Abstract
Introduction
Fedratinib, an oral, selective Janus kinase 2 inhibitor, has been shown to inhibit P-glycoprotein (P-gp), breast cancer resistance protein (BCRP), organic anion transporting polypeptide (OATP) 1B1, OATP1B3, organic cation transporter (OCT) 2, and multidrug and toxin extrusion (MATE) 1 and MATE2-K in vitro. The objective of this study was to evaluate the influence of fedratinib on the pharmacokinetics (PK) of digoxin (P-gp substrate), rosuvastatin (OATP1B1/1B3 and BCRP substrate), and metformin (OCT2 and MATE1/2-K substrate).
Methods
In this nonrandomized, fixed-sequence, open-label study, 24 healthy adult participants received single oral doses of digoxin 0.25 mg, rosuvastatin 10 mg, and metformin 1000 mg administered as a drug cocktail (day 1, period 1). After a 6-day washout, participants received oral fedratinib 600 mg 1 h before the cocktail on day 7 (period 2). An oral glucose tolerance test (OGTT) was performed to determine possible influences of fedratinib on the antihyperglycemic effect of metformin.
Results
Plasma exposure to the three probe drugs was generally comparable in the presence or absence of fedratinib. Reduced metformin renal clearance by 36% and slightly higher plasma glucose levels after OGTT were observed in the presence of fedratinib. Single oral doses of the cocktail ± fedratinib were generally well tolerated.
Conclusions
These results suggest that fedratinib has minimal impact on the exposure of P-gp, BCRP, OATP1B1/1B3, OCT2, and MATE1/2-K substrates. Since renal clearance of metformin was decreased in the presence of fedratinib, caution should be exercised in using coadministered drugs that are renally excreted via OCT2 and MATEs.
Trial registration
Clinicaltrials.gov NCT04231435 on January 18, 2020.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fedratinib is an oral, selective inhibitor of wild-type and mutationally activated Janus kinase (JAK) 2 and FMS-like tyrosine kinase 3. The JAK/signal transducer and activation of transcription (STAT) pathway is involved in cytokine signaling, and dysregulation of this pathway may play a role in hematologic malignancies [1, 2]. Fedratinib inhibits dysregulated JAK2 signaling that drives the pathogenesis of myeloproliferative neoplasms, including myelofibrosis (MF) [3]. Previous studies have demonstrated that, in addition to having a manageable safety profile, fedratinib significantly reduced splenomegaly, normalized peripheral cell counts, including leukocytes and platelets, and improved symptom burden in patients with MF [4]. In 2019, fedratinib was approved for the treatment of adult patients with intermediate-2 or high-risk primary or secondary MF by the US Food and Drug Administration (FDA) [5].
Patients with MF have a mean age of 61–66 years [6], and a majority have comorbidities of which the most common are cardiovascular disease, diabetes, hypertension, liver disease, and previous solid tumors [7,8,9]. Because of these comorbidities, patients with MF frequently take several medications concomitantly with treatment for their hematologic disease—making characterization of potential fedratinib drug–drug interactions (DDI) particularly important. Fedratinib is metabolized by cytochrome P450 (CYP) enzymes, predominantly by CYP3A4 [10, 11], and concomitant administration of fedratinib with strong CYP3A4 inhibitors significantly increased fedratinib exposure [12]. Fedratinib inhibits several CYP enzymes, including CYP3A4, and fedratinib treatment can result in increased plasma levels of other drugs metabolized by CYP3A4 and other CYP enzymes [10, 13].
Along with drug-metabolizing enzymes, cell membrane transporter proteins involved in tissue uptake and excretion of small molecules also frequently influence drug pharmacokinetics (PK) and pharmacodynamics (PD) in a clinically relevant fashion [14,15,16]. Transporters often involved in DDI include P-glycoprotein (P-gp), breast cancer resistance protein (BCRP), organic anion transporting polypeptide (OATP) 1B1, OATP1B3, organic cation transporter (OCT) 2, and multidrug and toxin extrusion (MATE) 1 and MATE2-K [15].
Fedratinib has been shown to inhibit P-gp, BCRP, OATP1B1/1B3, OCT2, and MATE1/2-K in vitro [5, 13]; thus, a clinical investigation of the transporter-mediated DDI potential was performed. The primary objective of this study was to evaluate the influence of fedratinib on the PK of probe substrates of these transporters, including digoxin, a substrate of P-gp; rosuvastatin, a substrate of OATP1B1/1B3 and BCRP; and metformin, a substrate of OCT2 and MATE1/2-K. In addition, the impact of fedratinib on metformin-mediated antihyperglycemic effects was evaluated using an oral glucose tolerance test (OGTT) [17, 18]. The secondary objective was to evaluate the safety and tolerability of fedratinib coadministration with digoxin, rosuvastatin, and metformin in healthy adult participants.
Materials and methods
Study and ethical considerations
This was a nonrandomized, open-label study in healthy adult participants. The protocol complied with recommendations of the 18th World Health Congress (Helsinki, 1964) and all applicable amendments. The protocol and its amendment were submitted to an institutional review board (Salus Independent Review Board, Austin, TX) for review and written approval. Written informed consent was obtained prior to the conduct of any study-related procedures.
Study population
Healthy adult male and female participants between 18 and 65 years of age who had a body mass index ≥ 18 and ≤ 33 kg/m2 were enrolled. Participants were considered healthy based on medical history, physical examination, clinical laboratory tests, vital signs, and 12-lead electrocardiogram (ECG), with supine systolic blood pressure ≥ 90 and ≤ 140 mmHg, supine diastolic blood pressure ≥ 50 and ≤ 90 mmHg, and pulse rate ≥ 45 and ≤ 100 beats per minute at screening. Conducting the study in healthy adult participants mitigated the potential confounding effects of the hematologic disease state, comorbidities, and concomitant medications, and obviated the significant enrollment challenges associated with performing the study in patients with MF.
