Abstract
Purpose
We conducted a phase 1 trial of the HSP90 inhibitor onalespib in combination with the CDK inhibitor AT7519, in patients with advanced solid tumors to determine the safety profile and maximally tolerated dose, pharmacokinetics, preliminary antitumor activity, and to assess the pharmacodynamic (PD) effects on HSP70 expression in patient-derived PBMCs and plasma.
Methods
This study followed a 3 + 3 trial design with 1 week of intravenous (IV) onalespib alone, followed by onalespib/AT7519 (IV) on days 1, 4, 8, and 11 of a 21-days cycle. PK and PD samples were collected at baseline, after onalespib alone, and following combination therapy.
Results
Twenty-eight patients were treated with the demonstration of downstream target engagement of HSP70 expression in plasma and PBMCs. The maximally tolerated dose was onalespib 80 mg/m2 IV + AT7519 21 mg/m2 IV. Most common drug-related adverse events included Grade 1/2 diarrhea (79%), fatigue (54%), mucositis (57%), nausea (46%), and vomiting (50%). Partial responses were seen in a palate adenocarcinoma and Sertoli–Leydig tumor; a colorectal and an endometrial cancer patient both remained on study for ten cycles with stable disease as the best response. There were no clinically relevant PK interactions for either drug.
Conclusions
Combined onalespib and AT7519 is tolerable, though below monotherapy RP2D. Promising preliminary clinical activity was seen. Further benefit may be seen with the incorporation of molecular signature pre-selection. Further biomarker development will require the assessment of the on-target impact on relevant client proteins in tumor tissue.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The 90 kDa heat-shock protein (HSP90) is a member of a class of evolutionarily conserved molecular chaperones that have an integral role in the maintenance of protein homeostasis within cells. These chaperones assist in the folding, stabilization, activation, and proteolytic turnover of newly synthesized “client” proteins [1,2,3]. Among these client proteins are those involved in aberrant cellular functions responsible for the hallmarks of cancer, including angiogenesis and glucose metabolism [4, 5], growth factor independence [2], cell cycle progression [6], tissue invasion and metastases [7], avoidance of apoptosis [8], and acquired drug resistance [9]. By protecting the aberrant survival of these proteins, HSP90 enables tumor progression and resistance to therapies. Inhibition of HSP90 accordingly results in the degradation of these aberrant proteins through the ubiquitin–proteasome pathway, resulting in tumor cell death [10].
Upregulation of the heat-shock response, in particular HSP70 and HSP27, occurs in response to HSP90 inhibition, and is postulated as a biomarker of HSP90 inhibition and target engagement [11]. Increasing evidence implicates this compensatory upregulation of HSP70 as a resistance mechanism to HSP90 inhibition based on the anti-apoptotic activity of HSP70 at the pre-mitochondrial, mitochondrial, and post-mitochondrial level [12]. HSP70 has been shown to block the recruitment of procaspase-9 to the Apaf-1 apoptosome [8, 13] and to directly antagonize apoptosis-inducing factor (AIF) [14]. Induction of HSP70 has been linked to the activity of the positive transcription elongation factor (P-TEFb), a multiprotein complex containing the catalytic subunit CDK9 that phosphorylates the C-terminal domain of RNA polymerase II and facilitates elongation of the HSP70 transcript [15]. Several in vitro studies have shown that ablation of HSP70 activity enhances HSP90 inhibitor-mediated cell death [16,17,18,19].
Onalespib is a non-ansamycin small molecule inhibitor of HSP90, with an IC50 = 0.7 nM that has demonstrated modest anti-tumor activity in patients with advanced solid tumors using both twice-weekly and once-weekly schedules [19,20,21]. AT7519 is a small molecule inhibitor of CDKs 1,2,4,5, and 9 (IC50 range 11–220 nM) that has demonstrated preliminary activity in patients with advanced solid tumors, chronic lymphocytic leukemias, and mantle cell lymphomas [22,23,24].
In vitro studies supporting the rationale for the combination in a colorectal cancer cell line show dose-dependent inhibition of onalespib-induced HSP70 upregulation by AT7519 (Supplementary Fig. 1A). Combination treatment with onalespib + AT7519 results in a rapid reduction of phosphorylated RNA polymerase II, a result of CDK9 inhibition, suggesting an impaired cellular survival mechanism. In these studies, downstream modulation of client proteins, particularly HER-2 and pAKT, were seen (Supplementary Fig. 1B). Similar data were confirmed in in vitro and in vivo models of NSCLC driven by ELM4-ALK, mutant EGFR, and mutant KRAS [25]. Based on extensive preclinical evidence supporting synergism of the combination, we conducted a phase I trial of the HSP90 inhibitor onalespib in combination with the CDK inhibitor AT7519, in patients with advanced solid tumors to determine the safety profile and maximally tolerated dose and to assess the pharmacodynamic (PD) effects on HSP70 expression in patient-derived PBMCs and plasma.
Patients and methods
Study population
Patients with advanced solid tumors without approved curative therapy or effective palliative therapy, ≥ 18 years of age, with an Eastern Cooperative Oncology Group (ECOG) performance status 0–1, and evaluable or measurable disease per Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 were eligible for the study. All patients were required to have adequate organ function defined by absolute neutrophil count ≥ 1.5 × 109/L, platelet count ≥ 100 × 109/L, total bilirubin ≤ 1.5 × institutional upper limit of the reference range (ULRR), aspartate aminotransferase (AST) and alanine aminotransferase (ALT) ≤ 2.5 × ULRR, creatinine clearance ≥ 50 mL/min by Cockcroft–Gault formula, creatinine phosphokinase within ULRR, QTc < 450 msec for men or < 470 msec for women by Fridericia correction, and left ventricular ejection fraction ≥ 50% on echocardiogram. Patients were required to have completed previous cancer therapy at least 3 weeks prior to study entry, and adverse events from prior therapy must have recovered to eligibility levels with the exception of alopecia. There were no limits on the number of prior therapies. Exclusion criteria included untreated brain metastases or carcinomatous meningitis, pregnancy or lactation, pre-existing retinal disease on an ophthalmologic exam, grade ≥ 2 peripheral neuropathy, or HIV status requiring antiretroviral therapy due to the potential for PK drug interactions.
