor-β responses: a novel mechanism for maintaining and amplifying fibrosis in scleroderma. Am J Pathol 182:192–205
Wang Y, Fan PS, Kahaleh B (2006) Association between enhanced type I collagen expression and epigenetic repression of the FLI1 gene in scleroderma fibroblasts. Arthritis Rheum 54:2271–2279
Mavalia C, Scaletti C, Romagnani P, Carossino AM, Pignone A, Emmi L et al (1997) Type 2 helper T-cell predominance and high CD30 expression in systemic sclerosis. Am J Pathol 151:1751–1758
Giacomelli R, Matucci-Cerinic M, Cipriani P, Ghersetich I, Lattanzio R, Pavan A et al (1998) Circulating Vdelta1+ T cells are activated and accumulate in the skin of systemic sclerosis patients. Arthritis Rheum 41:327–334
Kahaleh MB, Fan PS, Otsuka T (1999) Gammadelta receptor bearing T cells in scleroderma: enhanced interaction with vascular endothelial cells in vitro. Clin Immunol 91:188–195
Hill MB, Phipps JL, Cartwright RJ, Milford Ward A, Greaves M, Hughes P (1996) Antibodies to membranes of endothelial cells and fibroblasts in scleroderma. Clin Exp Immunol 106:491–497
Rosenbaum J, Pottinger BE, Woo P, Black CM, Loizou S, Byron MA et al (1988) Measurement and characterisation of circulating anti-endothelial cell IgG in connective tissue diseases. Clin Exp Immunol 72:450–456
Salojin KV, Le Tonquèze M, Saraux A, Nassonov EL, Dueymes M, Piette JC et al (1997) Antiendothelial cell antibodies: useful markers of systemic sclerosis. Am J Med 102:178–185
Sgonc R, Gruschwitz MS, Boeck G, Sepp N, Gruber J, Wick G (2000) Endothelial cell apoptosis in systemic sclerosis is induced by antibody-dependent cell-mediated cytotoxicity via CD95. Arthritis Rheum 43:2550–2562
Mihai C, Tervaert JW (2010) Anti-endothelial cell antibodies in systemic sclerosis. Ann Rheum Dis 69:319–324
Park C, Kim TM, Malik AB (2013) Transcriptional regulation of endothelial cell and vascular development. Circ Res 112:1380–1400
Fischer C, Schneider M, Carmeliet P (2006) Principles and therapeutic implications of angiogenesis, vasculogenesis and arteriogenesis. Handb Exp Pharmacol 176:157–212
Dimmeler S, Zeiher AM, Schneider MD (2005) Unchain my heart: the scientific foundations of cardiac repair. J Clin Invest 115:572–583
Prater DN, Case J, Ingram DA, Yoder MC (2007) Working hypothesis to redefine endothelial progenitor cells. Leukemia 21:1141–1149
Estes ML, Mund JA, Ingram DA, Case J (2010) Identification of endothelial cells and progenitor cell subsets in human peripheral blood. Curr Protoc Cytom Chapter 9:Unit 9.33.31-11.