Study design and treatment
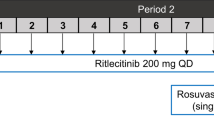
In this nonrandomized, fixed-sequence, open-label study (NCT04231435), the study duration was approximately 8 weeks from screening through the follow-up telephone call (Supplementary Fig S1). On day 1 (period 1), participants were administered a cocktail of single doses of digoxin (1 × 0.25-mg tablet), rosuvastatin (1 × 10-mg tablet), and metformin (1 × 1000-mg tablet). Based on the terminal elimination half-life (t1/2) of the probe drugs [19,20,21], a 6-day washout was considered sufficient to prevent carryover to the second dose of probe drugs. After the 6-day washout period, participants received fedratinib (6 × 100-mg capsules) 1 h before a cocktail of digoxin (1 × 0.25-mg tablet), rosuvastatin (1 × 10-mg tablet), and metformin (1 × 1000-mg tablet) on day 7 (period 2). For the OGTT, a glucose dose of 75 g was administered orally 2 h after the oral administration of the digoxin, rosuvastatin, and metformin cocktail. The antiemetic palonosetron (0.25 mg) was administered as a 30-s intravenous infusion approximately 30 min before fedratinib administration on day 7 (period 2) to reduce the potential for fedratinib-related nausea and vomiting. Palonosetron was also administered approximately 90 min before digoxin, metformin, and rosuvastatin administration on day 1 (period 1) to ensure similar conditions of probe drug administration in each study period. Palonosetron was selected because its DDI profile made unlikely any confounding PK interactions with the probe drugs [22, 23], while its intravenous route of administration avoided potential physical absorption effects on the probe drugs in the lumen of the digestive tract. Participants were discharged from the clinical site on day 22 and received a follow-up telephone call within 2 to 5 days of discharge.
PK and PD sampling times and bioanalytical methods
Blood samples were collected for measurement of plasma digoxin, rosuvastatin, and metformin concentrations on days 1 and 7 before dosing, and 0.5, 1, 1.5, 2, 3, 4, 6, 8, 12, 24, 48, 72, and 96 h post dose of the digoxin, rosuvastatin, and metformin cocktail. To measure fedratinib plasma concentrations, blood samples were collected predose, at 0.5 h post dose of fedratinib, and at 0.5, 1, 1.5, 2, 3, 4, 6, 8, 12, 24, 48, 72, 96, 120, 168, and 192 h post dose of the digoxin, rosuvastatin, and metformin cocktail. To determine urine metformin concentrations, urine samples were collected before dosing and at 0–2, 2–4, 4–6, 6–8, 8–12, 12–24, and 24–48 h post dose of the digoxin, rosuvastatin, and metformin cocktail. Blood samples were collected for measurement of plasma glucose for OGTT before and at 15, 30, 45, 60, 90, 120, and 180 min after oral glucose administration.
Following liquid–liquid extraction for digoxin, supported-liquid extraction for rosuvastatin, or protein precipitation extraction for metformin, plasma concentrations of digoxin, rosuvastatin, and metformin were determined using validated reversed-phase liquid chromatography–tandem mass spectrometry (LC–MS/MS) assays with lower limit of quantitation (LLOQ; calibration range) of 0.05 (0.05–10) ng/mL, 0.02 (0.02–20) ng/mL, and 2 (2–2000) ng/mL, respectively (Covance Bioanalytical Services, LLC, Indianapolis, IN). Intraday precision values were ≤ 8.0% for digoxin, ≤ 7.7% for rosuvastatin, and ≤ 4.4% for metformin. Interday precision values were ≤ 4.7% for digoxin, ≤ 6.0% for rosuvastatin, and ≤ 5.2% for metformin. Interday accuracy values were within ± 3.3% for digoxin, within ± 4.8% for rosuvastatin, and within ± 4.5% for metformin. Plasma fedratinib concentrations were determined using a validated LC−MS/MS assay [10, 24]. Following a dilution procedure for metformin, urine concentrations of metformin were determined using a validated reversed-phase LC–MS/MS assay with LLOQ (calibration range) of 5 (5–2500) μg/mL (WuXi AppTec, Plainsboro, NJ). The intraday precision, interday precision, and interday accuracy values were ≤ 9.96%, ≤ 7.09%, and within ± 10.5%, respectively.
PK variables
PK parameters were estimated for digoxin, rosuvastatin, metformin, and, when applicable, fedratinib. Plasma PK parameters included maximum observed plasma concentration (Cmax), time to Cmax (tmax), area under the plasma concentration–time curve (AUC) from time 0 to the last time point with a measurable concentration (AUC0−t), AUC from time 0 to infinity (AUC0−∞), t1/2, apparent total plasma clearance, and apparent total volume of distribution during the terminal phase. Urinary PK parameters for metformin included total amount excreted in the urine, percentage of metformin excreted in the urine, and renal clearance (CLr).
Statistical methods
Approximately 24 participants were planned to be enrolled so that ≥ 12 participants would complete the study. An analysis of variance model with treatment as a fixed effect and participant as a random effect was performed separately for each comparison on the natural log-transformed Cmax, AUCs, and CLr (metformin only). The geometric means along with ratios of the geometric means (expressed as a percentage) and associated 90% CIs were presented for digoxin, rosuvastatin, and metformin plus fedratinib (“cocktail + fedratinib,” test) versus digoxin, rosuvastatin, and metformin alone (“cocktail,” reference). For tmax, the Wilcoxon signed-rank test, Hodges–Lehmann estimate, and 90% CIs were calculated for the median difference between treatments.
Safety assessment
Safety was monitored throughout the study. Safety assessments included treatment-emergent adverse events (TEAEs), physical examinations, vital sign measurements, 12-lead ECG, glucose level measurements (via finger sticks) as clinically indicated, and clinical laboratory information (eg, hematology, chemistry, urinalysis). TEAEs were recorded from the time of consent form signing to study completion and up to 30 days after the last dose.
