Abstract
Cytokines play important roles in tumorigenesis and progression of cancer cells, while their functions in drug resistance remain to be illustrated. We successfully generated doxorubicin (Dox)-resistant CRC HCT-116 and SW480 cells (namely HCT-116/Dox and SW480/Dox, respectively). Cytokine expression analysis revealed that IL-8, while not FGF-2, EGF, TGF-β, IL-6, or IL-10, was significantly increased in Dox-resistant CRC cells as compared with their corresponding parental cells. Targeted inhibition of IL-8 via siRNAs or its inhibitor reparixin can increase the Dox sensitivity of HCT-116/Dox and SW480/Dox cells. The si-IL-8 can decrease the mRNA and protein expression of multidrug resistance 1 (MDR1, encoded by ABCB1), while has no effect on the expression of multidrug resistance-associated protein 1 (ABCC1), in CRC Dox-resistant cells. IL-8 can increase the phosphorylation of p65 and then upregulate the binding between p65 and promoter of ABCB1. BAY 11-7082, the inhibitor of NF-κB, suppressed the recombination IL-8 (rIL-8) induced upregulation of ABCB1. It confirmed that NF-κB is involved in IL-8-induced upregulation of ABCB1. rIL-8 also increased the phosphorylation of IKK-β, which can further activate NF-κB, while specific inhibitor of IKK-β (ACHP) can reverse rIL-8-induced phosphorylation of p65 and upregulation of MDR1. These results suggested that IL-8 regulates the Dox resistance of CRC cells via modulation of MDR1 through IKK-β/p65 signals. The targeted inhibition of IL-8 might be an important potential approach to overcome the clinical Dox resistance in CRC patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer (CRC) is the third leading cause of cancer-related death worldwide, which caused about 0.6 million deaths per year [5]. Chemotherapy is one of the major approaches for CRC treatment. However, chemoresistance is the primary reason for the treatment failure of advanced CRC [11]. Doxorubicin (Dox) is a DNA-intercalating anthracycline antibiotic which has been widely used for the treatment of CRC [3]. However, the drug resistance of Dox predominantly inhibits its clinical use for advanced CRC treatment [6]. A better understanding about the mechanisms responsible for Dox resistance will be of great help for its clinical application.
Cytokines and their corresponding receptors have been reported to be involved in the chemoresistance CRC [11]. Recent studies showed that interleukin (IL)-8 levels can predict tumor recurrence of CRC [12] and promote the proliferation, migration, invasion and chemoresistance of CRC cells [16]. Dox treatment can increase the expression of IL-8 and tumor necrosis factor-alpha (TNF-α) in human cancer cells [15]. The anti-cancer drug-induced IL-8 can increase the expression of ATP-binding cassette (ABC) transporters and then lead to poor chemotherapeutic response [18], while inhibition of IL-6 and IL-8 can increase the chemosensitivity of human multidrug-resistant (MDR) breast cancer cells [24]. In addition, IL-8 has also been reported to be involved in the resistance of docetaxel [23], cisplatin [28], and antiangiogenic agent Sunitinib [7]. However, its roles in CRC chemoresistance, particularly for Dox resistance, have not been well illustrated.
ATP-binding cassette (ABC) transporter is the major cause for chemoresistance [33]. It has been reported that ABCB1/MDR1/P-gp and ABCC1/MRP1 are the mostly implicated transporters involved in drug resistance [2]. In the present study, the expression of major cytokine was measured in Dox-resistant CRC cells. The roles of IL-8 on CRC progression and Dox resistance have been investigated. Our data showed that IL-8 can trigger the Dox resistance of CRC cells via induction of MDR1 through activation of p65.