Richardson MR, Yoder MC (2011) Endothelial progenitor cells: quo vadis? J Mol Cell Cardiol 50:266–272
Yamaguchi Y, Okazaki Y, Seta N, Satoh T, Takahashi K, Ikezawa Z et al (2010) Enhanced angiogenic potency of monocytic endothelial progenitor cells in patients with systemic sclerosis. Arthritis Res Ther 12:R205
Yamaguchi Y, Kuwana M (2013) Proangiogenic hematopoietic cells of monocytic origin: roles in vascular regeneration and pathogenic processes of systemic sclerosis. Histol Histopathol 28:175–183
Lau LF (2011) CCN1/CYR61: the very model of a modern matricellular protein. Cell Mol Life Sci 68:3149–3163
Grote K, Salguero G, Ballmaier M, Dangers M, Drexler H, Schieffer B (2007) The angiogenic factor CCN1 promotes adhesion and migration of circulating CD34+ progenitor cells: potential role in angiogenesis and endothelial regeneration. Blood 110:877–885
Saigusa R, Asano Y, Taniguchi T, Yamashita T, Takahashi T, Ichimura Y et al (2015) A possible contribution of endothelial CCN1 downregulation due to Fli1 deficiency to the development of digital ulcers in systemic sclerosis. Exp Dermatol 24:127–132
Distler O, Del Rosso A, Giacomelli R, Cipriani P, Conforti ML, Guiducci S et al (2002) Angiogenic and angiostatic factors in systemic sclerosis: increased levels of vascular endothelial growth factor are a feature of the earliest disease stages and are associated with the absence of fingertip ulcers. Arthritis Res 4:R11
Michalska-Jakubus M, Kowal-Bielecka O, Chodorowska G, Bielecki M, Krasowska D (2011) Angiopoietins-1 and -2 are differentially expressed in the sera of patients with systemic sclerosis: high angiopoietin-2 levels are associated with greater severity and higher activity of the disease. Rheumatology (Oxford) 50:746–755
Fleming JN, Nash RA, McLeod DO, Fiorentino DF, Shulman HM, Connolly MK et al (2008) Capillary regeneration in scleroderma: stem cell therapy reverses phenotype? PLoS One 3, e1452
Asano Y, Stawski L, Hant F, Highland K, Silver R, Szalai G et al (2010) Endothelial Fli1 deficiency impairs vascular homeostasis: a role in scleroderma vasculopathy. Am J Pathol 176:1983–1998
Noda S, Asano Y, Takahashi T, Akamata K, Aozasa N, Taniguchi T et al (2013) Decreased cathepsin V expression due to Fli1 deficiency contributes to the development of dermal fibrosis and proliferative vasculopathy in systemic sclerosis. Rheumatology (Oxford) 52:790–799
Noda S, Asano Y, Akamata K, Aozasa N, Taniguchi T, Takahashi T et al (2012) A possible contribution of altered cathepsin B expression to the development of skin sclerosis and vasculopathy in systemic sclerosis. PLoS One 7, e32272
Takahashi T, Asano Y, Noda S, Aozasa N, Akamata K, Taniguchi T et al (2015) A possible contribution of lipocalin-2 to the development of dermal fibrosis, pulmonary vascular involvement, and renal dysfunction in systemic sclerosis. Br J Dermatol. doi:10.1111/bjd.13779
Thalgott J, Dos-Santos-Luis D, Lebrin F (2015) Pericytes as targets in hereditary hemorrhagic telangiectasia. Front Genet 6:37
Tan FK, Zhou X, Mayes MD, Gourh P, Guo X, Marcum C et al (2006) Signatures of differentially regulated interferon gene expression and vasculotrophism in the peripheral blood cells of systemic sclerosis patients. Rheumatology (Oxford) 45:694–702
Yoshizaki A, Yanaba K, Iwata Y, Komura K, Ogawa A, Akiyama Y et al (2010) Cell adhesion molecules regulate fibrotic process via Th1/Th2/Th17 cell balance in a bleomycin-induced scleroderma model. J Immunol 185:2502–2515
Higashi-Kuwata N, Jinnin M, Makino T, Fukushima S, Inoue Y, Muchemwa FC et al (2010) Characterization of monocyte/macrophage subsets in the skin and peripheral blood derived from patients with systemic sclerosis. Arthritis Res Ther 12:R128
Yukawa S, Yamaoka K, Sawamukai N, Shimajiri S, Kubo S, Miyagawa I et al (2013) Dermal mast cell density in fingers reflects severity of skin sclerosis in systemic sclerosis. Mod Rheumatol 23:1151–1157