Results
Participants and participant disposition
A total of 24 healthy adults were enrolled, and 22 of those participants completed the study. Two participants (8.3%) discontinued; both discontinued prior to dosing with fedratinib, and neither discontinuation was considered related to study treatment. One participant discontinued on day 7 due to a diagnosis of trichomoniasis and the need for that infection to be treated with metronidazole. The second participant was found to have frequent premature atrial contractions on predose ECG (and subsequent rhythm strip) on day 7. This was considered not clinically significant (and no adverse event was reported), but the participant did not receive planned study treatment on day 7 and discontinued from the study on day 8 (due to investigator’s discretion). All 24 enrolled participants were included in the safety, PK, and PD populations, but the two discontinued participants provided data for period 1 only.
Demographics and baseline characteristics are shown in Table 1. Overall, 15 (62.5%) participants were male and 9 (37.5%) were female, with a mean age of 34 years (20–61). There were 13 (54.2%) White, 10 (41.7%) Black or African American, and 1 (4.2%) Asian participants.
Influence of fedratinib on digoxin, rosuvastatin, and metformin plasma PK
Mean plasma concentration–time profiles of digoxin, rosuvastatin, metformin, and fedratinib following single oral administration of the drug cocktail in period 1 and single oral administration of the cocktail + fedratinib 600 mg in period 2 are presented on linear and semilogarithmic scales in Fig. 1a–d. Following single oral doses of the cocktail, all three probe drugs exhibited a rapid absorption phase followed by a biphasic elimination phase with plasma concentrations measurable, on average, up to at least 72 h post dose. Following a single oral dose of the probe drug cocktail in the presence of fedratinib, concentrations of the probe drugs were generally comparable to that observed in the absence of fedratinib. PK parameters (geometric mean and geometric percent coefficient of variation [CV%]) are shown in Table 2.
Mean (± SD) plasma concentration–time profile of a digoxin, b rosuvastatin, c metformin, and d fedratinib following a single oral cocktail dose of digoxin (0.25 mg), rosuvastatin (10 mg), and metformin (1000 mg) when administered alone (open symbols) and when administered with fedratinib (600 mg, closed symbols), presented in linear (upper panel) and semilogarithmic (lower panel) scales. The dotted lines depict the lower limit of quantitation for digoxin (a; 0.0500 ng/mL), rosuvastatin (b; 0.0200 ng/mL), metformin (c; 2.00 ng/mL) and fedratinib (d; 1.00 ng/mL). Fedratinib was administered during the second experimental period on day 7 only
Geometric mean digoxin plasma exposures when the cocktail was administered with fedratinib were approximately 99%, 103%, and 111% for Cmax, AUC0−t, and AUC0−∞, respectively, of that when the cocktail was administered alone (Table 3). The 90% CI of the ratio of geometric means (cocktail + fedratinib/cocktail) lay within customary bioequivalence limits (80–125%) for all PK parameters. A statistically significant delay was observed in median tmax in the presence of fedratinib (1.92 h) compared with tmax in the absence of fedratinib (1.50 h) (P = 0.0162).
Geometric mean rosuvastatin plasma exposures when the cocktail was administered with fedratinib were approximately 80%, 101%, and 102%, for Cmax, AUC0−t, and AUC0−∞, respectively, of that when the cocktail was administered alone (Table 3). The 90% CI of the ratio of geometric means (cocktail + fedratinib/cocktail) lay within customary bioequivalence limits for AUC0−t and AUC0−∞. Furthermore, tmax for rosuvastatin was comparable in the presence or absence of fedratinib (P = 0.0552).
Geometric mean metformin plasma exposures when the cocktail was administered with fedratinib were approximately 88%, 100%, and 97%, for Cmax, AUC0−t, and AUC0−∞, respectively, of that when the cocktail was administered alone (Table 3). The 90% CI of the ratio of geometric means (cocktail + fedratinib/cocktail) lay within customary bioequivalence limits for AUC0−t and AUC0−∞. Furthermore, tmax for metformin was comparable in the presence or absence of fedratinib (P = 0.0693).
Influence of fedratinib on metformin urinary elimination
When the drug cocktail was administered alone, the geometric mean (geometric CV%) total amount of metformin excreted in the urine up to 48 h post dose was 324 mg (28%), or 32.4% of the total dose, whereas in the presence of cocktail + fedratinib, the amount excreted was 204 mg (30%), or 20.4% of the total dose. In the presence of cocktail + fedratinib, metformin CLr was significantly reduced by 35.8% despite no clinically relevant change in plasma metformin exposure (Table 3). The mean cumulative amount of metformin eliminated in urine is shown in Fig. 2.
Influence of fedratinib on antihyperglycemic effect of metformin
The metformin-mediated antihyperglycemic effects were evaluated by OGTT. Following a single dose of glucose in the absence and presence of fedratinib, plasma glucose concentrations gradually increased and returned to the baseline level over the 3-h observation period (Fig. 3). Both absolute and baseline-adjusted plasma glucose levels were higher in the presence of the cocktail + fedratinib from 0.75 h (after glucose administration) onward compared with cocktail alone. A small increase was seen in geometric mean (geometric CV%) absolute glucose AUC0−3 (17% higher) in the presence of cocktail + fedratinib vs in the presence of the cocktail alone (357 h × mg/dL [16.3%] vs 306 h × mg/dL [14.7%]), as shown in Supplementary Table S1.
Mean (± SD) plasma glucose concentration − time profiles by treatment. Baseline was defined as predose for each period and participant separately, i.e., period 1 (day 1) and period 2 (day 7) used predose records relative to that period (day). Absolute measured concentrations are shown in the upper panel. To estimate baseline-adjusted values, predose concentrations for each participant were subtracted from each postdose concentration; where baseline-adjusted concentrations were negative, they were set to zero
Safety evaluation
Overall, 15 of 24 participants (62.5%) reported ≥ 1 TEAE: nine of 24 participants (37.5%) after receiving the cocktail and 10 of 22 participants (45.5%) receiving cocktail + fedratinib. The majority of TEAEs were mild in severity. Moderate TEAEs (nausea, vomiting) were reported by two participants after they received the drug cocktail. No deaths or severe or serious AEs were reported. Overall, 10 of 24 participants (41.7%) reported ≥ 1 TEAE suspected of being related to any study drug, with the most common treatment-related AEs being diarrhea (n = 7) and nausea (n = 6). (Supplementary Table S2). All TEAEs resolved by the end of the study. No participant had a clinical laboratory, vital sign, or 12-lead ECG result that was reported as a TEAE.
