Abstract
The nicotinamide adenine dinucleotide phosphate (NADPH) oxidase of phagocytes is a multi-component electron transferase that uses cytoplasmic NADPH to convert molecular oxygen to superoxide anion, consequently delivering reactive oxygen species to the site of invading microorganisms. Together with soluble factors and other phagocyte-derived agents, the resultant toxic species kill and degrade the ingested microbe. Flavocytochrome b 558, a heterodimeric protein composed of gp91phox and p22phox, is the membrane component of the NADPH oxidase and was previously thought to be uniquely expressed in phagocytes. Based on structural homology with gp91phox, recent studies have defined a family of NADPH oxidase proteins (Nox) that is widely distributed throughout the plant and animal kingdoms and in many tissues in multicellular organisms. The goals of this review are to review features of the phagocyte NADPH oxidase that serve as a paradigm for exploiting oxidants for host defense, and to discuss contributions of other Nox proteins to innate immunity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Initiating an issue of Seminars in Immunopathology devoted to “Nox Enzymes in immuno-inflammatory pathologies” with an overview of the contributions of the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (NOX) family of proteins to immune cell function and innate immunity seems only just, given that structural homology with gp91phox of phagocytes defines membership in the newly recognized Nox protein family. Furthermore, not only does consideration of Nox proteins in immune cells recapitulate the history of the discovery of the protein family, but it also represents a logical format for the presentation of such a broad topic. NOX2, the 91-kDa membrane glycoprotein that is an essential component of the heterodimeric flavocytochrome b 558 of the phagocyte NADPH oxidase, is the patriarch of the NOX protein family. It was the object of intense study long before its homologs were discovered or the broad distribution of NADPH oxidases throughout the animal and plant kingdoms was recognized. Nearly five decades of study of the phagocyte NADPH oxidase has yielded considerable insight into its composition, structural organization, regulation, and biochemistry, thereby providing a model for comparing and contrasting features of the other family members. To that end, one goal of this contribution will be to summarize what are currently understood to be key features of the phagocyte oxidase in order to serve as a focal point for comparison with critical elements highlighted in subsequent chapters that discuss specific NOX proteins in other settings.
The second explicit goal of this article is to summarize the contributions of NOX proteins to the immune system that are mediated by cells that are not granulocytes, thereby expanding the appreciation of how NADPH oxidases participate in host defense in ways outside the context of phagocyte biology. With the recognition that expression of at least one member of the NOX family has been reported for nearly every tissue examined [1], demonstrating how widely expressed these proteins are in nature, one would anticipate that some would be represented in nonphagocytic members of the immune system. Current understanding of the presence and function of NOX proteins within the context of normal immune function in nonphagocytes will be presented, discussing NOX5 and Duox in lymphoid cells and epithelial cells, respectively. Emphasis will be placed on how NOX5 and Duox differ from NOX2 within the framework of human host defense.
Biochemical properties of the phagocyte NADPH oxidase
The appreciation that human polymorphonuclear leukocytes (PMN) utilize reactive oxygen species to kill ingested microorganisms reflects in large part the convergence of two independent scientific pursuits that occurred simultaneously during the middle decades of the last century. One body of work pursued a better understanding of the immunologic defect(s) that cause chronic granulomatous disease (CGD), an inherited disorder typically affecting male children [2]. Although it is likely that some cases of CGD were included in earlier reports of patients with frequent infections, the first explicit definition of the clinical syndrome and its features was in 1959 [3]. The four boys described presented with hypergammaglobulinemia, recurrent pyogenic infections, and granulomatous lesions in skin, lymph nodes, and liver. The patients had normal numbers of circulating granulocytes in peripheral blood and exhibited an appropriate leukocytosis during infection. Although the isolated phagocytes ingested bacteria normally, investigators still suspected that an underlying immunologic defect was responsible for the secondary infectious complications that affected patients experienced.
At nearly the same time that these clinical observations were being made, other investigators recognized that homogenates of resting guinea pig PMN generated H2O2 from oxygen in an NADPH-dependent fashion [4]. Moreover, homogenates made from PMN that had ingested particles were more active than those derived from resting PMN, providing the first hint that the burst in respiration was agonist-dependent [5, 6]. As a result of the intense efforts of investigators in several different countries, biochemical features of the phagocyte “respiratory burst” were identified. The enzyme is NADPH-dependent; for the human PMN, the K m is 33 μM NADPH and 930 μM for NADH [7]. Given that the intracellular concentrations of NADPH and NADH are both ∼70 μM, the enzyme uses NADPH under normal physiologic conditions.
The relative greater affinity for NADPH is not species-specific, as it holds for the phagocyte oxidase in guinea pig granulocytes (46 μM vs 580 μM [8]) and in rat alveolar macrophages (5 μM vs 1,000 μM [9]), to provide only two examples. Oxidase activity is resistant to inhibition by azide [10–13] or by cyanide [10, 12, 14–17], a property that early on indicated that the respiratory burst was not due to a sudden increase in mitochondrial activity, as was first suspected.
As an appreciation of the biochemical events that accompanied phagocyte stimulation grew, evidence mounted that CGD neutrophils had profound abnormalities in selected responses [18, 19]. The link between the inherited defect in CGD PMN and the cyanide-resistant, NADPH-dependent, leukocyte oxidase was made [20] and this recognition has provided investigators in the field with a biologically relevant context in which to evaluate hypotheses and a clinically important therapeutic target for biochemical manipulation.
In addition to its dependence on flavin and NADPH and its resistance to mitochondrial inhibitors, the PMN oxidase has other defined biochemical features. Oxygen is the substrate for the phagocyte oxidase, with a K m for oxygen of ∼10 μM, equivalent to ∼7 mmHg pO2 [21]. Thus the oxidase can function at very low oxygen tensions present in tissue, but might be compromised in clinical settings associated with extensive tissue necrosis. Nearly all of the oxygen consumed by PMN during phagocytosis can be recovered as superoxide anion [22] and the enzyme turnover is robust, ∼150–160 electrons heme−1 s−1 [23–27]. When quantitating extracellular superoxide anion as the superoxide dismutase-inhibitable reduction of ferricytochrome C [13], human PMN release 7–10 nmol min−110−6 PMN and 2–4 nmol min−110−6 PMN after stimulation with 100 nmol of phorbol myristate acetate and opsonized zymosan (20:1 particle to PMN), respectively.
However, the rate, amount, and duration of superoxide generation vary as a function of the state of the PMN (primed vs resting, in suspension or adherent) as well as the agonist (reviewed in [28]). NADPH availability in the cytosol limits oxidase activity under normal conditions [29]. As one might anticipate, the electron transferase activity of the activated oxidase necessitates charge compensation across the plasma or phagosomal membrane [30, 31]. Most evidence supports a proton channel as the dominant mediator of charge compensation during oxidase activity [32–34], although the electrophysiology is not completely elucidated. Drs. Rada and Ligeti review the obligate coupling oxidase activity with other electophysiological events elsewhere in this issue (Rada and Ligeti).
Flavocytochrome b 558: the membrane component of the phagocyte NADPH oxidase
Flavocytochrome b 558 is a heterodimeric integral membrane protein, comprised of a 91-kDa glycoprotein (gp91phox) and a 22-kDa protein (p22phox), that resides in the plasma membrane and membranes of secretory vesicles and of specific granules of PMN and in the plasma membrane of other granulocytes (review [2]). Within the context of a discussion of the Nox protein family, the extent and duration of the controversy that surrounded the identification of flavocytochrome b 558 as a component of the phagocyte oxidase seem both remarkable and ironic. First noted by several investigators in Japan [35–37], flavocytochrome b 558 was rediscovered by the Segal lab [38] and noted to be absent from patients with the X-linked form of CGD [39, 40]. Eventually, the heavy subunit of flavocytochrome b 558, gp91phox, was identified as the molecular defect in X-linked CGD (CYBB) [41, 42], whereas the gene encoding p22phox (CYBA) was subsequently localized to chromosome 16 [43]. With the birth of the Nox protein family [44], gp91phox was subsequently relegated to the designation Nox2, although both gp91phox and Nox2 are used nearly interchangeably.
As Nox2 has not been crystallized, the model for its disposition in the membrane has been derived from integrating data from many different analytical approaches (see discussion in [45]). A model proposed by Jesaitis integrates much of the available data from diverse sources and incorporates the structural features identified most consistently [46]. In this model, there are six transmembrane helices, two heme groups, and an extended cytosolic domain containing both NADPH and FAD binding sites. The heme groups are coordinated with histidine residues in parallel helices that are perpendicular to the plane of the membrane and are stacked. Since they have different mid-point potentials, −225 and −265 mV [47], this arrangement provides the means for shuttling electrons across the membrane.
The amount of flavocytochrome b 558 in membranes or detergent extractions of membranes can be quantitated using reduced-minus-oxidized difference spectroscopy, measuring from the peak at 558 nm to the trough at 540 nm and using Δɛ 558–540 of 21.6 mM−1 cm−1 [48]. Taken together, evidence indicates that Nox2 is the catalytic core of the phagocyte oxidase, functioning as the electron transferase to reduce molecular oxygen, although the precise mechanism by which electrons are transported is incompletely understood. Multiple steps are involved in the two electron transfer from NADPH to FAD, to the inner heme, to the outer heme, and finally to oxygen, as recently summarized in detail [49]. The immediate product of the NADPH oxidase is superoxide anion, although it rapidly undergoes spontaneous dismutation (pH optimum of 4.8) to yield H2O2, which in turns supports the generation of an array of reactive species (vide infra).