Materials and methods
Cell and cell culture
Human CRC HCT-116 and SW480 cells were purchased from American Type Culture Collection (ATCC) and cultured in the medium recommended by ATCC containing 10% FBS (Life Technologies, Grand Island, USA). Cells were maintained at 37 °C in a humidified atmosphere with 5% CO2. To generate the Dox-resistant cells, stepwise increasing concentrations of Dox (0.01–1 µM) were used to treat the HCT-116 and SW480 cells for 6 months. Finally, cells were exposed to 1 µM Dox to maintain their drug resistance and named as HCT-116/Dox and SW480/Dox, respectively. For experimentation of Dox-resistant cells, addition of Dox was stopped 3 days before the experiments.
Cell transfection
The siRNA negative control and siRNA for IL-8 were designed and chemically synthesized and purified by GenePharma (Shanghai, China). For cell transfection, the Dox-resistant cells were seeded in six-well plates with a confluence of 40–60%. After 12 h of attachment, cells were further transfected with siRNAs (working concentration 50 nmol/L) by use of Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol.
RNA extraction and qRT-PCR
Total RNAs were extracted by use of Trizol according to the manufacturer’s instructions. Then 2 µg of total RNA was used to generate cDNA by use of AMV-reverse transcriptase (Promega, Madison, WI). The relative gene expression levels were measured by use of a SYBR Green I Real-Time PCR kit (GenePharma) with the program of 95 °C for 10 min, followed by 35 cycles of 95 °C for 15 s, 60 °C for 20 s, and 72 °C for 45 s. The primers of targeted genes were as follows: FGF2, forward 5′-AGC GGC TGT ACT GCA AAA ACG G-3′ and reverse 5′-CCT TTG ATA GAC ACA ACT CCT CTC-3′; EGF, forward 5′-TGC GAT GCC AAG CAG TCT GTG A-3′ and reverse 5′-GCA TAG CCC AAT CTG AGA ACC AC-3′; TGF-β, forward 5′-TAC CTG AAC CCG TGT TGC TCT C-3′ and reverse 5′-GTT GCT GAG GTA TCG CCA GGA A-3′; IL-6, forward 5′-ATG GAT GCT ACC AAA CTG GAT-3′ and reverse 5′-TGA AGG ACT CTG GCT TTG TCT-3′; IL-8, forward 5′-CAC CTC AAG AAC ATC CAG AGC T-3′ and reverse 5′-CAA GCA GAA CTG AAC TAC CAT CG-3′; IL-10, forward 5′-GGT TGC CAA GCC TTA TCG GA-3′ and reverse 5′-ACC TGC TCC ACT GCC TTG CT-3′; and GAPDH, forward 5′-GCA CCG TCA AGG CTG AGA AC-3′ and reverse 5′-TGG TGA AGA CGC CAG TGG A-3′. The gene relative expression was calculated using the 2− ΔΔCt method. GAPDH was used to normalize the amount of cDNA.
Western blot analysis
Cells were lysed in lysis buffer containing 1 × Tris-buffered saline, 1% Nonidet P-40, 0.5% sodium deoxycholate, and 0.1% sodium dodecyl sulfate (SDS). The protein concentration was measured by use of the Bradford assay (Bio-Rad, ON, Canada). For each sample, 30 µg of cell lysate was separated by 10% SDS-polyacrylamide gel electrophoresis (PAGE), transferred to PVDF membranes (Millipore, Billerica, MA), and blocked by 5% nonfat milk (w/v) in phosphate-buffered saline (PBS) for 1 h. The primary antibodies against IL-8, MDR1, MRP1, p-p65, p65, p-IKK-α, IKK-α, p-IKK-β, and IKK-β (1:1000) were incubated at room temperature for 2 h and washed with PBS/0.1% Tween-20 three times. After incubating with horseradish peroxidase-conjugated secondary antibodies (Zhongshan Boil Tech Co, Beijing, China) for 1 h at room temperature, the membrane was visualized by an enhanced chemiluminescence system (Thermo Fisher Scientific, OL191210A, MA, USA). GAPDH was used as the loading control. Each experiment was repeated at least three times and the representative results are shown.