Matsushita T, Hasegawa M, Hamaguchi Y, Takehara K, Sato S (2006) Longitudinal analysis of serum cytokine concentrations in systemic sclerosis: association of interleukin 12 elevation with spontaneous regression of skin sclerosis. J Rheumatol 33:275–284
Yang X, Yang J, Xing X, Wan L, Li M (2014) Increased frequency of Th17 cells in systemic sclerosis is related to disease activity and collagen overproduction. Arthritis Res Ther 16:R4
Sato S, Hasegawa M, Takehara K (2001) Serum levels of interleukin-6 and interleukin-10 correlate with total skin thickness score in patients with systemic sclerosis. J Dermatol Sci 27:140–146
Nakashima T, Jinnin M, Yamane K, Honda N, Kajihara I, Makino T et al (2012) Impaired IL-17 signaling pathway contributes to the increased collagen expression in scleroderma fibroblasts. J Immunol 188:3573–3583
Chizzolini C, Rezzonico R, Ribbens C, Burger D, Wollheim FA, Dayer JM (1998) Inhibition of type I collagen production by dermal fibroblasts upon contact with activated T cells: different sensitivity to inhibition between systemic sclerosis and control fibroblasts. Arthritis Rheum 41:2039–2047
Chizzolini C, Parel Y, De Luca C, Tyndall A, Akesson A, Scheja A et al (2003) Systemic sclerosis Th2 cells inhibit collagen production by dermal fibroblasts via membrane-associated tumor necrosis factor alpha. Arthritis Rheum 48:2593–2604
Chizzolini C (1999) T lymphocyte and fibroblast interactions: the case of skin involvement in systemic sclerosis and other examples. Springer Semin Immunopathol 21:431–450
Ihn H, Sato S, Fujimoto M, Kikuchi K, Kadono T, Tamaki K et al (1997) Circulating intercellular adhesion molecule-1 in the sera of patients with systemic sclerosis: enhancement by inflammatory cytokines. Br J Rheumatol 36:1270–1275
Sfikakis PP, Tesar J, Baraf H, Lipnick R, Klipple G, Tsokos GC (1993) Circulating intercellular adhesion molecule-1 in patients with systemic sclerosis. Clin Immunol Immunopathol 68:88–92
Ihn H, Sato S, Fujimoto M, Takehara K, Tamaki K (1998) Increased serum levels of soluble vascular cell adhesion molecule-1 and E-selectin in patients with systemic sclerosis. Br J Rheumatol 37:1188–1192
Shahin AA, Anwar S, Elawar AH, Sharaf AE, Hamid MA, Eleinin AA et al (2000) Circulating soluble adhesion molecules in patients with systemic sclerosis: correlation between circulating soluble vascular cell adhesion molecule-1 (sVCAM-1) and impaired left ventricular diastolic function. Rheumatol Int 20:21–24
Kuryliszyn-Moskal A, Klimiuk PA, Sierakowski S (2005) Soluble adhesion molecules (sVCAM-1, sE-selectin), vascular endothelial growth factor (VEGF) and endothelin-1 in patients with systemic sclerosis: relationship to organ systemic involvement. Clin Rheumatol 24:111–116
Kizu A, Medici D, Kalluri R (2009) Endothelial-mesenchymal transition as a novel mechanism for generating myofibroblasts during diabetic nephropathy. Am J Pathol 175:1371–1373
Zeisberg EM, Tarnavski O, Zeisberg M, Dorfman AL, McMullen JR, Gustafsson E et al (2007) Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med 13:952–961
Li J, Qu X, Bertram JF (2009) Endothelial-myofibroblast transition contributes to the early development of diabetic renal interstitial fibrosis in streptozotocin-induced diabetic mice. Am J Pathol 175:1380–1388
Hashimoto N, Phan SH, Imaizumi K, Matsuo M, Nakashima H, Kawabe T et al (2010) Endothelial-mesenchymal transition in bleomycin-induced pulmonary fibrosis. Am J Respir Cell Mol Biol 43:161–172
Jimenez SA (2013) Role of endothelial to mesenchymal transition in the pathogenesis of the vascular alterations in systemic sclerosis. ISRN Rheumatol 2013:835948
Li Z, Jimenez SA (2011) Protein kinase Cδ and c-Abl kinase are required for transforming growth factor β induction of endothelial-mesenchymal transition in vitro. Arthritis Rheum 63:2473–2483