The unheralded essential partner: p22phox
In contrast to the electron transferase activity of Nox2, p22phox, the partner of Nox2 in flavocytochrome b 558, has no demonstrated enzymatic activity. However, the stability of both Nox2 and p22phox requires the formation of heterodimers, as the absence of either protein in myeloid cells results in the absence of both. Circulating PMN from patients with inherited defects or deficiency of Nox2 or p22phox lack both proteins [2, 50, 51], thus demonstrating their interdependence and the importance of heterodimer formation. Studies of flavocytochrome b 558 in human myeloid precursors demonstrate that solitary precursors of Nox2 or p22phox in the endoplasmic reticulum (ER) undergo proteolytic degradation, mediated in part by the proteasome [52]. In Epstein Barr virus-transformed B lymphocytes from patients with X-linked CGD, p22phox can be rescued from ER-associated degradation by transfection of normal Nox2 [53–55], and the reverse is also true, with normal p22phox able to rescue Nox2 precursors [56].
The requirement of heterodimer formation for stability of Nox2 and p22phox was exploited experimentally to identify the specific histidine residues that coordinate with the hemes in gp91phox [57]. Furthermore, as heterodimer formation is also prerequisite to exit the ER efficiently [52, 58], p22phox contributes to the subcellular localization of Nox2. It is important to recognize that ER quality control in myeloid cells may differ from that in other cell lines, as free gp91phox and p22phox each is more stable in cell lines such as COS7, epithelial cells, murine 3T3 fibroblasts, and CHO cells [58–60]. As demonstrated in Fig. 1, isolated membranes from CHO cells transfected with gp91phox express predominantly the 65-kDa precursor of gp91phox that resides in the ER [52, 58]. However, coexpression of both gp91phox and p22phox results in heterodimer formation and promotes maturation of the ER precursor into mature gp91phox. Surface staining of transfectants with 7D5, a murine monoclonal antibody that recognizes an extracellular epitope on flavocytochrome b 558 [61–63] demonstrates the augmented surface expression of flavocytochrome b 558 when p22phox is coexpressed with gp91phox (Fig. 1)
Augmented surface expression of gp91phox by coexpression of p22phox in CHO cells. A Light membranes from wild type CHO cells (WT) and CHO cells stably transfected with gp91phox (CHO 91) or with gp91phox and p22phox (CHO 91–21) were separated by SDS-PAGE and immunoblotted, using a murine monoclonal antibody directed against gp91phox (54.1) and a commercial rabbit polyclonal antibody directed against p22phox. In the transfectants expressing only gp91phox (CHO 91), the predominant species is the 65-kDa ER precursor, with very little mature gp91phox. However, coexpression of p22phox (CHO 91–22) significantly increases the maturation of the ER precursor and expression of gp91phox. B The same cells as shown in panel A were analyzed by FACS for staining with 7D5, a murine monoclonal antibody directed against an extracellular epitope in flavocytochrome b 558. Although there is detectable surface expression of gp91phox in CHO 91 cells, coexpression of p22phox increases surface expression more than ten-fold. Both panels are previously published [56] and shown with the publisher’s permission
p22 phox associates as well with Nox1, Nox3, and Nox4 [64–69]. In its association with Nox3, p22 phox apparently serves precisely the same role as it does for Nox2, extending the half-life of Nox3 and allowing export from the ER to the proper membrane destination. The critical importance of normal p22phox for both Nox2 and Nox3 is demonstrated by the consequences of an inherited missense mutation in p22phox, in which the histidine at codon 121 is replaced with tyrosine (Y121H). Mice with Y121H mutation have clinical disorders involving both their phagocytes and their vestibular system [219]. PMN from Y121H mice have the functional phenotype of CGD, with an absence of NADPH oxidase activity and increased susceptibility to infection. They also have a “head tilt” phenotype caused by the absence of otoconia in their inner ear, a manifestation of Nox3 dysfunction [70].
It is likely that humans with inherited deficiency of p22phox likewise have vestibular disease, but that visual compensation renders the symptoms subclinical. However, given this new discovery, it is important now to directly assess the contribution of p22phox to normal Nox3 function in humans, using sensitive and specific assays of inner ear function in patients not previously exposed to vestibulotoxic drugs such as aminoglycosides.
Despite these insights, the mechanism underlying the contribution of p22phox to Nox structure and function is unknown. The determinants of heterodimer formation are incompletely defined but under study [56].
Dependence of Nox2- p22 phox on cytosolic cofactors
Clinicians long recognized that only ∼1/2 the patients with CGD displayed an X-linked pattern of inheritance, thereby hinting that the phagocyte NADPH oxidase was a multicomponent system. Thus, the molecular identification of flavocytochrome b 558 as the membrane component of the oxidase and genetic localization of Nox2 to the X chromosome solved only part of the phagocyte oxidase puzzle. The development of the broken cell superoxide system demonstrated that one or more protein(s) in the cytosol of resting phagocytes was required to support Nox2-dependent superoxide production [71]. In the broken cell system, the light membranes and organelle-free cytosol from unstimulated phagocytes are combined in the presence of anionic amphiphiles and NADPH to generate superoxide. Subsequent studies from several laboratories identified three cytosolic proteins that are absolutely essential for Nox2 activity in human PMN: p47phox [72, 73], p67phox [72, 73], and the small molecular weight G protein Rac2 [74]. The inherited absence of Nox2, p22phox, p47phox, p67phox, or Rac2 provides the molecular basis for all cases of CGD [75].
The significance of a 47-kDa phosphoprotein in stimulated PMN was first suggested by Segal, who noted that PMA stimulates the phosphorylation of several cytosolic proteins in human PMN, including one of ∼44-kDa [76]. Phosphorylation of the 44-kDa protein was temporally linked to oxidase activation and these findings were confirmed by other investigators [77–81]. An activation-induced phosphoprotein of the same size was identified in the cytosol of PMN from other species [82–84], and it was suspected that the 44-kDa phosphoprotein might serve as the proximal electron-transferring molecule in the oxidase or some other component whose phosphorylation is a prerequisite for oxidase activity. The link between the 44-kDa phosphoprotein with a functional oxidase was extended by clinical data, as patients with autosomal CGD lacked it, whereas those with X-linked CGD had normal phosphorylation of the 44-kDa protein [76, 85–87].
Many of these predictions proved true, as the subsequent identification and molecular characterization of p47phox [72, 73] revealed several functionally important structural features, including two src-homology (SH3) motifs, a proline-rich region (PRR), and a region with clustered arginine and serine residues, likely targets for serine phosphorylation. Of note, an N-terminal region of p47phox proved to contain a novel phosphoinositide-binding motif [88] that was later found in a wide variety of proteins and subsequently designated as PX domain, to acknowledge its initial description in phox proteins p47phox and later p40phox [89–93]. In general, the PX, SH3, and PRR motifs mediate intermolecular associations, including protein–protein and protein-phospholipid interactions, and thus are essential structural features in the multicomponent NADPH oxidase complex. Residing in the cytoplasm of resting PMN in a complex with p67phox and p40phox, p47phox adopts a conformation that renders several of its interactive domains cryptic and unavailable to support interactions with their partners at the membrane.
For example, the PX domain of p47phox is destined to associate with the SH3 domain [94] in flavocytochrome b 558 but is masked by intramolecular interactions when in the cytoplasm of resting PMN [92]. However, phosphorylation of the serine residues in the arginine-rich autoinhibitory region (AIR) relaxes the conformational constraints on p47phox, exposing the PX and SH3 (Fig. 2). As a consequence, PMN stimulation and concomitant phosphorylation of p47phox trigger its translocation from cytoplasm to membrane [95–97], where it docks with flavocytochrome b 558 [98]. This agonist-dependent translocation of p47phox as a prerequisite for oxidase assembly [99] and provides the phagocyte with a novel mechanism for regulating oxidant generation, based on the spatial segregation of critical components when the cell is in the resting state. In this way, p47phox serves to organize the assembly of the Nox2 oxidase, which serves as a general feature for the Nox protein family (vide infra).
Conformational changes in p47phox during neutrophil stimulation. As discussed in the text, p47phox possesses structural motifs that mediate intermolecular interactions, including a PX domain, two SH3 regions, and a PRR. In addition, there is an arginine- and serine-rich region known as the autoinhibitory region (AIR). However, intramolecular interactions in p47phox result in its adopting a conformation in the cytoplasm of resting neutrophils that render the potential intermolecular domains cryptic. Agonist-dependent phosphorylation of the AIR, indicated by red stars, alters the conformation of p47phox, exposing interactive sites that can mediate association of p47phox with the membrane and with flavocytochrome b 558 and thereby support assembly of a functioning oxidase
In contrast to p47phox, which lacks intrinsic enzymatic activity, p67phox regulates NADPH reduction of FAD and thus serves as an essential activating cofactor [100]. When PMN are stimulated, p67phox translocates to the membrane in association with p47phox and p40phox. Whereas p47phox can translocate in the absence of p67phox, p67phox fails to become membrane-associated in PMN that lack p47phox [99].
Rac2, the third essential cytosolic component of the human PMN NADPH oxidase, exists in the cytoplasm of resting cells in the GDP state bound to RhoGDI. PMN stimulation triggers phosphorylation of RhoGDI with subsequent dissociation from Rac-GTP [101] and translocation of Rac-GTP to the membrane independent of the p47phox–p67phox–p40phox complex [102]. At the membrane, Rac2 contributes to oxidase activity, interacting with p67phox, Nox2, or both [103, 104]. Thus, agonist-dependent, post translational modifications mediate the translocation of both cytosolic complexes and thereby regulate oxidase activity by controlling oxidase assembly.