Cell proliferation assay
Cell proliferation assay was conducted by use of Cell Counting Kit-8 (Dojindo, Kumamoto, Japan) according to the manufacturer’s instructions. Briefly, cells (5000/well) were seeded in the 96-well plates at a volume of 100 µl. After incubating for the indicated times, 10 µl CCK-8 solution was added to each well and further incubated at 37 °C for 2 h. The absorbance at 450 nm for each well was measured by use of a Universal Microplate Reader (EL x800; Bio-Tek Instruments Inc., Winooski, VT, USA).
Cell retention of Dox
To detect the retention of Dox, cells were seeded in six-well plates and treated with 1 µM Dox for 8 h. After carefully washing with PBS six times, cells were trypsinized and collected by centrifugation. Then cells were resuspended in ice-cold PBS for measuring the fluorescence intensity by a flow cytometer as described previously [34].
Chromatin immunoprecipitation (ChIP)
The ChIP assay was conducted according to the previous study [31] to measure the binding between p65 and promoter of ABCB1 using the Chromatin Immunoprecipitation (ChIP) Assay Kit (Millipore Corporation). Briefly, cells were harvested, cross-linked by 1% formaldehyde, and sonicated for 30 min. After centrifugation at 15,000g for 15 min, the solubilized chromatin was precleared with protein G-agarose for 60 min and then incubated with p65 antibody or rabbit IgG overnight at 4 °C. Total combined DNA in samples and inputs was extracted and used as the template for PCR. The primers to amplify the promoter of ABCB1 were forward primer: 5′-CAA CTC GTC AAA GGA ATT AT-3′ and reverse primer: 5′-TTG TAC CTT TGA TCA ACA CC-3′.
Enzyme-linked immunosorbent assay (ELISA)
The concentration of IL-8 in culture medium was examined by ELISA using human IL-8 CytoSet™ (Biosource, Camarillo, USA) according to the manufacturer’s instructions. Human recombinant IL-8 (rIL-8) was used as the standard. The concentrations of IL-8 were measured using a microplate reader (Molecular Devices, Sunnyvale, CA, USA) at 450 nm.
Statistical analysis
SPSS version 13 (SPSS Inc., Chicago, IL, USA) was used to analyze the data. Student’s t test was used to compare the difference between two groups. p value less than 0.05 was considered as statistically significant (*p < 0.05, **p < 0.01).
Results
Characterization of Dox-resistant CRC cells
We measured the Dox sensitivity of HCT-116/Dox and SW480/Dox and their corresponding parental cell lines by use of CCK-8 kit. Our data showed that the sensitivity of both HCT-116/Dox and SW480/Dox was much less than that of their corresponding parental cell lines (Fig. 1a, b). The IC50 values for HCT-116 and HCT-116/Dox cells were 0.92 and 14.59 µM, respectively (Fig. 1a). The IC50 values for SW480 and SW480/Dox cells were 1.15 and 17.65 µM, respectively (Fig. 1b). These data suggested that the Dox-resistant CRC cells have been established successfully.
IL-8 expression is significantly increased in Dox-resistant CRC cells
Considering numerous studies revealed that cytokines were involved in chemoresistance of cancer cells, the expression of fibroblast growth factor-2 (FGF-2), epidermal growth factor (EGF), transforming growth factor beta (TGF-β), IL-6, IL-8, and IL-10 was measured in CRC/Dox cells and their corresponding parental cells by qRT-PCR. Our results showed that the mRNA expression of IL-8 and IL-10 was increased in HCT-116/Dox cells as compared to that in HCT-116 cells (Fig. 2a), while only IL-8 was significantly increased in SW480/Dox cells as compared to that in SW480 cells (Fig. 2b). The upregulation of IL-8, while not IL-10, was further confirmed in HCT-116/Dox and SW480/Dox cells by western blot analysis (Fig. 2c). ELISA supported that the levels of IL-8 in HCT-116/Dox and SW480/Dox cells were significantly greater than that in their corresponding parental cell lines (Fig. 2d). These data revealed that IL-8 was significantly greater in Dox-resistant CRC cells.