Plasma creatinine was measured on day − 1 and at 12 h post dose of the cocktail on day 1 (period 1) and day 7 (period 2)—all at approximately the same time of day. Mean plasma concentrations of creatinine on baseline (day − 1), and post treatment on day 1 (12 h after dosing of cocktail) were similar (80.6 μmol/L and 79.4 μmol/L, respectively) and within the normal range for men and women. However, an increase from baseline was observed on day 7 (12 h after dosing of cocktail + fedratinib), and the mean value (94.9 μmol/L) was within the normal range for men (67–112 μmol/L) but above the normal range for women (50–88 μmol/L) (Supplementary Fig. S2).
Discussion
Fedratinib has inhibitory effects on multiple transporters in vitro, including P-gp, BCRP, OATP1B1, OATP1B3, OCT2, MATE1, and MATE2-K. The purpose of this study was to evaluate the influence of fedratinib on the PK of clinical probe substrates of these transporters (digoxin, rosuvastatin, and metformin), as well as the impact of fedratinib on metformin-mediated antihyperglycemic effects, with the goal of understanding the potential for transporter-mediated DDI with fedratinib in a clinical setting.
In vitro evaluations indicated that fedratinib inhibits P-gp (half-maximal inhibitory concentration [IC50], 10.1 µM), BCRP (IC50, 29.9 µM), OATP1B1 (IC50, 16.4 µM), OATP1B3 (IC50, 9.51 µM), OCT2 (IC50, 0.78 µM), MATE1 (IC50, 0.352 µM), and MATE-2 K (IC50, 0.227 µM) [13, 25]. Based on the FDA DDI guidance [16], fedratinib has the potential to inhibit P-gp and BCRP in vivo because the Igut /IC50 value is ≥ 10, where Igut = 400 mg/250 mL = 3050 µM or 1.6 mg/mL, where Igut is the intestinal luminal concentration of the interacting drug calculated as the dose/250 mL. Fedratinib also has the potential to inhibit OCT2, MATE1, and MATE2-K in vivo because the Imax,u/IC50 value is ≥ 0.1, where Imax,u = 0.171 µM (Imax [3.8 µM or 2000 ng/mL] × fu,p [0.045]), where Imax,u is the maximal unbound plasma concentration of the interacting drug at steady state, Imax is the maximal plasma concentration of the interacting drug at steady state, and fu,p is the fraction unbound in the plasma. R values for OATP1B1 and OATP1B3 were close to 1.1, where R = 1 + (fu,p [0.045] × Iin,max [27 µM])/IC50), where Iin,max is the estimated maximum plasma inhibitor concentration at the inlet to the liver. Thus, this study was designed to evaluate the influence of fedratinib on the PK of clinical probe substrates for these transporters. Key design considerations included choice and administration (i.e., as a cocktail) of probe drugs, duration of washout period, and fedratinib dose.
The probe drugs chosen in this study were digoxin (P-gp substrate), rosuvastatin (OATP1B1/OATP1B3 and BCRP substrate), and metformin (OCT2 and MATE1/2-K substrate), each of which is a prototypic clinical substrate for transporters according to the FDA [26]. Utility of these probe drugs administered together as a cocktail has been previously demonstrated [27, 28]—supporting use of the cocktail as a screening tool in an initial drug interaction study—with significant changes in bioavailability triggering more focused follow-up DDI investigation. Although increased systemic exposure of rosuvastatin has been observed after coadministration with clinically relevant doses of metformin (by up to 54% and 23% at 500 and 750 mg, respectively) in healthy participants [29, 30], the interaction between metformin and rosuvastatin should not confound the results of this study because metformin does not inhibit OATP1B1, OATP1B3, or BCRP [31, 32], where the potential interaction between fedratinib and rosuvastatin could occur. The underlying mechanism for increased systemic exposure of rosuvastatin by metformin is unclear but it was indicated that metformin leads to an increase in rosuvastatin bioavailability rather than a change in systemic clearance of rosuvastatin [27]. Thus, DDI effect of fedratinib on rosuvastatin could be assessed. Moreover, both treatment arms had the same doses of rosuvastatin and metformin; therefore, one treatment arm serves as a control for another treatment arm, balancing out any potential interaction between these two agents. A 6-day washout period between cocktail administrations was used in this study. Though this might be shorter than five times the upper range of published t1/2 estimates for digoxin (26–45 h) [21], all predose plasma samples for digoxin as well as rosuvastatin and metformin in period 2 were below the LLOQ of their respective assays, validating the use of the 6-day washout period in this study.
The fedratinib dose to be administered prior to the cocktail in period 2 had to be chosen such that clinically relevant concentrations could be achieved in a single dose (no supportive multiple-dose experience having been available in healthy participants) in safe and tolerable fashion. Single fedratinib doses of up to 680 mg have been administered in healthy participants, with doses up to 500 mg having been generally well-tolerated [33]. Vomiting at 680 mg was considered a tolerability, rather than a safety, issue; thus, prophylaxis with an antiemetic was considered acceptable mitigation for fedratinib-related vomiting and nausea. Accordingly, 600 mg was chosen as the fedratinib dose in this study, with antiemetic prophylaxis, to maximize fedratinib exposure and tolerability. The Cmax values at 600 mg were expected to provide concentrations higher than the IC50 values for OCT2 and MATE1/2-K, while the gut/portal concentrations at 600 mg are expected to be higher than those achieved with a clinically recommended 400-mg dose. Single oral doses of the drug cocktail components alone and coadministered with fedratinib were generally safe and well-tolerated by the healthy adults in this study. Most TEAEs reported were mild in severity. The primarily gastrointestinal findings associated with fedratinib administration in this study were consistent with previous reports from clinical trials [33, 34]. Palonosetron was chosen as a prophylactic antiemetic in this study instead of ondansetron, which has typically been used in clinical studies of fedratinib [35] because ondansetron is a potent inhibitor of MATE1 (Ki, 0.035 μM) and MATE2-K (Ki, 0.015 μM) in vitro [36] and decreased the renal clearance of metformin by 37% in healthy participants [37]. Palonosetron is also reported as an inhibitor of MATE1 (IC50, 2.48 µM) and MATE2-K (IC50, 11.55 µM) in vitro [38]. However, the inhibitory potency of palonosetron on MATEs is weaker than those of ondansetron and fedratinib in vitro. Therefore, palonosetron is unlikely to have a significant impact on the influence on metformin renal clearance by fedratinib given that mean plasma palonosetron concentrations associated with the 0.25-mg intravenous dose fall well below its IC50s for these transporters [39]. Furthermore, palonosetron was administered in both periods in this study (cocktail only and cocktail + fedratinib) such that any potential small effects on MATE1 and MATE2-K function would cancel out in the comparisons between periods.