Whereas spatial segregation dominates regulation of Nox activity in PMN, there is evidence for limited transcriptional control, predominantly in monocytes or macrophages [105–107]. Regular prophylactic administration of interferon γ is considered the standard of care for patients with CGD because of its dramatic beneficial impact on the occurrence and severity of infections [108], although the underlying mechanisms are unknown. In the few patients with CGD due to splicing defects in the gene encoding gp91phox, interferon γ treatment partially restores normal transcription of gp91phox in myeloid precursors [109, 110]. However, there is no detectable transcription of gp91phox in mature PMN and the findings on patients with defects in splicing CYBB do not apply in general to the positive clinical impact of γ interferon in the infectious complications of CGD. In contrast to the arrangement of Nox2 regulation in PMN, it is very likely that transcriptional control of oxidase activity will figure prominently in the context of the other Nox protein family members.
Immune consequences of activation of the Nox2 oxidase
Phagocyte oxidase activation accompanies phagocytosis and contributes to the complex biochemical environment in the phagosome that is inhospitable to ingested microbes. These oxygen-dependent events are only part of the general response of phagocytes, which collectively comprise the many events that culminate in killing and degrading the ingested potential pathogen [111]. As mentioned above, the proximal product of the Nox2 oxidase is superoxide anion that quickly dismutates to generate H2O2. Although H2O2 alone exerts modest antimicrobial action, it contributes to innate host defense by serving as an essential cofactor in the MPO– H2O2–chloride system in the normal PMN phagosome [112, 113]. In the presence of H2O2, MPO forms compound I and catalyzes the two electron oxidation of Cl− to Cl+ and the production of HOCl.
The HOCl produced can chlorinate a wide range of biological substrates [114–117], including both susceptible targets in microbes [118–121] that compromise the viability of microbes, as well as host proteins [122], some of which provide extended antimicrobial activity. For example, chloramines can be produced by the action of MPO-derived HOCl on amines [123–125], some of which decompose to produce bioreactive aldehydes [115, 126–128].
Within the context of a consideration of Nox2 and host defense, it is important to recognize that superoxide anion produced by the NADPH oxidase also has effects that are indirect yet integral for effective antimicrobial action. Evidence suggests that superoxide interacts directly with MPO [129, 130] and thereby extends the functional life of the HOCl-generating system with the phagosome. In addition, Nox2 has been implicated in the process of antigen presentation by dendritic cells [131], thereby expanding the role of Nox2 activity to proper engagement and functioning of adaptive immunity, a link that may likewise include B cells and normal humoral immunity [132]. A recent study of Aspergillus infection in p47phox-deficient mice indicated that the absence of superoxide-dependent tryptophan catabolism results in immunologic dysregulation and a hyperinflammatory state [133]. The excessive IL-17 production from an expanded subpopulation of γδ-T cells in the infected p47phox knockout mice links Nox2-dependent superoxide production to a generalized immune response to fungi, and perhaps other microbes, that extends well beyond the phagocyte or its phagosome.
Nox2 vs other family members: compare and contrast
Several excellent, comprehensive reviews of the Nox family have been recently published [1, 134–136], providing the curious reader opportunities to examine in detail how closely the phagocyte oxidase paradigm summarized above predicts the attributes of the newly defined Nox2 homologs. However, to provide a context for appreciating the contributions to follow, several features merit highlighting.
As noted before, Nox1, Nox2, Nox3, and Nox4 pair with p22phox to form heterodimers in membranes. The other Nox protein family members, namely Nox5, Duox1, and Duox2, are not associated with p22phox. Many non phagocytic cells with Nox family members depend as well on cytosolic components that are necessary for optimal activity. These structural homologs of p47phox and p67phox serve as organizing and activating elements and are designated as NoxO1 and NoxA1, respectively, to highlight their function within the Nox complex. To date, only Nox1 and Nox3 employ NoxO1 and NoxA1 as cofactors, although human Nox3 functions independently of NoxA1, whereas murine Nox3 requires both cofactors for optimal activity. Although the basis for the species specificity for Nox3 dependence on particular cofactors is not known, the additional 21 amino acid insert in the C-terminal region of human NoxO1 may contribute.
The absence of the AIR from NoxO1 is one structural difference between p47phox and the NoxO1 homolog that has known functional consequences. As discussed earlier (Fig. 2), domains in p47phox that are destined for intramolecular associations in the assembled oxidase are cryptic in cytosolic p47phox in unstimulated cells. Agonist-dependent phosphorylation of several serines in AIR results in conformational changes that relieve constraints on interactive domains and allow assembly at the membrane. The absence of AIR from NoxO1 renders its interactive protein motifs always accessible and supports constitutive association of NoxO1 with the available docking sites at the membrane. In some situations, the active Nox complex may assemble not at the cell surface but on membrane-bound intracellular organelles, such as endosomes, and thus provide a source of oxidants that may support redox-dependent signal transduction [137].
The contribution of Rac to the organization and activity of the nonphagocyte Nox proteins also differs among the protein family members. Whereas Rac1 supports Nox1 [67, 138–142], there is evidence supporting a role for Rac in the activity of endogenous Nox 4 [143, 144], but not when expressed in transfected cells [69]. Both Nox5 and the Duoxes are independent of Rac1 [145, 146] and there are conflicting data with reference to Nox3, with evidence supporting [67] and not supporting [65, 138] a contribution of Rac1 to Nox3 activity. Analyses of the evolution of the Nox protein family strongly suggest that the EF hand-containing family members Nox5 and Duox are most ancient [147], with Nox3 most recent to have evolved, thus indicating that the earliest Nox proteins were independent of cytosolic factors entirely.
Nox 5 in host defense
As noted, Nox5 and Duox represent ancient members of the Nox protein family [147]. Nox5 functions independently of protein factors and requires only Ca+2 to trigger its NADPH-dependent activity [145]. Ca+2-dependent activation reflects in part binding of calmodulin to Nox5, thereby inducing a conformational change and increased sensitivity to calcium [148]. Protein kinase C activation can stimulate Nox5 as well [149], although the kinetics of activation differ from those seen when a Ca+2 ionophore is used as an agonist [150]. Stimulation of Nox5-expressing COS7 cells with phorbol myristate acetate promotes H2O2 generation at a relatively slow rate, in comparison to the response provoked by ionomycin. Nox5 activation under these conditions requires phosphorylation of threonine 494 and serine 498, residues in the C-terminal region of Nox5 between the putative NADPH and FAD binding sites [149, 150].
Although the specific kinase mediating phosphorylation in the COS7 system is not known, the PKC-β inhibitor LY379196 blocks both phosphorylation and activity of Nox5. As a consequence of its phosphorylation, Nox5 can be activated at lower concentrations of Ca+2. Thus, Nox5 phosphorylation provides one mechanism to modulate sensitivity to physiologic agonists that regulate intracellular Ca+2concentration.
Nox5 is highly expressed in testis, spleen, and lymph nodes [151], with lower levels of expression noted in endothelium, smooth muscle, pancreas, placenta, ovary, uterus, stomach, the HaCaT human keratinocyte cell line [152], and several fetal tissues [153, 154]. Expression in the B-cell and T cell-rich regions of spleen and lymph node seemed to foretell a role for Nox5 in some aspect of immune function, but Nox5 is absent from circulating lymphocytes [151]. In contrast to its absence from normal mature lymphocytes in peripheral blood, malignant B cells in hairy cell leukemia express Nox5 in plasma membrane and exhibit Ca+2-dependent H2O2 production [155]. The mechanisms underlying functional expression of Nox5 in hairy cell leukemia B cells are not known. The capacity to express Nox5 is not shared by other B-cell malignancies, including chronic lymphocytic leukemia, marginal zone leukemia, and mantle cell leukemia, and is not induced by integrin-dependent adhesion to vitronectin or fibronectin.
Perhaps Nox5 in splenic lymphocytes contributes to normal differentiation and proliferation of developing lymphocytes, functions limited in normal circulating lymphocytes and potentially deranged in selected B cell malignancies such as hairy cell leukemia. Nox5-dependent H2O2 production regulates growth and apoptosis in the cultured malignant prostatic cell line DU 145 [156], thus supporting a potential role for Nox5 in events relevant to cell proliferation. However, at this time, no data link Nox5 function with host defense or immunological events.
Duox in mucosal immunity
Although non phagocyte Nox proteins have been implicated in many biological functions across a broad array of tissues and organ systems [1, 49, 136, 157–159], the immune system has been relatively underrepresented to date [157, 160, 161]. The localization of Nox1 to the gastrointestinal tract [44, 139, 162–164] promised to link local oxidant production in the gut with host defense, but a convincing demonstration for a role for Nox1 local host defense has not emerged. However, extensive evidence supports an important role for Duox and its homologs as ancient participants in innate immunity.
Before discussing the details of Duox contribution to host defense, two generalizations should be made regarding principles that apply to the system. First, H2O2 and derived reactive oxygen species (ROS) figure prominently not only in Nox2-dependent phagocyte function but also in host defense systems in a wide variety of multicellular organisms. In the plant kingdom, oxidants generated by respiratory burst oxidase homologs (Rboh) mediate a wide-variety of biological processes, including pathogen recognition, direct antimicrobial activity, growth and development, and wound healing [165–168]. Oxidants can act as virulence factors as well, as illustrated by their role in fungal pathogenicity of rice blast disease [169]. Invertebrates likewise rely on H2O2 and H2O2-dependent systems to defend themselves against infection [170–172].