Study design and treatment administration
This study was conducted through the National Cancer Institute’s Experimental Therapeutics Clinical Trials Network (ETCTN) and opened at four sites. The primary objective was to determine the safety and tolerability of the combination. Secondary objectives were to describe the PK and to evaluate the PD effects of the addition of AT7519 to onalespib on HSP70 expression in PBMCs and plasma as proof-of-principle of target effect. The trial followed a 3 + 3 dose-escalation design evaluating sequential stepwise dose escalation of each agent, incorporating a 7-days lead-in (cycle “0”) of onalespib alone administered on days 1 and 4, to accommodate PK and PD evaluation of onalespib single-agent activity versus the combination. Thereafter, both agents were administered as a 1-h intravenous infusion twice weekly (days 1, 4, 8, and 11) every 21 days. The first dose level evaluated onalespib at 40 mg/m2 + AT7519 at 21 mg/m2 based on published monotherapy pharmacokinetics of the agents [19, 22]. Starting doses were below the monotherapy RP2D due to concerns for the potential for overlapping liver toxicities. The dose-escalation strategy favored increasing onalespib first based on the observation that higher doses of onalespib led to greater HSP70 induction in these studies, whereas the primary role of AT7519 was to limit the transcription of HSP70 through reduction in RNA polymerase II activation.
Dose-limiting toxicity definitions and study assessments
Safety was assessed via monitoring of toxicities during the lead-in cycle 0 + cycle 1 according to National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) v.4.03. Dose-limiting toxicities (DLT) were defined as Grade 4 neutropenia > 5 days or febrile neutropenia, Grade 4 thrombocytopenia, and any Grade 3–4 non-hematologic toxicities related to study drug and occurring during the lead-in and/or cycle 1. Grade 3 ≥ nausea, vomiting, diarrhea, or electrolyte derangements were considered dose-limiting if refractory to management and not improved to Grade ≤ 2 within 48 h. Any Grade 2 or higher ocular toxicity that did not resolve to Grade 1 or less within 2 weeks of withholding treatment was considered dose-limiting. Additionally, more than one individual dose omission during the DLT window as a result of drug-related toxicity was considered dose limiting. A Safety Monitoring Committee comprised of the Study Chair and the Principal Investigators at each participating site determined the dose escalations according to the toxicity observed in the previous cohorts. The MTD was defined as the dose level at which no more than one of six evaluable patients experienced a DLT during the lead-in period and the first cycle of treatment. At the MTD, up to ten patients could be enrolled in an expansion phase to further evaluate safety, tolerability, and PD endpoints.
A physical examination, assessment of vital signs, pertinent tumor biomarker assessments, hematology and chemistry assessments were performed at screening, day 1 of cycle 0, prior to each treatment (days 1, 4, 8, and 11) and day 15 of cycle 1, and days 1 and 8 of each 21-days cycle starting with cycle 2. Electrocardiograms were performed at the start of each cycle. Based on published ocular and cardiac toxicities of the agents [19, 22] ophthalmologic exam and echocardiogram were performed at screening, after two cycles, and as clinically indicated. Radiologic assessments by computerized tomography or magnetic resonance imaging were performed at screening and every 2 months during the first four cycles, and every three cycles thereafter if patients continued to receive treatment.
Pharmacokinetic assessments
Blood samples were collected in 4-mL plastic Vacutainer tubes with spray-dried K2EDTA (Becton, Dickinson and Co., Franklin Lakes, NJ) prior to dosing and designated timepoints after onalespib infusion on cycle 0 day 1, and on cycle 1 days 1 and 11 after sequential infusion of onalespin and AT7519. Blood collection tubes were centrifuged to harvest the plasma which was stored in cryovials at − 80 °C. Concentrations of onalespib and AT7519 in plasma samples were determined concurrently by ion-pair reversed-phase high-performance liquid chromatography with tandem mass spectrometric detection. The assay was validated as recommended by the US Food and Drug Administration Guidance for Industry, Bioanalytical Method Validation (May 2018). Plasma samples (50 µL) were spiked with a solution of the internal standard and prepared for analysis by vigorously mixing with three volumes of acetonitrile and centrifuging to pellet the precipitated proteins. The supernatant was removed and diluted with 1.5-volumes of 0.2% (v/v) heptafluorobutyric acid in water. An aliquot (10 µL) of the final sample solution was injected onto a Phenomenex (Torrance, CA) Luna 5 µm C18(2) analytical HPLC column maintained at 40 °C and separated by gradient elution using a mobile phase composed of 0.2% (v/v) heptafluorobutyric acid in acetonitrile and water delivered at 1.0 mL/min. An Agilent Technologies (Santa Clara, CA) model G6410B triple quadrupole mass spectrometer with an electrospray ionization interface was operated in the multiple-reaction monitoring mode to detect the m/z 410.2 → 132.1, m/z 382.1 → 84.2, and m/z 324.2 → 293.2 transitions for onalespib (retention time 5.4 min), AT7519 (4.6 min), and the internal standard (4.0 min), respectively. Quantitation was based upon integrating the extracted ion chromatogram for each transition to provide peak areas and calculating the ratio of the analyte-to-internal standard peak area for each study sample, quality control sample, and calibration standard.
Time points were determined as the difference between the blood sample collection time and the starting time of the infusion for each drug. Plasma concentration (free base equivalent)–time data were analyzed by noncompartmental methods using model 202 for constant infusion drug input in WinNonlin Professional version 5.0.1 (Pharsight Corp, Mountain View, CA). Pharmacokinetic parameters are reported as the geometric mean (geometric %CV) of the values for individual patients at each dose level. GraphPad Prism for Windows, version 8.3.0 (GraphPad Software, La Jolla, CA) was used for the statistical comparison of mean pharmacokinetic parameters using the paired or unpaired two-tailed t test, as appropriate, after logarithmic transformation of the data. P < 0.05 was the criterion for statistical significance.