Evaluation of the plasma PK parameters of digoxin (P-gp substrate), rosuvastatin (OATP1B1, OATP1B3, and BCRP substrate), and metformin (OCT2, MATE1, and MATE2-K substrate), generally suggested no clinically meaningful DDI, based on exposure in systemic circulation (plasma), between these probe drugs and fedratinib. Systemic plasma exposure to all 3 probe drugs was generally comparable after a single administration of the drug cocktail in the presence or absence of fedratinib. No apparent differences in dose-normalized Cmax and AUCs of fedratinib were observed between this study (fedratinib 600 mg) and the recent study with CYP3A4 inducers (fedratinib 500 mg, [40]). However, a short but statistically significant delay in tmax was observed for digoxin in the presence of fedratinib (1.92 vs 1.50 h), which corresponds to lower digoxin concentrations in the presence of fedratinib in early hours after administration (0.5–1.5 h after digoxin administration) although digoxin concentrations showed higher interindividual variability in the early hours after administration than the later timepoints. This finding is not explained by P-gp inhibition, and exposure metrics of digoxin such as Cmax and AUCs was comparable in the presence or absence of fedratinib. Wiebe et al. [41] recently reported that the effect of single-dose verapamil (a potent P-gp inhibitor) on digoxin systemic exposure was less than that expected from multiple-dose studies, suggesting that potential test compounds inhibiting P-gp could also be dosed attaining near steady-state concentration from a single dose before administering the cocktail. In this study, the impact of single-dose fedratinib on the PK of digoxin was investigated, however, 600 mg (1.5 times the clinically recommended dose) was used as fedratinib dose and no increase in digoxin exposure was observed in the presence of this higher than clinically recommended fedratinib dose while single-dose verapamil still had minor effect on digoxin exposure [41]. In addition, lower digoxin concentration in the presence of fedratinib in early hours after administration further confirms no P-gp inhibition by fedratinib since P-gp inhibition would be expected to have the opposite effect. Taken together, fedratinib is unlikely to inhibit P-gp in vivo at a clinical dose of 400 mg once daily.
Although no clinically meaningful DDI based on plasma PK for metformin was observed, a statistically significant 36% reduction in metformin CLr was observed in the presence of fedratinib. Metformin is transported from the blood compartment into renal proximal tubular cells primarily by OCT2 [42]. MATE1 and MATE2‐K are also involved in the efflux of metformin from renal proximal tubular cells to urine [43]. The combination of uptake by OCT2 and secretion by MATEs contributes to renal clearance of metformin. A decrease in metformin CLr in the presence of fedratinib could thus be due to the inhibition of MATEs and OCT2 by fedratinib in vivo. The involvement of MATE2-K is less clear, as inhibition of MATE2-K by the selective MATE2-K inhibitor nizatidine had no effect on metformin CLr [44]. It appears that equipotent inhibition of MATE1 and MATE2-K by fedratinib, unlike selective inhibition of MATE2-K by nizatidine, has translated into reduced CLr of metformin in this study. This inhibition of OCT2 and MATEs by fedratinib appears to be consistent with a slight increase from baseline in plasma creatinine, another endogenous OCT and MATE substrate, observed 12 h after single administration of the cocktail + fedratinib, as has been observed for other drugs, such as trimethoprim, an inhibitor of OCT2 and MATEs [45]. One explanation for the lack of a clinically relevant impact of fedratinib on plasma metformin exposure could be that fedratinib inhibits renal secretion of metformin via MATEs but not uptake of metformin from blood into renal proximal tubular cells via OCT2 in vivo. Fedratinib has higher inhibition potencies against MATE1/2-K versus OCT2, which is consistent with other known inhibitors of OCT2 and MATEs [46]. Furthermore, the DDI between cationic drugs and cimetidine (an inhibitor of OCT2 and MATEs) at their clinical doses is suggested to be due to competitive inhibition of MATEs by cimetidine instead of OCT2 [47]. Inhibition of apical efflux transporters may result in increased intracellular drug concentrations in renal proximal tubular cells [48], which should be taken into consideration when fedratinib is administered in combination with potentially nephrotoxic drugs that are also OCT2 and MATE substrates.
In addition to fedratinib’s effects on plasma and urinary metformin PK, its influence on the antihyperglycemic PD effect of metformin was also evaluated using an OGTT. The OGTT was included in both periods in which metformin was administered. Plasma glucose levels were higher in the presence of fedratinib (i.e., absolute glucose AUC0−3 was 17% higher). During the OGTT evaluation period (2–5 h after metformin administration), geometric mean plasma metformin concentrations were lower in the presence of fedratinib compared with the absence of fedratinib (at 2, 3, and 4 h post dose of metformin). The slight decrease in early metformin exposure by fedratinib might in part explain the slight decrease in antihyperglycemic PD effect of metformin after OGTT. A fedratinib-alone treatment period with the OGTT was not incorporated in this study because the phase 3 JAKARTA trial (in which patients with MF received placebo, fedratinib 400 mg, or fedratinib 500 mg once daily) demonstrated neither frequent adverse events of hyperglycemia, nor any adverse events of hypoglycemia, nor clinically meaningful changes from baseline in mean glucose levels in placebo or fedratinib dose groups (Bristol Myers Squibb, Data on file).