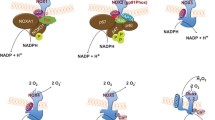
Reactive oxygen species generation is linked to innate immunity in insects, as exemplified by the reduced survival of the mosquito Anopheles gambiae to bacterial challenge when fed antioxidants [173]. Second, ROS mediate their functional effects by creating post-translational changes in specific biological targets. Often the chemistry utilizes ROS as a substrate for generating the effective mediator, rather than serving directly to modify the target. The oxygen- and MPO-dependent antimicrobial system of human PMN serves as the paradigm, with MPO as the peroxidase, Nox2 as the H2O2-generator, and Cl− or Br− as the halogen to produce HOCl or HOBr, respectively [174]. Application of this model extends beyond immune cells, and the functional consequences of the structural changes produced by this system reflect both the chemical nature and the cell type or tissue specifically targeted (Table 1). For example, thyroid hormone biosynthesis by thyrocytes depends on H2O2 from Duox2 and thyroperoxidase (TPO) to iodinate thyroglobulin [175–178].
Defects or deficiencies in either Duox2 or TPO can cause congenital hypothyroidism [179–182]. Using similar chemistry, ovoperoxidase catalyzes H2O2-dependent diotyrosine generation and cross-linking of the fertilization envelope of sea urchin eggs [183, 184], using H2O2 generated by the Duox homolog Udx1 [185]. Duox-derived H2O2 supports tyrosine cross-linking in the cuticle of Caenorhabditis elegans as well [186]. These functionally important structural variants on the phagocyte oxidase paradigm are likely the first of many tissue-specific adaptations of members of the Nox protein family.
Like Nox5, Duox 1 and 2 (referred below collectively as Duox) possess EF-hands, two compared to the four in Nox5, are regulated by calcium, and are independent of p22phox, Rac, or cytosolic activating or organizing proteins [134, 187–189]. In contrast to Nox5, Duox has been implicated in serving an important function in innate immunity at mucosal surfaces, most notably in the gastrointestinal and pulmonary tracts. Duox 2 is distributed along the length of the gastrointestinal tract [190], from oral salivary glands to the rectum [188], with sites of increased expression in the apical membranes of enterocytes and in the brush border of cecum and sigmoid colon [190]. The antibacterial activity of saliva had long been known [191] and early studies identified lactoperoxidase (LPO) as the responsible peroxidase [192], although the H2O2 source was unknown. The LPO-dependent system (reviewed in [192]) includes H2O2, LPO, and the pseudohalide thiocyanate (SCN−), which is widely distributed in mammalian tissues and body fluids. In adult humans, serum concentrations of SCN− are in the micromolar range (0.034–0.05 mM) with concurrent salivary concentrations much higher (0.8–1.5 mM [192]). Even before the source of H2O2 was identified, the biological properties of this system were characterized in detail [125, 192].
With the identification of Duox as the H2O2 source, the organization of the salivary gland antimicrobial system could be defined [188]. Although there was ample evidence that LPO-Duox H2O2–SCN system operates in vitro and generates antimicrobial activity that might contribute to mucosal innate immunity, the clearest link to the in vivo relevance for the Duox-based host defense in the gastrointestinal tract comes from studies in Drosophila. Selectively silencing expression of drosophila Duox (dDuox) in gut epithelial cells increases the mortality of adult flies ingesting food contaminated with Gram positive or Gram negative bacteria [193].
As in the gut, all elements of the LPO–H2O2–SCN antimicrobial system have been detected in airway epithelium [157, 187–189, 194–200], where it contributes to local host defense. LPO is largely secreted by bronchial submucosal glands in the airways of humans [201] and other animals [202, 203], and, under experimental conditions that exclude contamination by saliva, is present at 3–12 μg/ml [201]. Just as thiocyanate can be detected in saliva, lower airway secretions obtained from intubated patients contain amounts of SCN (e.g. ∼0.5 mM [201]) sufficient to support the antimicrobial action of the LPO–H2O2–SCN system [195, 204–206]. Expressed in the apical membranes of airway epithelial cells, Duox2 provides the H2O2 to complete the system and there generates agent(s) with bacteriostatic and bactericidal activities [125, 207, 208]. It merits noting that the reactive species produced by the LPO–H2O2–SCN system that mediates antimicrobial activity is not precisely known, as the major product(s) have been difficult to identify. Depending on the analytical technique used, candidates include HOSCN [209, 210], CN− [210], NCS-O-SCN− [209], and –OSC N [211]. Precise identification of the reactive product(s) that mediate cytotoxicity will facilitate unraveling the mechanisms underlying antimicrobial action of the LPO–H2O2–SCN system.
Regardless of the identity of the active species, the system spares airway epithelial cells while generating sufficiently reactive products to kill bacteria [199]. This relative sparing of mammalian cells during antimicrobial action contrasts with the collateral tissue toxicity often seen with HOCl or HOBr generated during inflammation [212–214]. Thus, the LPO–H2O2–SCN system is ideally suited for targeted host defense at mucosal surfaces. One can speculate as well that the low level of antimicrobial activity in this system serves the host well in rapidly clearing small inocula of airborne organisms that might gain access to the lower airway during respiration. If the number or virulence of aerosolized organisms exceeds the capacity of the LPO–H2O2–SCN system, other local factors, including soluble agents and alveolar macrophages, and recruited PMN are enlisted, resulting in a bona fide inflammatory response.
However, most low-level microbial challenges would be handled by the mucosal surface system below the threshold of airway inflammation. Ongoing studies have linked the LPO–H2O2 (Duox)–SCN antimicrobial system to the local host defense dysfunction seen in patients with cystic fibrosis [199]. Evidence linking thiocyanate transport to a functioning CFTR [197] provides a potential molecular mechanism underlying the depressed antimicrobial activity of epithelial airway fluid from cystic fibrosis patients.
In addition to supporting the generation of bioreactive agents that directly damage or kill microbes, Duox participates in innate host defense in the airway in other ways. Duox expression has been linked to regulation of mucous production in the airway. Production of mucins MUC1 and MUC5AC by stimulated airway epithelium involves expression of Duox1 [215–217], demonstrating a second contribution of Duox to maintaining the integrity of the airway and thereby contributing to host defense. Data also implicate Duox1 in augmenting endotoxin-elicited IL-8 production by airway epithelial cells [218], suggesting that in situations where Duox-mediated direct antimicrobial action may be insufficient, the system can relay signal to recruit additional host defense participants into the airway.
Summary
The multicomponent NADPH-dependent oxidase system, once thought to be uniquely expressed in phagocytes and dedicated to support innate host defense, represents instead a specialized application of ROS biochemistry. The recognition of the Nox protein family, based on structural homology with gp91phox, has extended the appreciation of the widespread role of ROS and their products in biology. This overview of the contributions of Nox proteins to immunity includes examples of how ROS directly mediate antimicrobial activity as well as evidence that ROS support signaling events that modulate the quantity and quality of the host response. The discovery that the phagocyte oxidase is but one member of a larger, ancient protein family provides new and exciting challenges, which promise to expand our understanding of many facets of plant and animal biology.
References
Bedard K, Krause K-H (2007) The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 87:245–313
Dinauer MC, Nauseef WM, Newburger PE (2001) Inherited disorders of phagocyte killing. In: Scriver CR et al (ed) The metabolic and molecular bases of inherited diseases. McGraw-Hill, New York, USA, pp 4857–4887
Bridges RA, Berendes H, Good RA (1959) A fatal granulomatous disease of childhood. Am J Dis Child 97:387–408
Iyer GYN, Quastel JH (1963) NADPH and NADH oxidation by guinea pig polymorphonuclear leukocytes. Can J Biochem Physiol 41:427–434
Baldridge C, Gerard R (1933) The extra respiration of phagocytosis. Am J Physiol 103:235–236
Iyer GYN, Islam DMF, Quastel JH (1961) Biochemical aspects of phagocytosis. Nature 192:535–541