Pharmacodynamic assessments
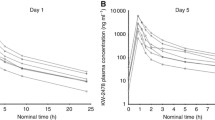
PBMCs and plasma were isolated from peripheral blood prior to onalespib during cycle 0 (C0D1), 2–4 h after completion of onalespib administration during cycle 0 day 4 (C0D4), and 2–4 h after completion of onalespib and AT7519 administration during cycle 1 day 11 (C0D11) based on optimal sampling time of putative biomarkers in preclinical modeling. The level of HSP70 protein expression was measured by western blot in PBMCs as reported previously [26]; soluble HSP70 protein level was measured by ELISA in plasma (Fig. 1). The significance of changes between C0D1, C0D4, and C1D11 was evaluated using the Wilcoxon sign-rank test. Correlation between change in plasma levels and change in PBMCs was tested using Pearson correlation. Additional exploratory PD assays that were planned included evaluation of HSP90 inhibition in tumor biopsies by assessing transcriptional changes and expression of HSP70 as a comparison to PBMC and plasma expression levels, based on previously published data suggesting differential expression in PBMCs versus tumor [27]. An expansion cohort with paired tumor biopsies was not pursued, however, due to internal re-prioritization by the sponsor to discontinue further development of both agents necessitating early closure of the study.
HSP70 expression in patient-derived plasma and peripheral blood mononuclear cells (PBMCs). Peripheral blood samples were collected at baseline, 2–4 h after completion of onalespib on C0D4, and 2–4 h after completion of the combination of onalespib + AT7519 on C1D11, and separated into plasma and PBMC pellets. The level of soluble HSP70 protein expression was measured by ELISA in plasma (Panel a); HSP70 protein expression was measured by western blot in PBMCs and quantitated (Panel b) as reported previously [26]
Results
Patient disposition and characteristics
A total of 29 patients enrolled in the study between September 2016 through January 2019, including a 10-patient expansion cohort at the MTD for evaluation of pharmacodynamic endpoints (Table 1). Twenty-eight patients received at least one treatment, one patient was determined to be ineligible after enrollment, and accordingly was not treated. All patients who received at least one treatment were evaluable for safety. Twenty-three patients were evaluable for response by RECIST v1.1. Three patients were inevaluable for response due to disease progression after completion of the lead-in cycle, necessitating change in treatment to include radiotherapy, surgical intervention, or transition to hospice. Two patients were removed from the study during the first cycle due to dose-limiting toxicities. Eight patients remained on study for greater than four cycles, two patients remained on study for ten cycles.
Adverse events
The most common adverse events (AEs) attributed to treatment and occurring in ≥ 10% of patients are summarized in Table 2. Of these AEs, the majority were Grades 1 and 2 (92.3%), 7% were Grade 3, and 0.7% were Grade 4. Across all grades, the most common AEs experienced by patients were gastrointestinal and included diarrhea (22/28; 79%), oral mucositis (16/28; 57%), vomiting (14/28; 50%), nausea (13/28; 46%), and dry mouth (11/28; 39%). Fatigue was also a recurring AE, occurring at a frequency of 54% (15/28). Additional adverse events included hyperglycemia (10/28; 36%), increase in alkaline phosphatase (8/28; 29%), and anemia (10/28; 36%). At the 80 mg/m2 dose of onalespib, two patients (7%) reported blurry vision and “floaters;” however, no abnormalities were detected on ophthalmologic exams. Visual disturbances resolved with holding treatment and did not recur with rechallenge of treatment. Central ECG recordings revealed no change in cardiac repolarization or prolongation of QTc intervals. Additionally, no changes in echocardiogram ejection fraction were detected after two cycles in any patients. Two patients (7%) had Grade 1 increase in creatine phosphokinase consistent with previously reported toxicity of AT7519. The two dose-limiting toxicities at DL3 (onalespib 80 mg/m2 + AT7519 27 mg/m2) were Grade 3 increase in cardiac troponins and Grade 3 oral mucositis. Both patients were removed from further study treatment at physician discretion. No additional dose escalation was pursued, and the MTD was declared at DL2 with onalespib at 80 mg/m2 + AT7519 at 21 mg/m2.
Pharmacokinetic analyses
Mean values of the pharmacokinetic parameters for onalespib and AT7519 for 17 patients evaluated at the MTD of the combination (DL2) are presented in Table 3. The mean maximum plasma concentration (Cmax) of onalespib on day 1 (331 ng/mL) and day 11 (355 ng/mL) of cycle 1 was comparable to the mean Cmax for the cycle 0 dose (349 ng/mL), as expected, because these samples were all obtained near the end of the onalespib infusion and just before the AT7519 infusion was started during cycle 1. The mean total body clearance (CL) of onalespib was 53.8 L/h/m2 on cycle 0 when given alone, 57.5 L/h/m2 on day 1 and 62.5 L/h/m2 on day 11 when given together with AT7519 in cycle 1; these differences were not statistically significant (P = 0.49 for C1D1 vs. C0; P = 0.22 for C1D11 vs. C0). The mean CL of onalespib for the five patients treated with the 40 mg/m2 dose in DL1 was comparable to the corresponding values of the mean CL for the 80 mg/m2 dose. Finally, there was no marked difference between the mean CL of onalespib 80 mg/m2 when given together with 21 or 27 mg/m2 doses of AT7519 in dose levels 2 and 3, respectively.