IL-8 expression is significantly increased in Dox-resistant CRC cells. The mRNA expression of FGF, EGF, TGF-β, IL-6, IL-8, and IL-10 in HCT-116/Dox (a) and SW480/Dox (b) and their corresponding parental cell lines were measured by qRT-PCR; c the expression of IL-8 and IL-10 in HCT-116/Dox and SW480/Dox and their corresponding parental cell lines were measured by western blot analysis; d the relative expression of IL-8 in media of HCT-116/Dox and SW480/Dox and their corresponding parental cell lines were measured by ELISA. *p < 0.05; **p < 0.01
IL-8 is involved in the Dox resistance of CRC cells
We then investigated whether IL-8 is involved in Dox sensitivity of CRC cells. First, we used siRNAs specific for IL-8 to knock down its expression in HCT-116/Dox and SW480/Dox cells. si-IL-8-2 was chosen for further studies due to its higher efficiency (Fig. 3a). Our data found that si-IL-8-2 can significantly increase the Dox sensitivity of both HCT-116/Dox (IC50 3.2 µM) and SW480/Dox (IC50 4.5 µM) cells (Fig. 3b). In addition, si-IL-8-2 can significantly increase Dox retention in both HCT-116/Dox and SW480/Dox cells (Fig. 3c). Reparixin, the specific inhibitor of IL-8 receptor CXCR2, was used to confirm the roles of IL-8 on Dox resistance of CRC cells. The results showed that reparixin treatment can also increase Dox sensitivity of HCT-116/Dox (IC50 2.6 µM) cells (Fig. 3d). Recombinant IL-8 (rIL-8) can decrease the Dox sensitivity of HCT-116 (IC50 3.6 µM) cells (Fig. 3e). These data suggested that IL-8 is involved in Dox resistance of CRC cells.
IL-8 is involved in the Dox resistance of CRC cells. a HCT-116/Dox and SW480/Dox cells were transfected with negative control (NC) or si-IL-8-1/-2 for 24 h, and the expression of IL-8 was measured by western blot analysis; b HCT-116/Dox and SW480/Dox cells were transfected with negative control (NC) or si-IL-8-1/-2 for 24 h and then further exposed to increasing concentrations of Dox for 48 h; c HCT-116/Dox and SW480/Dox cells were transfected with negative control (NC) or si-IL-8-1/-2 for 24 h and then further treated with 1 µM Dox for 8 h, the cell retention of Dox was detected by flow cytometry; d HCT-116/Dox cells were pretreated with or without reparixin (10 µM) for 90 min and then further treated with increasing concentrations of Dox for 48 h; e HCT-116 cells were pretreated with or without rIL-8 (50 ng/ml) for 90 min and then further exposed to increasing concentrations of Dox for 48 h
IL-8 regulates the expression of multidrug resistance 1 (MDR1) in CRC cells
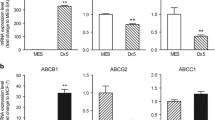
The mechanisms responsible for IL-8-regulated Dox resistance were further investigated. Our data showed that both multidrug resistance 1 (MDR1) and multidrug resistance-associated protein 1 (ABCC1) were significantly increased in HCT-116/Dox and SW480/Dox cells as compared with their corresponding parental cells (Fig. 4a). However, the si-IL8-2 can only decrease the expression of MDR1, while not MRP1, in both HCT-116/Dox and SW480/Dox cells (Fig. 4b). In addition, the si-IL8-2 also decreased the mRNA expression of ABCB1 (MDR1) in HCT-116/Dox and SW480/Dox cells (Fig. 4c). Consistently, rIL8 can increase the mRNA expression of ABCB1, while not ABCC1, in both HCT-116 and SW480 cells (Fig. 4d). All these data revealed that IL-8 can positively regulate the expression of MDR1 in CRC cells.