Gabig TG, Babior B (1979) The O2-forming oxidase responsible for the respiratory burst in human neutrophils. J Biol Chem 254:9070–9074
Cohen HJ, Newburger PE, Chovaniec ME (1980) NAD(P)H-dependent superoxide production by phagocytic vesicles from guinea pig and human granulocytes. J Biol Chem 255:6584–6588
Hoffman M, Autor AP (1980) Production of superoxide anion by an NADPH-oxidase from rat pulmonary macrophages. FEBS Lett 121:352–354
Klebanoff SJ, Hamon CB (1972) Role of myeloperoxidase-mediated antimicrobial systems in intact leukocytes. J Reticuloendothel Soc 12:170–196
Rosen H, Klebanoff SJ (1976) Chemiluminescence and superoxide production by myeloperoxidase-deficient leukocytes. J Clin Invest 58:50–60
Nauseef WM, Metcalf JA, Root RK (1983) Role of myeloperoxidase in the respiratory burst of human neutrophils. Blood 61:483–491
Jandl RC, Andre-Schwartz J, Borges-DuBois L et al (1978) Termination of the respiratory burst in human neutrophils. J Clin Invest 61:1176–1185
Sbarra AJ, Karnovsky ML (1959) The biochemical basis of phagocytosis. I. Metabolic changes during the ingestion of particles by polymorphonuclear leukocytes. J Biol Chem 234:1355–1362
Cohn ZA, Morse SI (1960) Functional and metabolic properties of polymorphonuclear leukocytes. I. Observations on the requirements and consequences of particle ingestion. J Exp Med 111:667–687
Becker H, Munder G, Fischer H (1958) Über den Leukocytosenstoffwechsel bei der Phagocytose. Hoppe Seyler Z Physiol Chem 313:266–275
Weening RS, Roos D, Loos JA (1974) Oxygen consumption of phagocytizing cells in human leukocyte and granulocyte preparations: a comparative study. J Lab Clin Med 83:570–576
Holmes B, Page AR, Good RA (1967) Studies of the metabolic activity of leukocytes from patients with a genetic abnormality of phagocytic function. J Clin Invest 46:1422–1432
Quie PG, White JG, Holmes B et al (1967) In vitro bactericidal capacity of human polymorphonuclear leukocytes: diminished activity in chronic granulomatous disease of childhood. J Clin Invest 46:668–679
Hohn DC, Lehrer RI (1975) NADPH oxidase deficiency in X-linked chronic granulomatous disease. J Clin Invest 55:707–713
Gabig TG, Bearman SI, Babior BM (1979) Effects of oxygen tension and pH on the respiratory burst of human neutrophils. Blood 53:1133–1139
Root RK, Metcalf JA (1977) H2O2 release from human granulocytes during phagocytosis. J Clin Invest 60:1266–1279
Koshkin V, Lotan O, Pick E (1997) Electron transfer in the superoxide-generating NADPH oxidase complex reconstituted in vitro. Biochim Biophys Acta 1319:139–146
Rotrosen D, Yeung CL, Leto TL et al (1992) Cytochrome b558: the flavin-binding component of the phagocyte NADPH oxidase. Science 256:1459–1462
Abo A, Boyhan A, West I et al (1992) Reconstitution of neutrophil NADPH oxidase activity in the cell-free system by four components: p67-phox, p47-phox, p21rac1, and cytochrome b -245. J Biol Chem 267:16767–16770
Cross AR, Curnutte JT (1995) The cytosolic activating factors p47phox and p67phox have distinct roles in the regulation of electron flow in NADPH oxidase. J Biol Chem 270:6543–6548
Knoller S, Shpungin S, Pick E (1991) The membrane-associated component of the amphiphile-activated, cytosol-dependent superoxide-forming NADPH oxidase of macrophages is identical to cytochrome b559. J Biol Chem 266:2795–2804
DeCoursey TE, Ligeti E (2005) Regulation and termination of NADPH oxidase activity. Cell Mol Life Sci 62:2173–2193
Cohen HJ, Chovaniec ME (1978) Superoxide production by digitonin-stimulated guinea pig granulocytes. J Clin Invest 61:1088–1096
Murphy R, DeCoursey TE (2006) Charge compensation during the phagocyte respiratory burst. Biochim Biophys Acta 1757:996–1011
Demaurex N, Petheö G (2005) Electon and proton transport by NADPH oxidases. Phil Trans R Soc B 360:2315–2325
DeCoursey TE (2007) Electrophysiology of the phagocyte respiratory burst. Am J Physiol Cell Physiol 293:C30–C32
Femling JK, Cherny VV, Morgan D et al (2006) The antibacterial activity of human neutrophils and eosinophils requires proton channels but not BK channels. J Gen Physiol 127:659–672
Essin K, Salanova B, Kettritz R et al (2007) Large-conductance calcium-activated potassium channel activity is absent in human and mouse neutrophils and is not required for innate immunity. Am J Physiol Cell Physiol 293:C45–C54
Hattori H (1961) Studies on the labile, stable Nadi oxidase and peroxidase staining reactions in the isolated particles of horse granulocyte. Nagoya J Med Sci 23:362–378
Shinagawa Y, Tanaka C, Teroaka A et al (1966) A new cytochrome in neutrophilic granules of rabbit leucocyte. J Biochem 59:622–624
Shinagawa Y, Tanaka C, Teroaka A (1966) Electron microscopic and biochemical study of the neutrophilic granules from leucocytes. J Electron Microsc (Tokyo) 15:81–85
Harper AM, Dunne MJ, Segal AW (1984) Purification of cytochrome b-245 from human neutrophils. Biochem J 219:519–527
Segal AW, Jones OT, Webster D et al (1978) Absence of a newly described cytochrome b from neutrophils of patients with chronic granulomatous disease. Lancet 2:446–449
Segal AW, Jones OTG (1978) Novel cytochrome b system in phagocytic vacuoles of human granulocytes. Nature 276:515–517
Royer-Pokora B, Kunkel LM, Monaco AP et al (1986) Cloning the gene for an inherited human disorder—chronic granulomatous disease—on the basis of its chromosomal location. Nature 322:32–38
Dinauer MC, Orkin SH, Brown R et al (1987) The glycoprotein encoded by the X-linked chronic granulomatous disease locus is a component of the neutrophil cytochrome b complex. Nature 327:717–720
Dinauer MC, Pierce EA, Bruns GAP et al (1990) Human neutrophil cytochrome b light chain (p22-phox). Gene structure, chromosomal location, and mutations in cytochrome-negative autosomal recessive chronic granulomatous disease. J Clin Invest 86:1729–1737
Suh Y-A, Arnold RS, Lassegue B et al (1999) Cell transformation by the superoxide-generating oxidase Mox1. Nature 401:79–82
Taylor RM, Baniulis D, Burritt JB et al (2006) Analysis of human phagocyte flavocytochrome B by mass spectrometry. J Biol Chem 281:37045–37056
Taylor RM, Burritt JB, Baniulis D et al (2004) Site-specific inhibitors of NADPH oxidase activity and structural probes of flavocytochrome b: characterization of six monoclonal antibodies to the p22phox subunit. J Immunol 173:7349–7357
Cross AR, Rae J, Curnutte JT (1995) Cytochrome b-245 of the neutrophil superoxide-generating system contains two nonidentical hemes. Potentiometric studies of a mutant form of gp91phox. J Biol Chem 270:17075–17077
Cross AR, Higson FK, Jones OTG (1982) The enzymic reduction and kinetics of oxidation of cytochrome b of neutrophils. Biochem J 204:479–485
Cross AR, Segal AW (2004) The NADPH oxidase of professional phagocytes-prototype of the NOX electron transport chain systems. Biochim Biophys Acta 1657:1–22
Segal AW (1987) Absence of both cytochrome b-245 subunits from neutrophils in X-linked chronic granulomatous disease. Nature 326:88–91
Parkos CA, Dinauer MC, Jesaitis AJ et al (1989) Absence of both the 91kD and 22kD subunits of human neutrophil cytochrome b in two genetic forms of chronic granulomatous disease. Blood 73:1416–1420
DeLeo FR, Burritt JB, Yu L et al (2000) Processing and maturation of flavocytochrome b558 include incorporation of heme as a prerequisite for heterodimer assembly. J Biol Chem 275:13986–13993
Kume A, Dinauer MC (1994) Retrovirus-mediated reconstitution of respiratory burst activity in X-linked chronic granulomatous disease cells. Blood 84:3311–3316
Maly FE, Schuerer-Maly CC, Quilliam L et al (1993) Restitution of superoxide generation in autosomal cytochrome-negative chronic granulomatous disease (A220 CGD)-derived B lymphocyte cell lines by transfection with p22phox cDNA. J Exp Med 178:2047–2053
Porter CD, Parkar MH, Verhoeven AJ et al (1994) p22phox-deficient chronic granulomatous disease: reconstitution by retrovirus-mediated expression and identification of a biosynthetic intermediate of gp91phox. Blood 84:2767–2775
Zhu Y, Marchal C, Casbon A-J et al (2006) Deletion mutagenesis of p22phox subunit of flavocytochrome b558: identification of regions critical for gp91phox maturation and NADPH oxidase activity. J Biol Chem 281:30336–30346
Biberstine-Kinkade KJ, DeLeo FR, Epstein RI et al (2001) Heme-ligating histidines in flavocytochrome b558. J Biol Chem 276:31105–31112
Yu L, DeLeo FR, Biberstine-Kinkade KJ et al (1999) Biosynthesis of flavocytochrome b558. J Biol Chem 274:4364–4369
Biberstine-Kinkade KJ, Yu L, Stull N et al (2002) Mutagenesis of p22phox histidine 94. J Biol Chem 277:30368–30374
Yu L, Dinauer MC (1997) Biosynthesis of the phagocyte NADPH oxidase cytochrome b 558. J Biol Chem 272:27288–27294
Nakamura M, Murakami M, Koga T et al (1987) Monoclonal antibody 7D5 raised to cytochrome b558 of human neutrophils: immunocytochemical detection of the antigen in peripheral phagocytes of normal subjects, patients with chronic granulomatous disease, and their carrier mothers. Blood 69:1404–1408