Mean values of the pharmacokinetic parameters for AT7519 21 mg/m2 were similar on days 1 and 11 of cycle 1 for patients evaluated at DL2. The mean Cmax was 459 ng/mL on day 1 and 509 ng/mL on day 11. The mean CL was 9.1 and 8.6 L/h/m2 on days 1 and 11, respectively. Mean values of the pharmacokinetic parameters for the 21 mg/m2 dose of AT7519 in four of the patients that concurrently received onalespib 40 mg/m2 in dose level 1 were similar to the values shown for the dose given together with onalespib 80 mg/m2.
Pharmacodynamic analyses
HSP70 expression in serial plasma and PBMC samples analyzed by ELISA and quantified western blot are shown in Fig. 1. Increased HSP70 protein expression was demonstrated in both plasma and PBMCs across all dose levels after onalespib treatment, consistent with downstream signaling after target engagement in response to HSP90 inhibition. Decreases in HSP70 expression with the addition of AT7519 to onalespib were variable across all samples. Statistical analyses were limited by the small number of patient samples (5/28) who met the criteria for comparative analysis: treatment completion through C1D11, collection of all three samples at C0D1, C0D4, and C1D11, and adequate collection sampling for isolation of both plasma and PBMCs for each timepoint. Pearson correlation of change in HSP70 levels in plasma and PBMC at time points C0D1 (P value = 0.89), C0D4 (P value = 0.12), and C1D11 (P value = 0.08) did not achieve statistical significance.
Antitumor activity
Twenty-three patients (82%) had measurable disease and were evaluable for the best response to therapy by RECIST v1.1. Sixteen patients (69.5%) had disease progression during the first two cycles, including 7 of 12 patients with stable disease (SD) by tumor metrics but with clinical progression in non-measurable sites. Two patients achieved a partial response (PR). One patient with an adenocarcinoma of the palate who achieved a confirmed PR remained on study for 15 cycles. One patient with a Sertoli–Leydig tumor showed marked tumor regression (Fig. 2). This response was not confirmed as a partial response as the patient withdrew from the study after six cycles without confirmatory scans, opting to pursue definitive resection of the remaining tumor. Two additional patients, a colon cancer patient and an endometrial cancer patient both remained on study with SD for a total of ten cycles each. The colon cancer patient progressed after ten cycles whereas the endometrial cancer patient withdrew consent for further treatment due to recurring intolerable Grade 2 fatigue.
Clinical response in a patient with Sertoli–Leydig tumor harboring AKT3 amplification and STK11 R304W mutation. Computed axial tomography (CT) coronal images are shown, yellow circles highlight the index mesenteric mass; baseline (left panel), following two cycles (middle panel), following six cycles (right panel)
Discussion
In this study, we show onalespib and AT7519 can be given safely in combination with no clinically relevant pharmacokinetic interactions of the agents, and report preliminary antitumor activity with PD confirmation of target engagement and proof-of-principle of modulation of HSP70 expression with the addition of AT7519. Clinical development of first-generation HSP90 inhibitors has been challenging due to prominent ocular and liver toxicities. In vivo modeling suggests prolonged retinal HSP90 inhibition causes photoreceptor cell death and ocular toxicity, and is postulated to vary by individual agent retina-to-plasma exposure rather than a class-wide effect [28, 29]. No clinically significant ocular toxicities were reported in this study, including those patients receiving therapy for > 6 months, supporting the premise that ocular toxicity may be dependent on the drug-specific retinal elimination rate and less of a concern for second-generation agents such as onalespib [29]. Five patients (18%) in this study experienced low-grade rises in liver transaminases, though no Grade 3 or 4 events occurred. Although the majority of toxicities were Grade 1/2, the recurring nature of these toxicities resulted in the omission of the second treatment of the week or treatment delays for patients who remained on study for longer than six cycles. In our experience, twice-weekly administration of this combination proved to be a challenging treatment schedule.
This study additionally highlights the complexities in developing targeted combinatorial dosing strategies and early phase clinical trial design. The administration schedule of days 1, 4, 8, 11 every 21 days did not result in clinically relevant alterations in the plasma pharmacokinetics of either drug. Mean values of the CL and apparent biological half-life of onalespib were within the ranges previously reported for the initial phase I clinical trial of this agent given as monotherapy [19]. Additionally, AT7519 exhibits linear pharmacokinetics at doses ranging from 14.4 to 32.4 mg/m2 when given as a single agent to adult patients with solid tumors [22]. In our experience, administration of AT7519 as a 1 h infusion immediately after completing the 1 h infusion of onalespib has no effect on the plasma pharmacokinetics of the latter, at the doses evaluated in this study.
The DLT events reported in this study, mucositis in one patient and cardiac troponin increase in a second patient, are postulated to reflect a pharmacodynamic interaction as abrogation of HSP70 upregulation by the addition of AT7519 may result in enhanced onalespib-specific on-target effect and toxicity. Although the study was not designed to assess for dose-dependent pharmacodynamic interaction, the occurrence of both these events at the same dose level guided the decision to terminate escalation. This highlights the limitations of a 3 + 3 design and would argue in favor of a modified adaptive trial design or the time-to-event Bayesian optimal interval design in the identification of the optimal dose for combination studies [30]. Alternate schedule of administration and intermediate doses were not pursued due to the study sponsor’s internal decision to discontinue further development of both agents. The combinatorial MTD was accordingly declared at onalespib 80 mg/m2 + AT7519 21 mg/m2.
Even at doses below the RP2D for each individual agent administered on a twice-weekly schedule, 120 mg/m2 for onalespib and 27 mg/m2 for AT7519 [19, 22], antitumor responses were seen. While we did not preselect for specific mutations due to the expansive nature of the HSP90 interactome which includes more than 200 client proteins, notable clinical responses of interest occurred in two patients with tumors harboring alterations in AKT on retrospective analysis of Next-Generation sequencing of archival tumor. One patient with endometrial cancer achieved durable clinical benefit with SD lasting > 10 months on study had an AKT E17K activating mutation. A second patient with a Sertoli–Leydig tumor was found to have both AKT amplification and STK11 R304W alteration on molecular profiling of her tumor. In vitro data suggest both STK11 and AKT form intracellular complexes with HSP90 and Cdc37 [31, 32]. In these studies, treatment with HSP90 inhibitors resulted in proteasome-mediated degradation of the respective protein. Changes in HSP70 expression could not be evaluated in these two patients as the C1D11 sample could not be collected on the patient with endometrial cancer and the baseline samples for the patient with Sertoli–Leydig tumor were too degraded for comparative analysis. Neither patient had progressed on study at the time of study discontinuation. The patient with endometrial cancer withdrew after 10 cycles due to recurring Grade 2 fatigue and the patient with Sertoli–Leydig tumor withdrew after achieving an unconfirmed PR, to pursue definitive resection of her remaining tumor.