ten Freyhaus H, Dumitrescu D, Bovenschulte H, Erdmann E, Rosenkranz S (2009) Significant improvement of right ventricular function by imatinib mesylate in scleroderma-associated pulmonary arterial hypertension. Clin Res Cardiol 98:265–267
Sfikakis PP, Gorgoulis VG, Katsiari CG, Evangelou K, Kostopoulos C, Black CM (2008) Imatinib for the treatment of refractory, diffuse systemic sclerosis. Rheumatology (Oxford) 47:735–737
ten Freyhaus H, Dumitrescu D, Berghausen E, Vantler M, Caglayan E, Rosenkranz S (2012) Imatinib mesylate for the treatment of pulmonary arterial hypertension. Expert Opin Investig Drugs 21:119–134
Celermajer DS, Sorensen KE, Gooch VM, Spiegelhalter DJ, Miller OI, Sullivan ID et al (1992) Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet 340:1111–1115
Joannides R, Haefeli WE, Linder L, Richard V, Bakkali EH, Thuillez C et al (1995) Nitric oxide is responsible for flow-dependent dilatation of human peripheral conduit arteries in vivo. Circulation 91:1314–1319
Peng X, Haldar S, Deshpande S, Irani K, Kass DA (2003) Wall stiffness suppresses Akt/eNOS and cytoprotection in pulse-perfused endothelium. Hypertension 41:378–381
Li M, Chiou KR, Bugayenko A, Irani K, Kass DA (2005) Reduced wall compliance suppresses Akt-dependent apoptosis protection stimulated by pulse perfusion. Circ Res 97:587–595
Lekakis J, Papamichael C, Mavrikakis M, Voutsas A, Stamatelopoulos S (1998) Effect of long-term estrogen therapy on brachial arterial endothelium-dependent vasodilation in women with Raynaud’s phenomenon secondary to systemic sclerosis. Am J Cardiol 82(1555–1557):A8
Lekakis J, Mavrikakis M, Papamichael C, Papazoglou S, Economou O, Scotiniotis I et al (1998) Short-term estrogen administration improves abnormal endothelial function in women with systemic sclerosis and Raynaud’s phenomenon. Am Heart J 136:905–912
Szucs G, Tímár O, Szekanecz Z, Dér H, Kerekes G, Szamosi S et al (2007) Endothelial dysfunction precedes atherosclerosis in systemic sclerosis—relevance for prevention of vascular complications. Rheumatology (Oxford) 46:759–762
Bartoli F, Blagojevic J, Bacci M, Fiori G, Tempestini A, Conforti ML et al (2007) Flow-mediated vasodilation and carotid intima-media thickness in systemic sclerosis. Ann N Y Acad Sci 1108:283–290
Rollando D, Bezante GP, Sulli A, Balbi M, Panico N, Pizzorni C et al (2010) Brachial artery endothelial-dependent flow-mediated dilation identifies early-stage endothelial dysfunction in systemic sclerosis and correlates with nailfold microvascular impairment. J Rheumatol 37:1168–1173
Rossi P, Granel B, Marziale D, Le Mée F, Francès Y (2010) Endothelial function and hemodynamics in systemic sclerosis. Clin Physiol Funct Imaging 30:453–459
Cypiene A, Laucevicius A, Venalis A, Dadoniene J, Ryliskyte L, Petrulioniene Z et al (2008) The impact of systemic sclerosis on arterial wall stiffness parameters and endothelial function. Clin Rheumatol 27:1517–1522
Giannattasio C, Pozzi M, Gardinali M, Gradinali M, Montemerlo E, Citterio F et al (2007) Effects of prostaglandin E1alpha cyclodextrin [corrected] treatment on endothelial dysfunction in patients with systemic sclerosis. J Hypertens 25:793–797
Cotton SA, Herrick AL, Jayson MI, Freemont AJ (1999) Endothelial expression of nitric oxide synthases and nitrotyrosine in systemic sclerosis skin. J Pathol 189:273–278
Dooley A, Gao B, Bradley N, Abraham DJ, Black CM, Jacobs M et al (2006) Abnormal nitric oxide metabolism in systemic sclerosis: increased levels of nitrated proteins and asymmetric dimethylarginine. Rheumatology (Oxford) 45:676–684
Andersen GN, Mincheva-Nilsson L, Kazzam E, Nyberg G, Klintland N, Petersson AS et al (2002) Assessment of vascular function in systemic sclerosis: indications of the development of nitrate tolerance as a result of enhanced endothelial nitric oxide production. Arthritis Rheum 46:1324–1332