Burritt JB, DeLeo FR, McDonald CL et al (2001) Phage display epitope mapping of human neutrophil flavocytochrome b558. Identification of two juxtaposed extracellular domains. J Biol Chem 276:2053–2061
Yamauchi A, Yu LX, Pötgens AJG et al (2001) Location of the epitope for 7D5, a monoclonal antibody raised against human flavocytochrome b 558, to the extracellular peptide portion of primate gp91phox. Microbiol Immunol 45:249–257
Nakano Y, Banfi B, Jesaitis AJ et al (2007) Critical roles for p22 phox in the structural maturation and subcellular targeting of Nox3. Biochem J 403:97–108
Ueno N, Takeya R, Miyano K et al (2005) The NADPH oxidase NOX3 constitutively produces superoxide in a p22phox-dependent manner: its regulation by oxidase organizers and activators. J Biol Chem 280:23328–23339
Kawahara T, Ritsick D, Cheng G et al (2005) Point mutations in the proline-rich region of p22phox are dominant inhibitors of NOX1- and NOX2-dependent reactive oxygen generation. J Biol Chem 280:31859–31869
Ueyama T, Geiszt M, Leto TL (2006) Involvement of Rac1 in activation of multicomponent Nox1- and Nox3-based NADPH oxidases. Mol Cell Biol 26:2160–2174
Ambasta RK, Kumar P, Griendling KK et al (2004) Direct interaction of the novel nox proteins with p22phox is required for the formation of a functionally active NADPH oxidase. J Biol Chem 279:45935–45941
Martyn KD, Frederick LM, von Loehneysen K et al (2006) Functional analysis of Nox4 reveals unique characteristics compared to other NADPH oxidases. Cell Signal 18:69–82
Banfi B, Malgrange B, Knisz J et al (2004) NOX3, a superoxide-generating NADPH oxidase of the inner ear. J Biol Chem 279:46065–46072
Bromberg Y, Pick E (1984) Unsaturated fatty acids stimulate NADPH-dependent superoxide production by cell-free system derived from macrophages. Cell Immunol 88:213–221
Volpp BD, Nauseef WM, Clark RA (1988) Two cytosolic neutrophil NADPH oxidase components absent in autosomal chronic granulomatous disease. Science 242:1295–1298
Nunoi H, Rotrosen D, Gallin JI et al (1988) Two forms of autosomal chronic granulomatous disease lack distinct neutrophil cytosol factors. Science 242:1298–1301
Knaus UG, Heyworth PG, Evans T et al (1991) Regulation of phagocyte oxygen radical production by the GTP-binding protein Rac2. Science 254:1512–1515
Clark RA, Malech HL, Gallin JI et al (1989) Genetic variants of chronic granulomatous disease: prevalence of deficiencies of two cytosolic components of the NADPH oxidase system. N Engl J Med 321:647–652
Segal AW, Heyworth PG, Cockcroft S et al (1985) Stimulated neutrophils from patients with autosomal recessive chronic granulomatous disease fail to phosphorylate a Mr-44,000 protein. Nature 316:547–549
Okamura N, Curnutte JT, Roberts RL et al (1988) Relationship of protein phosphorylation to the activation of the respiratory burst in human neutrophils. J Biol Chem 263:6777–6782
Okamura N, Malawista SE, Roberts RL et al (1988) Phosphorylation of the oxidase-related 48K phosphoprotein family in the unusual autosomal cytochrome-negative and X-linked cytochrome-positive types of chronic granulomatous disease. Blood 72:811–816
Andrews PC, Babior BM (1984) Phosphorylation of cytosolic proteins by resting and activated human neutrophils. Blood 64:883–890
Heyworth PG, Badwey JA (1990) Continuous phosphorylation of both the 47 and the 49 kDa proteins occurs during superoxide production by neutrophils. Biochim Biophys Acta Mol Cell Res 1052:299–305
Heyworth PG, Karnovsky ML, Badwey JA (1989) Protein phosphorylation associated with synergistic stimulation of neutrophils. J Biol Chem 264:14935–14939
Gennaro R, Florio C, Romeo D (1985) Activation of protein kinase C in neutrophil cytoplasts. FEBS Lett 180:185–190
Okamura N, Ohashi S, Nagahisa N et al (1984) Changes in protein phosphorylation in guinea pig polymorphonuclear leukocytes by treatment with membrane-perturbing agents which stimulate superoxide anion production. Arch Biochem Biophys 228:270–277
White JR, Huang C-K, Hill JM Jr et al (1984) Effect of phorbol 12-myristate 13-acetate and its analogue 4a-phorbol 12,13-didecanoate on protein phosphorylation and lysosomal enzyme release in rabbit neutrophils. J Biol Chem 259:8605–8611
Heyworth PG, Segal AW (1986) Further evidence for the involvement of a phosphoprotein in the respiratory burst oxidase of human neutrophils. Biochem J 239:723–731
Teahan CG, Totty N, Casimir CM et al (1990) Purification of the 47 kDa phosphoprotein associated with the NADPH oxidase of human neutrophils. Biochem J 267:485–489
Hayakawa T, Suzuki K, Suzuki S et al (1986) A possible role for protein phosphorylation in the activation of respiratory burst in human neutrophils. Evidence from studies with cells from patients with chronic granulomatous disease. J Biol Chem 261:9109–9115
Ago T, Takeya R, Hiroaki H et al (2001) The PX domain as a novel phosphoinositide-binding module. Biochem Biophys Res Commun 287:733–738
Ellson C, Gobert-Gosse S, Anderson K et al (2001) PtdIns(3)P regulates the neutrophil oxidase complex by binding to the PX domain of p40phox. Nat Cell Biol 3:679–682
Kanai F, Liu H, Field S et al (2000) The PX domains of p47phox and p40phox bind to lipid products of PI(3)K. Nat Cell Biol 3:675–678
Ellson C, Davidson K, Anderson K et al (2006) Ptdlns3P binding to the PX domain of p40phox is a physiological signal in NADPH oxidase activation. EMBO J 25:4468–4478
Karathanassis D, Stahelin RV, Bravo J et al (2002) Binding of the PX domain of p47phox to phosphatidylinositol 3,4-bisphosphate and phosphatidic acid is masked by an intramolecular interaction. EMBO J 21:5057–5068
Zhan Y, Virbasius JV, Song X et al (2002) The p40phox and p47phox PX domains of NADPH oxidase target cell membranes via direct and indirect recruitment by phosphoinositides. J Biol Chem 277:4512–4518
Hiroaki H, Ago T, Ito T et al (2001) Solution structure of the PX domain, a target of the SH3 domain. Nat Struct Biol 8:526–530
Nauseef WM, Volpp BD, McCormick S et al (1991) Assembly of the neutrophil respiratory burst oxidase. Protein kinase C promotes cytoskeletal and membrane association of cytosolic oxidase components. J Biol Chem 266:5911–5917
Faust LP, El Benna J, Babior BM et al (1995) The phosphorylation targets of p47phox, a subunit of the respiratory burst oxidase. Functions of the individual target serines as evaluated by site-directed mutagenesis. J Clin Invest 96:1499–1505
Inanami O, Johnson JL, McAdara JK et al (1998) Activation of the leukocyte NADPH oxidase by phorbol ester requires the phosphorylation of p47PHOX on serine 303 or 304. J Biol Chem 273:9539–9543
Nauseef WM (2004) Assembly of the phagocyte NADPH oxidase. Histochem Cell Biol 122:277–291
Heyworth PG, Curnutte JT, Nauseef WM et al (1991) Neutrophil NADPH oxidase assembly. Membrane translocation of p47-phox and p67-phox requires interaction between p47-phox and cytochrome b558. J Clin Invest 87:352–356
Nisimoto Y, Motalebi S, Han CH et al (1999) The p67phox activation domain regulates electron flow from NADPH to flavin in flavocytochrome b558. J Biol Chem 274:22999–23005
DerMardirossian C, Schnelzer A, Bokoch GM (2004) Phosphorylation of RhoGDI by Pak1 mediates dissociation of Rac GTPase. Mol Cell 15:117–127
Heyworth PG, Bohl BP, Bokoch GM et al (1994) Rac translocates independently of the neutrophil NADPH oxidase components p47phox and p67phox. Evidence for its interaction with flavocytochrome b 558 . J Biol Chem 269:30749–30752
Bokoch GM, Diebold BA (2002) Current molecular models for NADPH oxidase regulation by Rac GTPase. Blood 100:2692–2696
Sarfstein R, Gorzalczany Y, Mizrahi A et al (2004) Dual role of rac in the assembly of NADPH oxidase: tethering to the membrane and activation of p67phox. J Biol Chem 279:16007–16016
Gauss KA, Nelson-Overton LK, Siemsen DW et al (2007) Role of NF-kB in transcriptional regulation of the phagocyte NADPH oxidase by tumor necrosis factor-a. J Leukoc Biol 82:729–741
Anrather J, Racchumi G, Iadecola C (2006) NF-kB regulates phagocytic NADPH oxidase by inducing the expression of gp91phox. J Biol Chem 281:5657–5667
Dusi S, Donini M, Lissandrini D et al (2001) Mechanisms of expression of NADPH oxidase components in human cultured monocytes: role of cytokines and transcriptional regulators involved. Eur J Immunol 31:929–938
Intl Chron Granulom Dis Study Grp (1991) A controlled trial of interferon gamma to prevent infection in chronic granulomatous disease. The International Chronic Granulomatous Disease Cooperative Study Group. N Engl J Med 324:509–516
Condino-Neto A, Newburger P (2000) Interferon-gamma improves splicing efficiency of CYBB gene transcripts in an interferon-responsive variant of chronic granulomatous disease due to a splice site consensus region mutation. Blood 95:3548–3554