The patient with palate adenocarcinoma who achieved a confirmed PR on study was found to have androgen receptor (AR) P392S alteration, a missense mutation which has been shown to predict for partial androgen insensitivity [33]. While this particular variant has not previously been identified in salivary cancers, other AR variants which have previously been implicated in resistance to androgen deprivation therapy (ADT) in prostate cancer have also recently been identified in salivary cancers [34]. HSP90 has been shown to play a critical role in stabilization of the AR and overexpression of HSP90 has been detected in prostate cancer cells [35]. It is conceivable to postulate that depletion of AR through HSP90 inhibition may explain this patient’s response and presents an opportunity to address ADT resistance in the treatment of salivary cancers [36].
For this patient, plasma analysis demonstrated an increase in soluble HSP70 expression after treatment with onalespib alone followed by a decrease in HSP70 expression after treatment with the combination of onalespib and AT7519 (Fig. 3). This is consistent with a proof-of-principle of transcriptional signaling downstream from target engagement following HSP90 inhibition with onalespib only, followed by downstream target effect of inhibition of CDK9-mediated transcription of HSP70 with combination therapy. Changes in HSP70 expression in PBMCs followed the same trend but were not statistically significant. Due to the small sample size of the study, definitive correlation of changes in HSP70 expression with response could not be verified in other patients. We additionally cannot rule out that variability of the timing of sample collection across multiple institutes may have contributed to the wide range in HSP70 expression across samples.
Clinical response a patient with adenocarcinoma of the palate with corresponding HSP70 measurements in plasma and PBMC. a Computed tomography scan demonstrating partial response of thoracic metastases (arrow) at baseline (Top) and at 13 cycles (Bottom). Right-sided panels show corresponding: b changes in soluble HSP70 protein expression in plasma as measured by ELISA at C0D1, C0D4 and C1D11; and c ImageQuant quantitated changes in HSP70 protein expression as measured by western blot in PBMCs at C0D1, C0D4 and C1D11
In this study, we have demonstrated the biologic activity of onalespib and AT7519 on HSP70 expression. The variability of HSP70 expression in plasma and PBMCs seen in this study highlights not only the challenges of biomarker development for this class of agents but also adds to the ongoing debate of whether PBMCs are an optimal surrogate for HSP90 inhibition, as putative driver client proteins may be differentially expressed in PBMCs versus tumor [10]. The intriguing preliminary responses seen in this study warrant further evaluation in tumors harboring mutations in HSP90 client proteins of interest, particularly AKT and AR, with incorporation of pharmacodynamic markers of HSP90 inhibition in tumor samples. The decision to halt additional agent development and study closure precluded the collection of tumor biopsies in a planned expansion cohort. Future biomarker development will require further validation of on-target impact on relevant client proteins within tumor tissue given the expansive nature of the HSP90 interactome, variability of client protein degradation within tumor, and differential PK/PD profiles of HSP90 inhibitors in tumor versus normal tissue, to determine the robustness of this parameter as a surrogate biomarker [37, 38]. Further validation of intratumoral target engagement and development of biomarker profiling of HSP90 inhibition will be needed to support the successful development of these classes of agents.
Data availability
The data on internal records will be provided upon request.
Code availability
Not applicable.
References
Pratt WB (1998) The hsp90-based chaperone system: involvement in signal transduction from a variety of hormone and growth factor receptors. Proc Soc Exp Biol Med 217(4):420–434. https://doi.org/10.3181/00379727-217-44252
Neckers L (2007) Heat shock protein 90: the cancer chaperone. J Biosci 32(3):517–530. https://doi.org/10.1007/s12038-007-0051-y
Lang BJ, Guerrero-Gimenez ME, Prince TL, Ackerman A, Bonorino C, Calderwood SK (2019) Heat shock proteins are essential components in transformation and tumor progression: cancer cell intrinsic pathways and beyond. Int J Mol Sci. https://doi.org/10.3390/ijms20184507
Bruns AF, Yuldasheva N, Latham AM, Bao L, Pellet-Many C, Frankel P, Stephen SL, Howell GJ, Wheatcroft SB, Kearney MT, Zachary IC, Ponnambalam S (2012) A heat-shock protein axis regulates VEGFR2 proteolysis, blood vessel development and repair. PLoS ONE 7(11):e48539. https://doi.org/10.1371/journal.pone.0048539
Shiota M, Kusakabe H, Izumi Y, Hikita Y, Nakao T, Funae Y, Miura K, Iwao H (2010) Heat shock cognate protein 70 is essential for Akt signaling in endothelial function. Arterioscler Thromb Vasc Biol 30(3):491–497. https://doi.org/10.1161/ATVBAHA.109.193631