IL-8 regulates the expression of MDR1 in CRC cells. a The expression of MDR1 and MRP1 in HCT-116/Dox and SW480/Dox cells and their parental cells were measured by western blot analysis; b HCT-116/Dox and SW480/Dox cells were transfected with negative control (NC) or si-IL-8-1/-2 for 48 h and then expression of MDR1 and MRP1 was measured by western blot analysis; c HCT-116/Dox and SW480/Dox cells were transfected with negative control (NC) or si-IL-8-1/-2 for 24 h, and mRNA expression of ABCB1 was measured by qRT-PCR; d HCT-116 or SW480 cells were treated with or without rIL-8 (50 ng/ml) for 24 h, and mRNA expression of ABCB1 and ABCCC was measured by qRT-PCR. *p < 0.05
IL-8 regulates the transcription of ABCB1 via activation of NF-κB
We further investigated the mechanisms responsible for IL-8-induced upregulation of ABCB1. Results showed that rIL-8 can increase the mRNA expression of ABCB1 in HCT-116 cells since treatment for 0.5 h (Fig. 5a). Consistently, reparixin also rapidly suppressed the expression of ABCB1 in HCT-116/Dox cells (Fig. 5b). NF-κB, which can be activated by IL-8, regulates the transcription of ABCB1 by binding to its promoter [32]. Our data showed that rIL-8 can increase the phosphorylation of p65 in HCT-116 and SW480 cells (Fig. 5c). In addition, ChIP assay showed that rIL-8 can increase the binding between p65 and promoter of ABCB1 in HCT-116 cells (Fig. 5d). However, BAY 11-7082, the inhibitor of NF-κB, suppressed the rIL-8-induced upregulation of ABCB1 mRNA in HCT-116 cells (Fig. 5e). These results indicated that IL-8 regulates the transcription of ABCB1 via activation of NF-κB.
IL-8 regulates the transcription of ABCB1 via activation of NF-κB. a, b HCT-116 cells were treated with rIL-8 (50 ng/ml) or reparixin (10 µM) for the indicated times, the mRNA of ABCB1 was measured by qRT-PCR; c HCT-116 or SW480 cells were treated with rIL-8 (50 ng/ml) for the indicated times, the phosphorylation and total expression of p65 were measured by western blot analysis; d HCT-116 cells were treated with or without rIL-8 (50 ng/ml) for 12 h, the binding between p65 and ABCB1 promoter was measured by ChIP assay; e HCT-116 cells were pretreated with or without BAY 11-7082 (10 µM) for 90 min and then exposed to rIL-8 (50 ng/ml) for 24 h, and the mRNA of ABCB1 was measured by qRT-PCR. **p < 0.01
IL-8 regulates NF-κB/ABCB1 pathways via phosphorylation of IKKβ
IKK-α and IKK-β, which can phosphorylate IκBα and p65, regulate the activities of NF-κB in cancer cells [30]. Our results showed that rIL-8 treatment can significantly increase the phosphorylation of IKK-β, while not IKK-α, in HCT-116 and SW480 cells (Fig. 6a). Furthermore, the specific inhibitor of IKK-β (ACHP) can reverse rIL-8 induced phosphorylation of p65 in HCT-116 cells (Fig. 6b). ACHP also abolished rIL-8-induced upregulation of ABCB1 in HCT-116 cells (Fig. 6c). These results suggested that IL-8 regulates NF-κB/ABCB1 pathways via phosphorylation of IKKβ.