Ishibashi F, Mizukami T, Kanegasaki S et al (2001) Improved superoxide-generating ability by interferon g due to splicing pattern change of transcripts in neutrophils from patients with a splice site mutation in CYBB gene. Blood 98:436–441
Nauseef WM (2007) How human neutrophils kill and degrade microbes: an integrated view. Immunol Rev 219:88–102
Hampton MB, Kettle AJ, Winterbourn CC (1998) Inside the neutrophil phagosome: oxidants, myeloperoxidase, and bacterial killing. Blood 92:3007–3017
Winterbourn CC, Hampton MB, Livesey JH et al (2006) Modeling the reactions of superoxide and myeloperoxidase in the neutrophil phagosome. J Biol Chem 281:39860–39869
Hawkins DL, Davies MJ (1999) Hypochlorite-induced oxidation of proteins in plasma: formation of chloramines and nitrogen-centered radicals and their role in protein fragmentation. Biochem J 340:539–548
Pattison DI, Davies MJ (2006) Reactions of myeloperoxidase-derived oxidants with biological substrates: gaining chemical insight into human inflammatory diseases. Curr Med Chem 13:3271–3290
Pattison DI, Davies MJ (2001) Absolute rate constants for the reactions of hypochlorous acid with protein side chains and peptide bonds. Chem Res Toxicol 14:1453–1464
Hawkins CL, Pattison DI, Davies MJ (2003) Hypochlorite-induced oxidation of amino acids, peptides, and proteins. Amino Acids 25:259–274
Hurst JK, Barrette WC Jr., Michel BR et al (1991) Hypochlorous acid and myeloperoxidase-catalyzed oxidation of iron-sulfur clusters in bacterial respiratory dehydrogenases. Eur J Biochem 202:1275–1282
Rakita RM, Michel BR, Rosen H (1990) Differential inactivation of Escherichia coli membrane dehydrogenases by a myeloperoxidase-mediated antimicrobial system. Biochemistry 29:1075–1080
Rakita RM, Michel BR, Rosen H (1989) Myeloperoxidase-mediated inhibition of microbial respiration: damage to Escherichia coli ubiquinol oxidase. Biochemistry 28:3031–3036
Rosen H, Crowley JR, Heinecke JW (2002) Human neutrophils use the myeloperoxidase-hydrogen peroxide–chloride system to chlorinate but not nitrate bacterial proteins during phagocytosis. J Biol Chem 277:30463–30468
Chapman ALP, Hampton MB, Senthilmohan R et al (2002) Chlorination of bacterial and neutrophil proteins during phagocytosis and killing of Staphylococcus aureus. J Biol Chem 277:9757–9762
Zgliczynski JM, Stelmaszynska T, Domanski J et al (1971) Chloramines as intermediates of oxidative reaction of amino acids by myeloperoxidase. Biochem Biophys Acta 235:419–424
Zgliczynski JM, Stelmaszynska T (1975) Chlorinating ability of human phagocytosing leucocytes. Eur J Biochem 56:157–162
Thomas EL, Bozeman PM, Learn DB (1991) Lactoperoxidase: structural and catalytic properties. In: Everse J, Everse KE, Grisham MB (eds) Peroxidases in chemistry and biology. vol. 1. CRC Press, Boca Raton, FL, pp 123–142
Weiss SJ, Klein R, Slivka A et al (1982) Chlorination of taurine by human neutrophils. Evidence for hypochlorous acid generation. J Clin Invest 70:598–607
Weiss SJ, Lampert MB, Test ST (1983) Long-lived oxidants generated by human neutrophils: characterization and bioactivity. Science 222:625–628
Weiss SJ, Test ST, Eckmann CM et al (1986) Brominating oxidants generated by human eosinophils. Science 234:200–202
Kettle AJ, Winterbourn CC (1999) Superoxide modulates the activity of myeloperoxidase and optimizes the production of hypochlorous acid. Biochem J 252:529–536
Kettle AJ, Anderson RF, Hampton MB et al (2007) Reactions of superoxide with myeloperoxidase. Biochemistry 46:4888–4897
Savina A, Jancic C, Hugues S et al (2006) NOX2 controls phagosomal pH to regulate antigen processing during cross presentation by dendritic cells. Cell 126:205–218
Bleesing JJ, Souto-carneiro MM, Savage WJ et al (2006) Patients with chronic granulomatous disease have a reduced peripheral blood memory B cell compartment. J Immunol 176:7096–7103
Romani L, Fallarino F, De Luca A et al (2008) Defective tryptophan catabolism underlies inflammation in mouse chronic granulomatous disease. Nature 451:211–216
Lambeth JD, Kawahara T, Diebold B (2007) Regulation of Nox and Duox enzymatic activity and expression. Free Radic Biol Med 43:319–331
Geiszt M (2006) NADPH oxidases: new kids on the block. Cardiovasc Res 71:289–299
Bedard K, Lardy B, Krause K-H (2007) NOX family NADPH oxidases: not just in mammals. Biochemie 89:1107–1112
Miller FJ Jr., Filali M, Huss GJ et al (2007) Cytokine activation of nuclear factor kB in vascular smooth muscle cells requires signaling endosomes containing Nox1 and CLC-3. Circ Res 101:663–671
Cheng G, Diebold BA, Hughes Y et al (2006) Nox1-dependent reactive oxygen generation is regulated by Rac1. J Biol Chem 281:17718–17726
Kawahara T, Kohjima M, Kuwano Y et al (2005) Helicobacter pylori lipopolysaccharide activates rac1 and transcription of NADPH oxidase NOX1 and its organizer NOXO1 in guinea pig gastric mucosal cells. Am J Physiol Cell Physiol 288:C450–C457
Miyano K, Ueno N, Takeya R et al (2006) Direct involvement of the small GTPase rac in activation of the superoxide-producing NADPH oxidase Nox1. J Biol Chem 281:21857–21868
Park HS, Lee S-H, Park D et al (2004) Sequential activation of phosphatidylinositol 3-kinase, beta Pix, Rac1, Nox1 in growth factor-induced production of H2O2. Mol Cell Biol 24:4384–4394
Takeya R, Ueno N, Kami K et al (2003) Novel human homologues of p47phox and p67phox participate in activation of superoxide-production NADPH oxidases. J Biol Chem 278:25234–25246
Gorin Y, Ricono JM, Kim N-H et al (2003) Nox4 mediates angiotensin II-induced activation of Akt/protein kinase B in mesangial cells. Am J Physiol Renal Physiol 285:F219–F229
Inoguchi T, Sonta T, Tsubouchi H et al (2003) Protein kinase C-dependent increase in reactive oxygen species (ROS) production in vascular tissues of diabetes: role of vascular NAD(P)H oxidase. J Am Soc Nephrol 14:S227–S232
Bánfi B, Tirone F, Durussel I et al (2004) Mechanism of Ca2 + activation of the NADPH oxidase 5 (NOX5). J Biol Chem 279:18583–18591
Ameziane-El-Hassani R, Morand S, Boucher J-L et al (2005) Dual oxidase-2 has an intrinsic Ca2 + -dependent H2O2-generating activity. J Biol Chem 280:30046–30054
Kawahara T, Quinn MT, Lambeth JD (2007) Molecular evolution of the reactive oxygen-generating NADPH oxidase (Nox/Duox) family of enzymes. BMC Evol Biol 7:109
Tirone F, Cox JA (2007) NADPH oxidase 5 (NOX5) interacts with and is regulated by calmodulin. FEBS Lett 581:1202–1208
Serrander L, Jaquet V, Bedard K et al (2007) NOX5 is expressed at the plasma membrane and generates superoxide in response to protein kinase C activation. Biochimie 89:1159–1167
Jagnandan D, Church JE, Banfi B et al (2007) Novel mechanism of activation of NADPH oxidase 5(NOX5): calcium-sensitization via phosphorylation. J Biol Chem 282:6494–6507
Bánfi B, Molnár G, Maturana A et al (2001) A Ca(2+)-activated NADPH oxidase in testis, spleen, and lymph nodes. J Biol Chem 276:37594–37601
Benedyk M, Sopalla C, Nacken W et al (2007) HaCaT keratinocytes overexpressing the S100 proteins S100A8 and S100A9 show increased NADPH oxidase and NF-kB activities. J Invest Dermatol 127:2001–2011
Cheng G, Cao Z, Xu X et al (2001) Homologs of gp91phox: cloning and tissue expression of Nox3, Nox4, and Nox5. Gene 269:131–140
Salles N, Szanto I, Herrmann F et al (2005) Expression of mRNA for ROS-generating NADPH oxidases in the aging stomach. Exp Gerontol 40:353–357
Kamiguti AS, Serrander L, Lin K et al (2005) Expression and activity of NOX5 in the circulating malignant B cells of hairy cell leukemia. J Immunol 175:8424–8430
Brar SS, Corbin Z, Kennedy TP et al (2003) NOX5 NAD(P)H oxidase regulates growth and apoptosis in DU 145 prostate cancer cells. Am J Physiol Cell Physiol 285:C353–C369
van der Vliet A (2008) NADPH oxidases in lung biology and pathology: host defense enzymes, and more. Free Radic Biol Med 44(6):938–955
Geiszt M, Leto TL (2004) The Nox family of NAD(P)H oxidases: host defense and beyond. J Biol Chem 279:51715–51718
Lambeth JD (2002) Nox/Duox family of nicotinamide adenine dinucleotide (phosphate) oxidases. Curr Opin Hematol 9:11–17
Grandvaux N, Soucy-Faulkner A, Fink K (2007) Innate host defense: nox and duox on phox's tail. Biochimie 89:1113–1122
Leto TL, Geiszt M (2006) Role of Nox family NADPH oxidases in host defense. Antioxid Redox Signal 8:1549–1561
Szanto I, Rubbia-Brandt L, Kiss P et al (2005) Expression of NOX1, a superoxide-generating NADPH oxidase, in colon cancer and inflammatory bowel disease. J Pathol 207:164–176
Bánfi B, Clark RA, Steger K et al (2003) Two novel proteins activate superoxide generation by the NADPH oxidase NOX1. J Biol Chem 278:3510–3513
Kawahara T, Kuwano Y, Teshima-Kondo S et al (2004) Role of nicotinamide adenine dinucleotide phosphate oxidase 1 in oxidative burst response to toll-like receptor 5 signaling in large intestinal epithelial cells. J Immunol 172:3051–3058
Torres MA, Jones JDG, Dangl JL (2006) Reactive oxygen species signaling in response to pathogens. Plant Physiol 141:373–378