Yamane K, Miyauchi T, Suzuki N, Yuhara T, Akama T, Suzuki H et al (1992) Significance of plasma endothelin-1 levels in patients with systemic sclerosis. J Rheumatol 19(10):1566–1571
Vancheeswaran R, Magoulas T, Efrat G, Wheeler-Jones C, Olsen I, Penny R et al (1994) Circulating endothelin-1 levels in systemic sclerosis subsets—a marker of fibrosis or vascular dysfunction? J Rheumatol 21:1838–1844
Marvi U, Chung L (2010) Digital ischemic loss in systemic sclerosis. Int J Rheumatol. doi:10.1155/2010/130717
Kahaleh MB (1994) Raynaud’s phenomenon and vascular disease in scleroderma. Curr Opin Rheumatol 6:621–627
Chung L, Fiorentino D (2006) Digital ulcers in patients with systemic sclerosis. Autoimmun Rev 5:125–128
Schiopu E, Impens AJ, Phillips K (2010) Digital ischemia in scleroderma spectrum of diseases. Int J Rheumatol. doi:10.1155/2010/923743
Noda S, Asano Y, Nishimura S, Taniguchi T, Fujiu K, Manabe I et al (2014) Simultaneous downregulation of KLF5 and Fli1 is a key feature underlying systemic sclerosis. Nat Commun 5:5797
Kundi R, Hollenbeck ST, Yamanouchi D, Herman BC, Edlin R, Ryer EJ et al (2009) Arterial gene transfer of the TGF-beta signalling protein Smad3 induces adaptive remodelling following angioplasty: a role for CTGF. Cardiovasc Res 84:326–335
Tashkin DP, Elashoff R, Clements PJ, Goldin J, Roth MD, Furst DE et al (2006) Cyclophosphamide versus placebo in scleroderma lung disease. N Engl J Med 354:2655–2666
Hoyles RK, Ellis RW, Wellsbury J, Lees B, Newlands P, Goh NS et al (2006) A multicenter, prospective, randomized, double-blind, placebo-controlled trial of corticosteroids and intravenous cyclophosphamide followed by oral azathioprine for the treatment of pulmonary fibrosis in scleroderma. Arthritis Rheum 54:3962–3970
Mouthon L, Berezne A, Guillevin L, Valeyre D (2010) Therapeutic options for systemic sclerosis related interstitial lung diseases. Respir Med 104(Suppl 1):S59–S69
Sakkas LI, Chikanza IC, Platsoucas CD (2006) Mechanisms of disease: the role of immune cells in the pathogenesis of systemic sclerosis. Nat Clin Pract Rheumatol 2:679–685
Casale R, Generini S, Luppi F, Pignone A, Matucci-Cerinic M (2004) Pulse cyclophosphamide decreases sympathetic postganglionic activity, controls alveolitis, and normalizes vascular tone dysfunction (Raynaud’s phenomenon) in a case of early systemic sclerosis. Arthritis Rheum 51:665–669
Apras S, Ertenli I, Ozbalkan Z, Kiraz S, Ozturk MA, Haznedaroglu IC et al (2003) Effects of oral cyclophosphamide and prednisolone therapy on the endothelial functions and clinical findings in patients with early diffuse systemic sclerosis. Arthritis Rheum 48:2256–2261
Caramaschi P, Volpe A, Pieropan S, Tinazzi I, Mahamid H, Bambara LM et al (2009) Cyclophosphamide treatment improves microvessel damage in systemic sclerosis. Clin Rheumatol 28:391–395
Furuya Y, Okazaki Y, Kaji K, Sato S, Takehara K, Kuwana M (2010) Mobilization of endothelial progenitor cells by intravenous cyclophosphamide in patients with systemic sclerosis. Rheumatology (Oxford) 49:2375–2380
Penn H, Quillinan N, Khan K, Chakravarty K, Ong VH, Burns A et al (2013) Targeting the endothelin axis in scleroderma renal crisis: rationale and feasibility. QJM 106:839–848
Frerix M, Stegbauer J, Dragun D, Kreuter A, Weiner SM (2012) Ulnar artery occlusion is predictive of digital ulcers in SSc: a duplex sonography study. Rheumatology (Oxford) 51:735–742
Matucci-Cerinic M, Denton CP, Furst DE, Mayes MD, Hsu VM, Carpentier P et al (2011) Bosentan treatment of digital ulcers related to systemic sclerosis: results from the RAPIDS-2 randomised, double-blind, placebo-controlled trial. Ann Rheum Dis 70:32–38
Korn JH, Mayes M, Matucci Cerinic M, Rainisio M, Pope J, Hachulla E et al (2004) Digital ulcers in systemic sclerosis: prevention by treatment with bosentan, an oral endothelin receptor antagonist. Arthritis Rheum 50:3985–3993