Park AM, Tsunoda I, Yoshie O (2018) Heat shock protein 27 promotes cell cycle progression by down-regulating E2F transcription factor 4 and retinoblastoma family protein p130. J Biol Chem 293(41):15815–15826. https://doi.org/10.1074/jbc.RA118.003310
Pavan S, Musiani D, Torchiaro E, Migliardi G, Gai M, Di Cunto F, Erriquez J, Olivero M, Di Renzo MF (2014) HSP27 is required for invasion and metastasis triggered by hepatocyte growth factor. Int J Cancer J Int du Cancer 134(6):1289–1299. https://doi.org/10.1002/ijc.28464
Beere HM, Wolf BB, Cain K, Mosser DD, Mahboubi A, Kuwana T, Tailor P, Morimoto RI, Cohen GM, Green DR (2000) Heat-shock protein 70 inhibits apoptosis by preventing recruitment of procaspase-9 to the Apaf-1 apoptosome. Nat Cell Biol 2(8):469–475. https://doi.org/10.1038/35019501
Ahn JY, Lee JS, Min HY, Lee HY (2015) Acquired resistance to 5-fluorouracil via HSP90/Src-mediated increase in thymidylate synthase expression in colon cancer. Oncotarget 6(32):32622–32633. https://doi.org/10.18632/oncotarget.5327
Trepel J, Mollapour M, Giaccone G, Neckers L (2010) Targeting the dynamic HSP90 complex in cancer. Nat Rev Cancer 10(8):537–549. https://doi.org/10.1038/nrc2887
Biamonte MA, Van de Water R, Arndt JW, Scannevin RH, Perret D, Lee WC (2010) Heat shock protein 90: inhibitors in clinical trials. J Med Chem 53(1):3–17. https://doi.org/10.1021/jm9004708
Garrido C, Brunet M, Didelot C, Zermati Y, Schmitt E, Kroemer G (2006) Heat shock proteins 27 and 70: anti-apoptotic proteins with tumorigenic properties. Cell Cycle 5(22):2592–2601. https://doi.org/10.4161/cc.5.22.3448
Saleh A, Srinivasula SM, Balkir L, Robbins PD, Alnemri ES (2000) Negative regulation of the Apaf-1 apoptosome by Hsp70. Nat Cell Biol 2(8):476–483. https://doi.org/10.1038/35019510
Ravagnan L, Gurbuxani S, Susin SA, Maisse C, Daugas E, Zamzami N, Mak T, Jaattela M, Penninger JM, Garrido C, Kroemer G (2001) Heat-shock protein 70 antagonizes apoptosis-inducing factor. Nat Cell Biol 3(9):839–843. https://doi.org/10.1038/ncb0901-839
Ni Z, Schwartz BE, Werner J, Suarez JR, Lis JT (2004) Coordination of transcription, RNA processing, and surveillance by P-TEFb kinase on heat shock genes. Mol Cell 13(1):55–65. https://doi.org/10.1016/s1097-2765(03)00526-4
Guo F, Rocha K, Bali P, Pranpat M, Fiskus W, Boyapalle S, Kumaraswamy S, Balasis M, Greedy B, Armitage ES, Lawrence N, Bhalla K (2005) Abrogation of heat shock protein 70 induction as a strategy to increase antileukemia activity of heat shock protein 90 inhibitor 17-allylamino-demethoxy geldanamycin. Cancer Res 65(22):10536–10544. https://doi.org/10.1158/0008-5472.CAN-05-1799
Powers MV, Clarke PA, Workman P (2008) Dual targeting of HSC70 and HSP72 inhibits HSP90 function and induces tumor-specific apoptosis. Cancer Cell 14(3):250–262. https://doi.org/10.1016/j.ccr.2008.08.002
Powers MV, Clarke PA, Workman P (2009) Death by chaperone: HSP90, HSP70 or both? Cell Cycle 8(4):518–526. https://doi.org/10.4161/cc.8.4.7583
Shapiro GI, Kwak E, Dezube BJ, Yule M, Ayrton J, Lyons J, Mahadevan D (2015) First-in-human phase I dose escalation study of a second-generation non-ansamycin HSP90 inhibitor, AT13387, in patients with advanced solid tumors. Clin Cancer Res 21(1):87–97. https://doi.org/10.1158/1078-0432.CCR-14-0979
Do K, Speranza G, Chang LC, Polley EC, Bishop R, Zhu W, Trepel JB, Lee S, Lee MJ, Kinders RJ, Phillips L, Collins J, Lyons J, Jeong W, Antony R, Chen AP, Neckers L, Doroshow JH, Kummar S (2015) Phase I study of the heat shock protein 90 (Hsp90) inhibitor onalespib (AT13387) administered on a daily for 2 consecutive days per week dosing schedule in patients with advanced solid tumors. Invest New Drugs 33(4):921–930. https://doi.org/10.1007/s10637-015-0255-1
Woodhead AJ, Angove H, Carr MG, Chessari G, Congreve M, Coyle JE, Cosme J, Graham B, Day PJ, Downham R, Fazal L, Feltell R, Figueroa E, Frederickson M, Lewis J, McMenamin R, Murray CW, O’Brien MA, Parra L, Patel S, Phillips T, Rees DC, Rich S, Smith DM, Trewartha G, Vinkovic M, Williams B, Woolford AJ (2010) Discovery of (2,4-dihydroxy-5-isopropylphenyl)-[5-(4-methylpiperazin-1-ylmethyl)-1,3-dihydrois oindol-2-yl]methanone (AT13387), a novel inhibitor of the molecular chaperone Hsp90 by fragment based drug design. J Med Chem 53(16):5956–5969. https://doi.org/10.1021/jm100060b
Chen EX, Hotte S, Hirte H, Siu LL, Lyons J, Squires M, Lovell S, Turner S, McIntosh L, Seymour L (2014) A Phase I study of cyclin-dependent kinase inhibitor, AT7519, in patients with advanced cancer: NCIC Clinical Trials Group IND 177. Br J Cancer 111(12):2262–2267. https://doi.org/10.1038/bjc.2014.565
Seftel MD, Kuruvilla J, Kouroukis T, Banerji V, Fraser G, Crump M, Kumar R, Chalchal HI, Salim M, Laister RC, Crocker S, Gibson SB, Toguchi M, Lyons JF, Xu H, Powers J, Sederias J, Seymour L, Hay AE (2017) The CDK inhibitor AT7519M in patients with relapsed or refractory chronic lymphocytic leukemia (CLL) and mantle cell lymphoma. A Phase II study of the Canadian Cancer Trials Group. Leuk Lymphoma 58(6):1358–1365. https://doi.org/10.1080/10428194.2016.1239259