IL-8 regulates NF-κB/ABCB1 pathways via phosphorylation of IKKβ. a HCT-116 or SW480 cells were treated with rIL-8 (50 ng/ml) for 30 min, the phosphorylation and total expression of IKK-α/β were measured by western blot analysis; b HCT-116 cells were pretreated with ACHP (10 µM) for 90 min and then further treated with or without rIL-8 (50 ng/ml) for 30 min, the phosphorylation and total expression of p65 were measured by western blot analysis; c HCT-116 cells were pretreated with ACHP (10 µM) for 90 min and then further treated with or without rIL-8 (50 ng/ml) for 24 h, the mRNA of ABCB1 was measured by qRT-PCR; d the schematic of IL-8 IL-8 regulated NF-κB/ABCB1 pathways via phosphorylation of IKKβ. **p < 0.01
Discussion
Although Dox does not represent the first-line treatment of colon cancers, the usage of Dox in combination with other anti-cancer agents has been proved to have a good therapeutic effect on advanced colorectal cancer patients [19]. Increasing evidences suggest that cytokines including IL-8 were involved in drug resistance of cancer cells. Our present study reveals that IL-8 was significantly increased in Dox-resistant CRC cells. Its specific siRNA or inhibitor can reverse the Dox resistance of CRC cells, while rIL-8 can decrease the Dox sensitivity of CRC cells. Further, IL-8 regulates the mRNA and protein expression of MDR1 via activation of IKKβ/NF-κB signals. All the present data suggest that IL-8 can mediate the Dox resistance of CRC cells via IKKβ/NF-κB-induced upregulation of MDR1 (Fig. 6d). Its targeted inhibition might be an important potential approach to overcome the clinical Dox resistance in CRC patients.
Our data show that IL-8 mediates the Dox resistance of CRC cells. Increasing concentrations of IL-8 have been observed in various types of cancers and are positively correlated with the progression of cancers [1, 17]. Laboratory data reveal that IL-8 can trigger the migration, proliferation and chemoresistance of cancer cells via autocrine or paracrine mechanisms [16, 28]. Our present study reveals that Dox-resistant CRC cells have significantly higher levels of IL-8 than their parental cells. Targeted inhibition of IL-8 can attenuate the Dox resistance of both HCT-116/Dox and SW480/Dox cells. This is consistent with previous studies that targeting IL-6 and IL-8 can increase the chemosensitization in multidrug-resistant human breast cancer cells [24]. It has been reported that the Dox treatment can induce the expression of IL-8 in human lung carcinoma cells [15]. Furthermore, the Dox-resistant cells can recruit monocytic myeloid-derived suppressor cells (MDSCs) and neutrophils to the tumor microenvironment by expressing IL-8 [29]. Then neutrophils secrete various molecules that support and promote tumor angiogenesis, progression and metastasis [4, 14]. Together with the published literatures, our present study confirms that IL-8 can trigger the Dox resistance of CRC cells and, therefore, suggests that inhibition of IL-8 might be helpful for chemotherapy.
Our present study revealed that IL-8 regulates the Dox resistance of CRC cells via IKKβ/NF-κB-induced upregulation of MDR1. In CRC cells, rIL-8 treatment increases the expression of MDR1, while not MRP1. Similarly, IL-8 significantly upregulates the mRNA and protein levels of MDR1, while not GSTpi, MRP, LRP and TopoI, in ovarian A2780 cells [28]. Our study reveals for the first time that IL-8 can increase the transcription of MDR1 by upregulating the promoter activities by the activation of NF-κB. NF-κB and other transcription factors such as AP-1 and SP-1 can induce the expression of ABCB1 [10]. Previous studies identified that there are several NF-κB-binding sites in human ABCB1 promoter [22, 25]. Our data revealed that IL-8 treatment can increase the phosphorylation of IKK-β, which can phosphorylate IκBα and then activate NF-κB in cancer cells [8]. The roles of IKK-β are further confirmed by the results that the specific inhibitor of IKK-β (ACHP) can reverse rIL-8-induced upregulation of ABCB1. The mechanisms of IL-8-induced phosphorylation of IKK-β in CRC cells need further investigations.