In conclusion, clinically meaningful influences of fedratinib on the plasma exposure of digoxin, rosuvastatin, and metformin were not observed. These results suggest that fedratinib has minimal impact on the exposure of P-gp, BCRP, OATP1B1/1B3, and OCT2/MATEs substrates. Since renal clearance of metformin was decreased in the presence of fedratinib, caution should be exercised for patients receiving fedratinib in combination with drugs that are renally excreted via OCT2 and MATEs.
Data availability
Data requests may be submitted to Celgene, a Bristol Myers Squibb Company, at https://vivli.org/ourmember/celgene/ and must include a description of the research proposal.
References
Vainchenker W, Constantinescu SN (2013) JAK/STAT signaling in hematological malignancies. Oncogene 32(21):2601–2613. https://doi.org/10.1038/onc.2012.347
Furqan M, Mukhi N, Lee B, Liu D (2013) Dysregulation of JAK-STAT pathway in hematological malignancies and JAK inhibitors for clinical application. Biomark Res 1(1):5. https://doi.org/10.1186/2050-7771-1-5
Pardanani A, Gotlib JR, Jamieson C, Cortes JE, Talpaz M, Stone RM, Silverman MH, Gilliland DG, Shorr J, Tefferi A (2011) Safety and efficacy of TG101348, a selective JAK2 inhibitor, in myelofibrosis. J Clin Oncol 29(7):789–796. https://doi.org/10.1200/JCO.2010.32.8021
Pardanani A, Tefferi A, Jamieson C, Gabrail NY, Lebedinsky C, Gao G, Liu F, Xu C, Cao H, Talpaz M (2015) A phase 2 randomized dose-ranging study of the JAK2-selective inhibitor fedratinib (SAR302503) in patients with myelofibrosis. Blood Cancer J 5(8):e335. https://doi.org/10.1038/bcj.2015.63
Bristol Myers Squibb INREBIC (fedratinib) label. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/212327s000lbl.pdf. Accessed 10 Dec 2020
Mehta J, Wang H, Iqbal SU, Mesa R (2014) Epidemiology of myeloproliferative neoplasms in the United States. Leuk Lymphoma 55(3):595–600. https://doi.org/10.3109/10428194.2013.813500
Breccia M, Palandri F, Luciano L, Benevolo G, Bonifacio M, Caocci G, Castagnetti F, Palumbo GA, Iurlo A, Landi F (2018) Identification and assessment of frailty in older patients with chronic myeloid leukemia and myelofibrosis, and indications for tyrosine kinase inhibitor treatment. Ann Hematol 97(5):745–754. https://doi.org/10.1007/s00277-018-3258-0
Bartoszko J, Panzarella T, McNamara CJ, Lau A, Schimmer AD, Schuh AC, Sibai H, Maze D, Yee KWL, Devlin R, Gupta V (2017) Distribution and impact of comorbidities on survival and leukemic transformation in myeloproliferative neoplasm-associated myelofibrosis: a retrospective cohort study. Clin Lymphoma Myeloma Leuk 17(11):774–781. https://doi.org/10.1016/j.clml.2017.06.031
Newberry KJ, Naqvi K, Nguyen KT, Cardenas-Turanzas M, Florencia Tanaka M, Pierce S, Verstovsek S (2014) Comorbidities predict worse prognosis in patients with primary myelofibrosis. Cancer 120(19):2996–3002. https://doi.org/10.1002/cncr.28857
Ogasawara K, LoRusso PM, Olszanski AJ, Rixe O, Xu C, Yin J, Palmisano M, Krishna G (2020) Assessment of effects of repeated oral doses of fedratinib on inhibition of cytochrome P450 activities in patients with solid tumors using a cocktail approach. Cancer Chemother Pharmacol 86(1):87–95. https://doi.org/10.1007/s00280-020-04102-3
Ogasawara K, Xu C, Kanamaluru V, Siebers N, Surapaneni S, Ridoux L, Palmisano M, Krishna G (2020) Excretion balance and pharmacokinetics following a single oral dose of [(14)C]-fedratinib in healthy subjects. Cancer Chemother Pharmacol 86(2):307–314. https://doi.org/10.1007/s00280-020-04121-0
Ogasawara K, Xu C, Kanamaluru V, Palmisano M, Krishna G (2020) Effects of repeated oral doses of ketoconazole on a sequential ascending single oral dose of fedratinib in healthy subjects. Cancer Chemother Pharmacol 85(5):899–906. https://doi.org/10.1007/s00280-020-04067-3
Wu F, Krishna G, Surapaneni S (2020) Physiologically based pharmacokinetic modeling to assess metabolic drug-drug interaction risks and inform the drug label for fedratinib. Cancer Chemother Pharmacol 86(4):461–473. https://doi.org/10.1007/s00280-020-04131-y
Giacomini KM, Huang SM (2013) Transporters in drug development and clinical pharmacology. Clin Pharmacol Ther 94(1):3–9. https://doi.org/10.1038/clpt.2013.86
International Transporter C, Giacomini KM, Huang SM, Tweedie DJ, Benet LZ, Brouwer KL, Chu X, Dahlin A, Evers R, Fischer V, Hillgren KM, Hoffmaster KA, Ishikawa T, Keppler D, Kim RB, Lee CA, Niemi M, Polli JW, Sugiyama Y, Swaan PW, Ware JA, Wright SH, Yee SW, Zamek-Gliszczynski MJ, Zhang L (2010) Membrane transporters in drug development. Nat Rev Drug Discov 9(3):215–236. https://doi.org/10.1038/nrd3028
U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) (2020) In Vitro Drug Interaction Studies—Cytochrome P450 Enzyme- and Transporter-Mediated Drug Interactions Guidance for Industry. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/vitro-drug-interaction-studies-cytochrome-p450-enzyme-and-transporter-mediated-drug-interactions. Accessed 11 Feb 2021
Hibma JE, Zur AA, Castro RA, Wittwer MB, Keizer RJ, Yee SW, Goswami S, Stocker SL, Zhang X, Huang Y, Brett CM, Savic RM, Giacomini KM (2016) The effect of famotidine, a MATE1-selective inhibitor, on the pharmacokinetics and pharmacodynamics of metformin. Clin Pharmacokinet 55(6):711–721. https://doi.org/10.1007/s40262-015-0346-3