Wyatt PG, Woodhead AJ, Berdini V, Boulstridge JA, Carr MG, Cross DM, Davis DJ, Devine LA, Early TR, Feltell RE, Lewis EJ, McMenamin RL, Navarro EF, O’Brien MA, O’Reilly M, Reule M, Saxty G, Seavers LC, Smith DM, Squires MS, Trewartha G, Walker MT, Woolford AJ (2008) Identification of N-(4-piperidinyl)-4-(2,6-dichlorobenzoylamino)-1H-pyrazole-3-carboxamide (AT7519), a novel cyclin dependent kinase inhibitor using fragment-based X-ray crystallography and structure based drug design. J Med Chem 51(16):4986–4999. https://doi.org/10.1021/jm800382h
Do KT, Hays JL, Liu SV, Chen AP, O’Sullivan G, Supko JG, Beebe K, Neckers L, Trepel JB, Lee M-J, Gannon C, Muzikansky A, Campos SM, Downey E, Wolanski A, Hedglin J, Arnaldez FI, Doroshow JH, Shapiro G (2019) Phase I trial of the combination of the heat shock protein-90 inhibitor onalespib (AT13387) and the cyclin-dependent kinase inhibitor AT7519M in patients with advanced solid tumors. J Clin Oncol 37(15_suppl):2619–2619. https://doi.org/10.1200/JCO.2019.37.15_suppl.2619
Rajan A, Kelly RJ, Trepel JB, Kim YS, Alarcon SV, Kummar S, Gutierrez M, Crandon S, Zein WM, Jain L, Mannargudi B, Figg WD, Houk BE, Shnaidman M, Brega N, Giaccone G (2011) A phase I study of PF-04929113 (SNX-5422), an orally bioavailable heat shock protein 90 inhibitor, in patients with refractory solid tumor malignancies and lymphomas. Clin Cancer Res 17(21):6831–6839. https://doi.org/10.1158/1078-0432.CCR-11-0821
Ramanathan RK, Egorin MJ, Erlichman C, Remick SC, Ramalingam SS, Naret C, Holleran JL, TenEyck CJ, Ivy SP, Belani CP (2010) Phase I pharmacokinetic and pharmacodynamic study of 17-dimethylaminoethylamino-17-demethoxygeldanamycin, an inhibitor of heat-shock protein 90, in patients with advanced solid tumors. J Clin Oncol 28(9):1520–1526. https://doi.org/10.1200/jco.2009.25.0415
Jhaveri K, Ochiana SO, Dunphy MP, Gerecitano JF, Corben AD, Peter RI, Janjigian YY, Gomes-DaGama EM, Koren J 3rd, Modi S, Chiosis G (2014) Heat shock protein 90 inhibitors in the treatment of cancer: current status and future directions. Expert Opin Investig Drugs 23(5):611–628. https://doi.org/10.1517/13543784.2014.902442
Zhou D, Liu Y, Ye J, Ying W, Ogawa LS, Inoue T, Tatsuta N, Wada Y, Koya K, Huang Q, Bates RC, Sonderfan AJ (2013) A rat retinal damage model predicts for potential clinical visual disturbances induced by Hsp90 inhibitors. Toxicol Appl Pharmacol 273(2):401–409. https://doi.org/10.1016/j.taap.2013.09.018
Yuan Y, Lin R, Li D, Nie L, Warren KE (2018) Time-to-event bayesian optimal interval design to accelerate Phase I trials. Clin Cancer Res 24(20):4921–4930. https://doi.org/10.1158/1078-0432.CCR-18-0246
Basso AD, Solit DB, Chiosis G, Giri B, Tsichlis P, Rosen N (2002) Akt forms an intracellular complex with heat shock protein 90 (Hsp90) and Cdc37 and is destabilized by inhibitors of Hsp90 function. J Biol Chem 277(42):39858–39866. https://doi.org/10.1074/jbc.M206322200
Boudeau J, Deak M, Lawlor MA, Morrice NA, Alessi DR (2003) Heat-shock protein 90 and Cdc37 interact with LKB1 and regulate its stability. Biochem J 370(Pt 3):849–857. https://doi.org/10.1042/BJ20021813
Kalfa N, Philibert P, Werner R, Audran F, Bashamboo A, Lehors H, Haddad M, Guys JM, Reynaud R, Alessandrini P, Wagner K, Kurzenne JY, Bastiani F, Breaud J, Valla JS, Lacombe GM, Orsini M, Daures JP, Hiort O, Paris F, McElreavey K, Sultan C (2013) Minor hypospadias: the “tip of the iceberg” of the partial androgen insensitivity syndrome. PLoS ONE 8(4):e61824. https://doi.org/10.1371/journal.pone.0061824
Kang H, Antonarakis ES, Luo J, Zheng Q, Rooper L, De Marzo AM, Westra WH, Lotan TL (2018) Detection of AR-V7 transcript with RNA in situ hybridization in human salivary duct cancer. Oral Oncol 84:134–136. https://doi.org/10.1016/j.oraloncology.2018.06.026
Tien AH, Sadar MD (2019) Keys to unlock androgen receptor translocation. J Biol Chem 294(22):8711–8712. https://doi.org/10.1074/jbc.H119.009180
Jansson KH, Tucker JB, Stahl LE, Simmons JK, Fuller C, Beshiri ML, Agarwal S, Fang L, Hynes PG, Alilin AN, Lake R, Abbey YC, Cawley J, Tice CM, Yin J, McKnight C, Klummp-Thomas C, Zhang X, Guha R, Hoover S, Simpson RM, Nguyen HM, Corey E, Thomas CJ, Proia DA, Kelly K (2018) High-throughput screens identify HSP90 inhibitors as potent therapeutics that target inter-related growth and survival pathways in advanced prostate cancer. Sci Rep 8(1):17239. https://doi.org/10.1038/s41598-018-35417-0
Eiseman JL, Lan J, Lagattuta TF, Hamburger DR, Joseph E, Covey JM, Egorin MJ (2005) Pharmacokinetics and pharmacodynamics of 17-demethoxy 17-[[(2-dimethylamino)ethyl]amino]geldanamycin (17DMAG, NSC 707545) in C.B-17 SCID mice bearing MDA-MB-231 human breast cancer xenografts. Cancer Chemother Pharmacol 55(1):21–32. https://doi.org/10.1007/s00280-004-0865-3
Kamal A, Thao L, Sensintaffar J, Zhang L, Boehm MF, Fritz LC, Burrows FJ (2003) A high-affinity conformation of Hsp90 confers tumour selectivity on Hsp90 inhibitors. Nature 425(6956):407–410. https://doi.org/10.1038/nature01913