Ichimura Y, Asano Y, Hatano M, Tamaki Z, Takekoshi T, Kogure A et al (2011) Significant attenuation of macrovascular involvement by bosentan in a patient with diffuse cutaneous systemic sclerosis with multiple digital ulcers and gangrene. Mod Rheumatol 21:548–552
Abdelsaid M, Kaczmarek J, Coucha M, Ergul A (2014) Dual endothelin receptor antagonism with bosentan reverses established vascular remodeling and dysfunctional angiogenesis in diabetic rats: relevance to glycemic control. Life Sci 118:268–273
Dréau D, Karaa A, Culberson C, Wyan H, McKillop IH, Clemens MG (2006) Bosentan inhibits tumor vascularization and bone metastasis in an immunocompetent skin-fold chamber model of breast carcinoma cell metastasis. Clin Exp Metastasis 23:41–53
Guiducci S, Bellando Randone S, Bruni C, Carnesecchi G, Maresta A, Iannone F et al (2012) Bosentan fosters microvascular de-remodelling in systemic sclerosis. Clin Rheumatol 31:1723–1725
Cutolo M, Zampogna G, Vremis L, Smith V, Pizzorni C, Sulli A (2013) Longterm effects of endothelin receptor antagonism on microvascular damage evaluated by nailfold capillaroscopic analysis in systemic sclerosis. J Rheumatol 40:40–45
Akamata K, Asano Y, Yamashita T, Noda S, Taniguchi T, Takahashi T et al (2015) Endothelin receptor blockade ameliorates vascular fragility in endothelial cell-specific Fli1 knockout mice by increasing Fli1 DNA-binding ability. Arthr Rheumatol. doi:10.1002/art.39062
Asano Y, Trojanowska M (2009) Phosphorylation of Fli1 at threonine 312 by protein kinase C δ promotes its interaction with p300/CREB-binding protein-associated factor and subsequent acetylation in response to transforming growth factor β. Mol Cell Biol 29:1882–1894
Asano Y, Czuwara J, Trojanowska M (2007) Transforming growth factor-β regulates DNA binding activity of transcription factor Fli1 by p300/CREB-binding protein-associated factor-dependent acetylation. J Biol Chem 282:34672–34683
Kubo M, Czuwara-Ladykowska J, Moussa O, Markiewicz M, Smith E, Silver RM et al (2003) Persistent down-regulation of Fli1, a suppressor of collagen transcription, in fibrotic scleroderma skin. Am J Pathol 163:571–581
Jordan S, Distler JH, Maurer B, Huscher D, van Laar JM, Allanore Y et al (2014) Effects and safety of rituximab in systemic sclerosis: an analysis from the European Scleroderma Trial and Research (EUSTAR) group. Ann Rheum Dis. doi:10.1136/annrheumdis-2013-204522
Sumida H, Asano Y, Tamaki Z, Aozasa N, Taniguchi T, Takahashi T et al (2014) Successful experience of rituximab therapy for systemic sclerosis-associated interstitial lung disease with concomitant systemic lupus erythematosus. J Dermatol 41:418–420
Khor CG, Chen XL, Lin TS, Lu CH, Hsieh SC (2014) Rituximab for refractory digital infarcts and ulcers in systemic sclerosis. Clin Rheumatol 33:1019–1020
Daoussis D, Antonopoulos I, Liossis SN, Yiannopoulos G, Andonopoulos AP (2012) Treatment of systemic sclerosis-associated calcinosis: a case report of rituximab-induced regression of CREST-related calcinosis and review of the literature. Semin Arthritis Rheum 41:822–829
Maslyanskiy AL, Lapin SV, Kolesova EP, Penin IN, Cheshuina MD, Feist E et al (2014) Effects of rituximab therapy on elastic properties of vascular wall in patients with progressive systemic sclerosis. Clin Exp Rheumatol 32(6 Suppl 86):S-228
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is a contribution to the Special Issue on Immunopathology of Systemic Sclerosis - Guest Editors: Jacob M. van Laar and John Varga
Rights and permissions
About this article
Cite this article
Asano, Y., Sato, S. Vasculopathy in scleroderma. Semin Immunopathol 37, 489–500 (2015). https://doi.org/10.1007/s00281-015-0505-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00281-015-0505-5