Zamek-Gliszczynski MJ, Chu X, Cook JA, Custodio JM, Galetin A, Giacomini KM, Lee CA, Paine MF, Ray AS, Ware JA, Wittwer MB, Zhang L, International Transporter Consortium (2018) ITC commentary on metformin clinical drug-drug interaction study design that enables an efficacy- and safety-based dose adjustment decision. Clin Pharmacol Ther 104(5):781–784. https://doi.org/10.1002/cpt.1082
Graham GG, Punt J, Arora M, Day RO, Doogue MP, Duong JK, Furlong TJ, Greenfield JR, Greenup LC, Kirkpatrick CM, Ray JE, Timmins P, Williams KM (2011) Clinical pharmacokinetics of metformin. Clin Pharmacokinet 50(2):81–98. https://doi.org/10.2165/11534750-000000000-00000
White CM (2002) A review of the pharmacologic and pharmacokinetic aspects of rosuvastatin. J Clin Pharmacol 42(9):963–970
Iisalo E (1977) Clinical pharmacokinetics of digoxin. Clin Pharmacokinet 2(1):1–16. https://doi.org/10.2165/00003088-197702010-00001
Helsinn Therapeutics (2020) ALOXI® (palonosetron HCl injection) label. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/021372s021lbl.pdf. Accessed 21 Jan 2021
University of Washington (2021) Drug Interaction Solutions. https://didb.druginteractionsolutions.org/drug/monograph/6225/ Accessed 9 Jan 2021
Ogasawara K, Zhou S, Krishna G, Palmisano M, Li Y (2019) Population pharmacokinetics of fedratinib in patients with myelofibrosis, polycythemia vera, and essential thrombocythemia. Cancer Chemother Pharmacol 84(4):891–898. https://doi.org/10.1007/s00280-019-03929-9
Center for Drug Evaluation and Research (2019) Application number: 212327Orig1s000 Multi-discipline review, Fedratinib. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2019/212327Orig1s000MultidisciplineR.pdf. Accessed 10 Dec 2020
U. S. Food and Drug Administration (2019) Drug development and drug interactions: table of substrates, inhibitors and inducers. https://www.fda.gov/drugs/drug-interactions-labeling/drug-development-and-drug-interactions-table-substrates-inhibitors-and-inducers#table3-3. Accessed 10 Dec 2020
Stopfer P, Giessmann T, Hohl K, Sharma A, Ishiguro N, Taub ME, Zimdahl-Gelling H, Gansser D, Wein M, Ebner T, Muller F (2016) Pharmacokinetic evaluation of a drug transporter cocktail consisting of digoxin, furosemide, metformin, and rosuvastatin. Clin Pharmacol Ther 100(3):259–267. https://doi.org/10.1002/cpt.406
Stopfer P, Giessmann T, Hohl K, Hutzel S, Schmidt S, Gansser D, Ishiguro N, Taub ME, Sharma A, Ebner T, Muller F (2018) Optimization of a drug transporter probe cocktail: potential screening tool for transporter-mediated drug-drug interactions. Br J Clin Pharmacol 84(9):1941–1949. https://doi.org/10.1111/bcp.13609
Lee D, Roh H, Son H, Jang SB, Lee S, Nam SY, Park K (2014) Pharmacokinetic interaction between rosuvastatin and metformin in healthy Korean male volunteers: a randomized, open-label, 3-period, crossover, multiple-dose study. Clin Ther 36(8):1171–1181. https://doi.org/10.1016/j.clinthera.2014.06.004
Stopfer P, Giessmann T, Hohl K, Sharma A, Ishiguro N, Taub ME, Jungnik A, Gansser D, Ebner T, Muller F (2018) Effects of metformin and furosemide on rosuvastatin pharmacokinetics in healthy volunteers: implications for their use as probe drugs in a transporter cocktail. Eur J Drug Metab Pharmacokinet 43(1):69–80. https://doi.org/10.1007/s13318-017-0427-9
Shin E, Shin N, Oh JH, Lee YJ (2017) High-dose metformin may increase the concentration of atorvastatin in the liver by inhibition of multidrug resistance-associated protein 2. J Pharm Sci 106(4):961–967. https://doi.org/10.1016/j.xphs.2016.11.020
Bachmakov I, Glaeser H, Fromm MF, Konig J (2008) Interaction of oral antidiabetic drugs with hepatic uptake transporters: focus on organic anion transporting polypeptides and organic cation transporter 1. Diabetes 57(6):1463–1469. https://doi.org/10.2337/db07-1515
Zhang M, Xu CR, Shamiyeh E, Liu F, Yin JY, von Moltke LL, Smith WB (2014) A randomized, placebo-controlled study of the pharmacokinetics, pharmacodynamics, and tolerability of the oral JAK2 inhibitor fedratinib (SAR302503) in healthy volunteers. J Clin Pharmacol 54(4):415–421. https://doi.org/10.1002/jcph.218
Pardanani A, Harrison C, Cortes JE, Cervantes F, Mesa RA, Milligan D, Masszi T, Mishchenko E, Jourdan E, Vannucchi AM, Drummond MW, Jurgutis M, Kuliczkowski K, Gheorghita E, Passamonti F, Neumann F, Patki A, Gao G, Tefferi A (2015) Safety and efficacy of fedratinib in patients with primary or secondary myelofibrosis: a randomized clinical trial. JAMA Oncol 1(5):643–651. https://doi.org/10.1001/jamaoncol.2015.1590
Ogasawara K, Vince B, Xu C, Zhang M, Palmisano M, Krishna G (2020) A phase I study of the effect of repeated oral doses of pantoprazole on the pharmacokinetics of a single dose of fedratinib in healthy male subjects. Cancer Chemother Pharmacol 85(5):995–1001. https://doi.org/10.1007/s00280-020-04074-4
Li Q, Guo D, Dong Z, Zhang W, Zhang L, Huang SM, Polli JE, Shu Y (2013) Ondansetron can enhance cisplatin-induced nephrotoxicity via inhibition of multiple toxin and extrusion proteins (MATEs). Toxicol Appl Pharmacol 273(1):100–109. https://doi.org/10.1016/j.taap.2013.08.024