Graham B, Curry J, Smyth T, Fazal L, Feltell R, Harada I, Coyle J, Williams B, Reule M, Angove H, Cross DM, Lyons J, Wallis NG, Thompson NT (2012) The heat shock protein 90 inhibitor, AT13387, displays a long duration of action in vitro and in vivo in non-small cell lung cancer. Cancer Sci 103(3):522–527. https://doi.org/10.1111/j.1349-7006.2011.02191.x
Squires MS, Feltell RE, Wallis NG, Lewis EJ, Smith DM, Cross DM, Lyons JF, Thompson NT (2009) Biological characterization of AT7519, a small-molecule inhibitor of cyclin-dependent kinases, in human tumor cell lines. Mol Cancer Ther 8(2):324–332. https://doi.org/10.1158/1535-7163.MCT-08-0890
Acknowledgements
We thank the participating patients and their families, the study investigators, the research nurses, and the study coordinators at all institutions for their invaluable contributions.
Funding
This trial was opened through the Experimental Therapeutics Clinical Trials Network (ETCTN) and supported in part with federal funds from the National Cancer Institute, National Institutes of Health UM1 grant # CA186709, and in part from Gateway for Cancer Research and the Center for Cancer Research, NCI.
Author information
Authors and Affiliations
Contributions
KTD, GOC, JLH, SVL, and APC: supervision/conduct the clinical trial and provision/analysis/interpretation of the data, review and/or revision of the manuscript. KTD, APC, JHD, GOC, TS, LN, JBT, KB, M-JL, JGS, and GIS: conception and design, development of methodology, acquisition of preclinical data, analysis/interpretation of data, writing, review, and/or revision of the manuscript. CG, JH, JL, PI, and AM: administrative, technical, or material support, analysis/interpretation of data, review and/or revision of the manuscript. SC acquisition of data and review/revision of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
KTD has served as a consultant/advisory board member Seattle Genetics and QED Therapeutics; consulting fees from Jackson Laboratories; and has received commercial research grants (to institution) from Eli Lilly. JLH reports serving as a consultant/advisory board member for Clovis, AstraZeneca, Merck, Tesaro, Ipsen, Deciphera and receiving commercial research grants (to Institution) Mersana, Deciphera, Sanofi, Amgen, Boston Biomedical, Karyopharm, Exelexis, Beigene, EMD-Serono. SVL reports serving as a paid consultant/advisory board member for AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Catalyst, Celgene, G1 Therapeutics, Genentech/Roche, Guardant Health, Janssen, Lilly, LOXO, MSD, Pfizer, PharmaMar, Regeneron, and Takeda and receiving commercial research grants (to Institution) from Alkermes, AstraZeneca, Bayer, Blueprint, Bristol-Myers Squibb, Corvus, Genentech/Roche, Eli Lilly, Lycera, Merck, Merus, Molecular Partners, Pfizer, Rain Therapeutics, RAPT, Spectrum and Turning Point Therapeutics. SC reports serving as a paid consultant/advisory board for GSK and Esai. GIS has received research funding from Eli Lilly, Merck KGaA/EMD-Serono, Merck, and Sierra Oncology. He has served on advisory boards for Pfizer, Eli Lilly, G1 Therapeutics, Roche, Merck KGaA/EMD-Serono, Sierra Oncology, Bicycle Therapeutics, Fusion Pharmaceuticals, Cybrexa Therapeutics, Astex, Almac, Ipsen, Bayer, Angiex, Daiichi Sankyo, Seattle Genetics, Boehringer Ingelheim, ImmunoMet, Asana, Artios, Atrin and Concarlo Holdings. In addition, he holds a patent entitled, “Dosage regimen for sapacitabine and seliciclib,” also issued to Cyclacel Pharmaceuticals, and a pending patent, entitled, “Compositions and Methods for Predicting Response and Resistance to CDK4/6 Inhibition,” together with Liam Cornell. TS and JL are employees of Astex. GOC, JGS, KB, LN, JBT, M-JL, CG, JH, AM, PI, JHD, and APC declare no conflicts of interest.
Ethics approval and consent to participate
This study was conducted in accordance with the International Conference on Harmonization, Good Clinical Practice guidelines, and the ethical principles outlined in the Declaration of Helsinki 2008. The study protocol, amendments, informed consent, and other information that required approval were reviewed and approved by the Central Institutional Review Board, National Cancer Institute, in accordance with the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use Good Clinical Practice and applicable country-specific requirements, including US 21 Code of Federal Regulations 312.3(b) for the constitution of independent ethics committees. All participants provided written informed consent prior to study entry.
Consent for publication
Participant consent for publication was obtained and on file at the respective participating site.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Do, K.T., O’Sullivan Coyne, G., Hays, J.L. et al. Phase 1 study of the HSP90 inhibitor onalespib in combination with AT7519, a pan-CDK inhibitor, in patients with advanced solid tumors. Cancer Chemother Pharmacol 86, 815–827 (2020). https://doi.org/10.1007/s00280-020-04176-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-020-04176-z