Kumar GNM, Iyer S, Knowles NR (2007) Strboh A homologue of NADPH oxidase regulates wound-induced oxidative burst and facilitates wound-healing in potato tubers. Planta 227:25–36
Foreman J, Demidchik V, Bothwell JHF et al (2003) Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 422:442–446
Dangl JL, McDowell JM (2006) Two modes of pathogen recognition by plants. Proc Natl Acad Sci U S A 103:8575–8576
Egan MJ, Wang Z-Y, Jones MA et al (2007) Generation of reactive oxygen species by fungal NADPH oxidases is required for rice blast disease. Proc Natl Acad Sci U S A 104:11772–11777
Lemaitre B, Hoffmann J (2007) The host defense of Drosophila melanogaster. Annu Rev Immunol 25:697–743
De Muñez FG-G, Lanz-Mendoza H, Hernández-Hernández FC (2007) Free radical generation during the activation of hemolymph prepared from the homopteran Dactylopius coccus. Arch Insect Biochem Physiol 65:20–28
Pereira LS, Oliveira PL, Barja-Fidalgo C et al (2001) Production of reactive oxygen species by hemocytes from the cattle tick Boophilus microplus. Exp Parasitol 99:66–72
Molina-Cruz A, DeJong RJ, Charles B et al (2008) Reactive oxygen species modulate Anopheles gambiae immunity against bacteria and Plasmodium. J Biol Chem 283:3217–3223
Dancer SJ (2004) How antibiotics can make us sick: the less obvious adverse effects of antimicrobial chemotherapy. Lancet 4:611–619
Dème D, Doussiere J, De Sandro V et al (1994) The Ca2 + /NADPH-dependent H2O2 generator in thyroid plasma membrane: inhibition by diphenyleneiodonium. Biochem J 301:75–81
Dupuy C, Ohayon R, Valent A et al (1999) Purification of a novel flavoprotein involved in the thyroid NADPH oxidase. J Biol Chem 274:37265–37269
de Deken X, Wang D, Dumont JE et al (2002) Characterization of ThOX proteins as components of the thyroid H2O2-generating system. Exp Cell Res 273:187–196
Dupuy C, Virion A, Ohayon R et al (1991) Mechanism of H2O2 formation catalyzed by NADPH oxidase in thyroid plasma membrane. J Biol Chem 266:3739–3743
Grasberger H, de Deken X, Miot F et al (2007) Missense mutations of dual oxidase 2 (DUOX2) implicated in congenital hypothyroidism have impaired trafficking in cells reconstituted with DUOX2 maturation factor. Mol Endocrinol 21:1408–1421
Moreno JC, Bikker H, Kempers MJE et al (2002) Inactivating mutations in the gene for thyroid oxidase 2 (thox2) and congenital hypothyroidism. N Engl J Med 347:95–102
Moreno JC, Visser TJ (2007) New phenotypes in thyroid dyshormonogenesis:hypothyroidism due to DUOX2 mutations. Endocr Dev 10:99–117
De Vijlder JJM, Bikker H, Ris Stalpers C et al (1997) Structure, function, and relevance of thyroid peroxidase in inherited diseases of the thyroid. Curr Opin Endocrinol Diabetes 4:328–332
Heinecke JW, Shapiro BM (1989) Respiratory burst oxidase of fertilization. Proc Natl Acad Sci U S A 86:1259–1263
Heinecke JW, Shapiro BM (1992) The respiratory burst oxidase of fertilization: a physiological target for regulation by protein kinase C. J Biol Chem 267:7959–7962
Wong JL, Créton R, Wessel GM (2004) The oxidative burst at fertilization is dependent upon activation of the dual oxidase Udx1. Dev Cell 7:801–814
Edens WA, Sharling L, Cheng G et al (2001) Tyrosine cross-linking of extracellular matrix is catalyzed by Duox, a multidomain oxidase/peroxidase with homology to the phagocyte oxidase subunit gp91phox. J Cell Biol 154:879–891
Ris-Stalpers C (2006) Physiology and pathophysiology of the duoxes. Antioxid Redox Signal 8:1563–1572
Geiszt M, Witta J, Baffi J et al (2003) Dual oxidases represent novel hydrogen peroxide sources supporting mucosal surface host defense. FASEB J 17:1502–1504
Donko A, Peterfi Z, Sum A et al (2005) Dual oxidases. Philos Trans R Soc Lond [Biol] 360:2301–2308
El Hassani RA, Benfares N, Caillou B et al (2005) Dual oxidase2 is expressed all along the digestive tract. Am J Physiol Gastrointest Liver Physiol 288:G933–G942
Klebanoff SJ, Clem WH, Luebke RG (1966) The peroxidase–thiocyanate–hydrogen peroxide antimicrobial system. Biochim Biophys Acta 117:63–72
Reiter N, Perraudin JP (1991) Lactoperoxidase: biological function. In: Everse J, Everse KE, Grisham MB (eds) Peroxidases in chemistry and biology. vol. 1. CRC Press, Boca Raton, FL, pp 143–180
Ha E-M, Oh C-T, Bae Y-S et al (2005) A direct role for dual oxidase in Drosophila gut immunity. Science 310:847–850
Conner GE, Salathe M, Forteza R (2002) Lactoperoxidase and hydrogen peroxide metabolism in the airway. Am J Respir Crit Care Med 166:S57–S61
El-Chemaly S, Salathe M, Baier S et al (2003) Hydrogen peroxide-scavenging properties of normal human airway secretions. Am J Respir Crit Care Med 167:425–430
Forteza R, Salathe M, Miot F et al (2005) Regulated hydrogen peroxide production by duox in human airway epithelial cells. Am J Respir Cel Mol Biol 32:462–469
Fragoso MA, Fernandez V, Forteza R et al (2004) Transcellular thiocyanate transport by human airway epithelia. J Physiol 561:183–194
Harper RW, Xu C, McManus M et al (2006) Duox2 exhibits potent heme peroxidase activity in human respiratory tract epithelium. FEBS Lett 580:5150–5154
Moskwa P, Lorentzen D, Excoffon KJ et al (2007) A novel host defense system of airways is defective in cystic fibrosis. Am J Respir Crit Care Med 175:174–183
Schwarzer C, Machen TE, Illek B et al (2004) NADPH oxidase-dependent acid production in airway epithelial cells. J Biol Chem 279:36454–36461
Wijkstrom-Frei C, El-Chemaly S, Ali-Rachedi R et al (2003) Lactoperoxidase and human airway host defense. Am J Respir Cell Mol Biol 29:206–212
Salathe M, Holderby M, Forteza R et al (1997) Isolation and characterization of a peroxidase from the airway. Am J Respir Cell Mol Biol 17:97–105
Christensen TG (1984) The distribution and function of peroxidases in the respiratory tract. Surv Synth Pathol Res 3:201–218
Ihalin R, Loimaranta V, Tenovuo J (2005) Origin, structure, and biological activities of peroxidases in human saliva. Arch Biochem Biophys 445:261–268
Vilja P, Lumikari M, Tenovuo J et al (1991) Sensitive immunometric assays for secretory peroxidase and myeloperoxidase in human saliva. J Immunol Methods 141:277–284
Tenovuo J, Makinen KK, Sievers G (1985) Antibacterial effect of lactoperoxidase and myeloperoxidase against Bacillus cereus. Antimicrob Agents Chemother 27:96–101
Thomas EL, Fishman M (1986) Oxidation of chloride and thiocyanate by isolated leukocytes. J Biol Chem 261:9694–9702
Thomas EL, Aune TM (1978) Lactoperoxidase, peroxide, thiocyanate antimicrobial system: correlation of sulfhydryl oxidation with antimicrobial action. Infect Immun 20:456–463
Pollock JR, Goff HM (1992) Lactoperoxidase-catalyzed oxidation of thiocyanate ion: a carbon-13 nuclear magnetic resonance study of the oxidation products. Biochim Biophys Acta 1159:279–285
Modi S, Deodhar SS, Behere DV et al (1991) Lactoperoxidase-catalyzed oxidation of thiocyanate by hydrogen peroxide: 15N nuclear magnetic resonance and optical spectral studies. Biochemistry 30:118–124
Løvaas E (1992) Free radical generation and coupled thiol oxidation by lactoperoxidase/SCN-/H2O2. Free Radic Biol Med 13:187–195
Senthilmohan R, Kettle AJ (2006) Bromination and chlorination reactions of myeloperoxidase at physiological concentrations of bromide and chloride. Arch Biochem Biophys 445:235–44
Gaut JP, Ydh GC, Tran HD et al (2001) Neutrophils employ the myeloperoxidase system to generate antimicrobial brominating and chlorinating oxidants during sepsis. Proc Natl Acad Sci U S A 98:11961–11966
Klebanoff SJ (2005) Myeloperoxidase: friend and foe. J Leukoc Biol 77:598–625
Shao MXG, Nadel JA (2005) Dual oxidase 1-dependent MUC5AC mucin expression in cultured human airway epithelial cells. Proc Natl Acad Sci U S A 102:767–772
Kuwahara I, Lillejoh EP, Koga T et al (2007) The signaling pathway involved in neutrophil elastase-stimulated MUC1 transcription. Am J Respir Cell Mol Bio 37:691–698
Yan F, Li W, Jono H et al (2008) Reactive oxygen species regulate Pseudomonas aeruginosa lipopolysaccharide-induced MuC5AC mucin expression via PKC-NADPH oxidase-ROS-TGF-a signaling pathways in human airway epithelial cells. Biochem Biophys Res Commun 366:513–519
Nakanaga T, Nadel JA, Ueki IF et al (2007) Regulation of interleukin-8 via an airway epithelial signaling cascade. Am J Physiol Lung Cell Mol Physiol 292:L1289–L1296
Nakano Y, Longo-Guess CM, Bergstrom DE, Nauseef WM, Jones SM, Banfi B (2008) Mutation of the Cyba gene encoding p22phox causes vestibular and immune defects in mice. J Clin Invest 118:1176–1185
Acknowledgements
Work in the Nauseef laboratory is supported by grants from the National Institutes of Health (AI 34879, HL 53592, AI 44642) and the Veterans Administration (Merit Review).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nauseef, W.M. Nox enzymes in immune cells. Semin Immunopathol 30, 195–208 (2008). https://doi.org/10.1007/s00281-008-0117-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00281-008-0117-4