Li Q, Yang H, Guo D, Zhang T, Polli JE, Zhou H, Shu Y (2016) Effect of ondansetron on metformin pharmacokinetics and response in healthy subjects. Drug Metab Dispos 44(4):489–494. https://doi.org/10.1124/dmd.115.067223
U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) (2017) Application number: 210493Orig1s000 MULTI-DISCIPLINE REVIEW (Akynzeo). https://www.accessdata.fda.gov/drugsatfda_docs/nda/2018/210493Orig1s000MultidisciplineR.pdf. Accessed 18 Jan 2021
Shah A, DeGroot T, Apseloff G (2006) Pharmacokinetic evaluation and safety profile of a 15-minute versus 30-second infusion of palonosetron in healthy subjects. J Clin Pharmacol 46(10):1139–1145. https://doi.org/10.1177/0091270006291625
Ogasawara K, Kam J, Thomas M, Liu L, Liu M, Xue Y, Surapaneni S, Carayannopoulos LN, Zhou S, Palmisano M, Krishna G (2021) Effects of strong and moderate CYP3A4 inducers on the pharmacokinetics of fedratinib in healthy adult participants. Cancer Chemother Pharmacol. https://doi.org/10.1007/s00280-021-04292-4
Wiebe ST, Giessmann T, Hohl K, Schmidt-Gerets S, Hauel E, Jambrecina A, Bader K, Ishiguro N, Taub ME, Sharma A, Ebner T, Mikus G, Fromm MF, Muller F, Stopfer P (2020) Validation of a drug transporter probe cocktail using the prototypical inhibitors rifampin, probenecid, verapamil, and cimetidine. Clin Pharmacokinet 59(12):1627–1639. https://doi.org/10.1007/s40262-020-00907-w
Kimura N, Okuda M, Inui K (2005) Metformin transport by renal basolateral organic cation transporter hOCT2. Pharm Res 22(2):255–259. https://doi.org/10.1007/s11095-004-1193-3
Tanihara Y, Masuda S, Sato T, Katsura T, Ogawa O, Inui K (2007) Substrate specificity of MATE1 and MATE2-K, human multidrug and toxin extrusions/H(+)-organic cation antiporters. Biochem Pharmacol 74(2):359–371. https://doi.org/10.1016/j.bcp.2007.04.010
Morrissey KM, Stocker SL, Chen EC, Castro RA, Brett CM, Giacomini KM (2016) The effect of Nizatidine, a MATE2K selective inhibitor, on the pharmacokinetics and pharmacodynamics of metformin in healthy volunteers. Clin Pharmacokinet 55(4):495–506. https://doi.org/10.1007/s40262-015-0332-9
Nakada T, Kudo T, Kume T, Kusuhara H, Ito K (2018) Quantitative analysis of elevation of serum creatinine via renal transporter inhibition by trimethoprim in healthy subjects using physiologically-based pharmacokinetic model. Drug Metab Pharmacokinet 33(1):103–110. https://doi.org/10.1016/j.dmpk.2017.11.314
Mathialagan S, Rodrigues AD, Feng B (2017) Evaluation of renal transporter inhibition using creatinine as a substrate in vitro to assess the clinical risk of elevated serum creatinine. J Pharm Sci 106(9):2535–2541. https://doi.org/10.1016/j.xphs.2017.04.009
Tsuda M, Terada T, Ueba M, Sato T, Masuda S, Katsura T, Inui K (2009) Involvement of human multidrug and toxin extrusion 1 in the drug interaction between cimetidine and metformin in renal epithelial cells. J Pharmacol Exp Ther 329(1):185–191. https://doi.org/10.1124/jpet.108.147918
Yin J, Wang J (2016) Renal drug transporters and their significance in drug-drug interactions. Acta Pharm Sin B 6(5):363–373. https://doi.org/10.1016/j.apsb.2016.07.013
Acknowledgements
The authors would like to thank all study participants and clinical study team members from both Bristol Myers Squibb and PPD. The clinical trial reported in this manuscript was designed and sponsored by Bristol Myers Squibb. Medical writing support was provided by Alex Loeb, PhD, of Chrysalis Medical Communications, Hamilton, NJ, and funded by Bristol Myers Squibb.
Funding
This trial was sponsored by Bristol Myers Squibb.
Author information
Authors and Affiliations
Contributions
KO and GK contributed to study design, data analysis, interpretation, and drafted manuscript. LNC, LL, and ML contributed to study design, data analysis, and interpretation. RNW-H and YX contributed to data acquisition and interpretation. MaT contributed to study design, data acquisition, and interpretation. MiT, SS, SZ, and MP contributed to study design and interpretation. All authors critically reviewed the draft manuscript and approved the final version to be published and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Conflict of interest
K.O., Ma.T., Mi.T., L.L, M.L, Y.X, S.S, L.N.C., S.Z., M.P., and G.K. are employees of, and hold equity ownership in, Bristol Myers Squibb. R.N.W–H. is an employee of PPD Development LP.
Ethics approval
The protocol complied with recommendations of the 18th World Health Congress (Helsinki, 1964) and all applicable amendments. The protocol and its amendment were submitted to an institutional review board (Salus Independent Review Board, Austin, TX) for review and written approval.
Consent to participate
Written informed consent was obtained prior to the conduct of any study-related procedures.
Consent for publication
All authors critically reviewed the draft manuscript and approved the final version to be published and agree to be accountable for all aspects of the work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ogasawara, K., Wood-Horrall, R.N., Thomas, M. et al. Impact of fedratinib on the pharmacokinetics of transporter probe substrates using a cocktail approach. Cancer Chemother Pharmacol 88, 941–952 (2021). https://doi.org/10.1007/s00280-021-04346-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-021-04346-7