It should be noted that the status of tumor suppressor adenomatous polyposis coli (APC) in HCT-116 and SW480 cells is different [21]. Specifically, APC1338 is a truncated product expressed by SW480, while HCT116 cells contain wild-type alleles of APC [21]. APC can regulate deoxycholate-induced IL-8 in CRC cells by modulating the activity of Wnt/β-catenin signaling [20]. Ectopic expression of IL-8 can enhance intestinal tumor development caused by a mutation in the APC gene [9]. In addition, APC loss in breast cancer leads to Dox resistance via STAT3 activation [27]. In the MMTV-PyMT mouse transgenic model, loss of APC results in resistance to both cisplatin and Dox in a Wnt/β-catenin-independent manner [26]. Considering that APC1338 in SW480 cells will highly activate the Wnt/β-catenin signaling [13], the roles of APC in IL-8-regulated Dox resistance of CRC cells need further investigation.
In conclusion, our present study characterizes that IL-8 is upregulated in Dox-resistant CRC cells. It can increase the transcription and expression of MDR1 via activation of NF-κB. IKK-β is involved in IL-8-induced MDR1 expression and phosphorylation of NF-κB. It suggests that IL-8 might be a potential target to overcome Dox resistance in CRC patients.
References
Benoy IH, Salgado R, Van Dam P, Geboers K, Van Marck E, Scharpe S, Vermeulen PB, Dirix LY (2004) Increased serum interleukin-8 in patients with early and metastatic breast cancer correlates with early dissemination and survival. Clin Cancer Res 10:7157–7162
Cascorbi I (2006) Role of pharmacogenetics of ATP-binding cassette transporters in the pharmacokinetics of drugs. Pharmacol Ther 112:457–473
Colombo V, Lupi M, Falcetta F, Forestieri D, D’Incalci M, Ubezio P (2011) Chemotherapeutic activity of silymarin combined with doxorubicin or paclitaxel in sensitive and multidrug-resistant colon cancer cells. Cancer Chemother Pharmacol 67:369–379
De Larco JE, Wuertz BR, Furcht LT (2004) The potential role of neutrophils in promoting the metastatic phenotype of tumors releasing interleukin-8. Clin Cancer Res 10:4895–4900
Fidler MM, Soerjomataram I, Bray F (2016) A global view on cancer incidence and national levels of the human development index. Int J Cancer 139:2436–2446
Gottesman MM, Fojo T, Bates SE (2002) Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer 2:48–58
Huang D, Ding Y, Zhou M, Rini BI, Petillo D, Qian CN, Kahnoski R, Futreal PA, Furge KA, Teh BT (2010) Interleukin-8 mediates resistance to antiangiogenic agent sunitinib in renal cell carcinoma. Cancer Res 70:1063–1071
Karin M, Greten FR (2005) NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol 5:749–759
Lee YS, Choi D, Kim NY, Yang S, Jung E, Hong M, Yang D, Lenz HJ, Hong YK (2014) CXCR2 inhibition enhances sulindac-mediated suppression of colon cancer development. Int J Cancer 135:232–237
Liptrott NJ, Owen A (2011) The role of cytokines in the regulation of drug disposition: extended functional pleiotropism? Expert Opin Drug Metab Toxicol 7:341–352
Longley DB, Johnston PG (2005) Molecular mechanisms of drug resistance. J Pathol 205:275–292
Lurje G, Zhang W, Schultheis AM, Yang D, Groshen S, Hendifar AE, Husain H, Gordon MA, Nagashima F, Chang HM, Lenz HJ (2008) Polymorphisms in VEGF and IL-8 predict tumor recurrence in stage III colon cancer. Ann Oncol 19:1734–1741
Min J, Liu L, Li X, Jiang J, Wang J, Zhang B, Cao D, Yu D, Tao D, Hu J et al (2015) Absence of DAB2IP promotes cancer stem cell like signatures and indicates poor survival outcome in colorectal cancer. Sci Rep 5:16578
Nagarsheth N, Wicha MS, Zou WP (2017) Chemokines in the cancer microenvironment and their relevance in cancer immunotherapy. Nat Rev Immunol 17:559–572
Niiya M, Niiya K, Kiguchi T, Shibakura M, Asaumi N, Shinagawa K, Ishimaru F, Kiura K, Ikeda K, Ueoka H, Tanimoto M (2003) Induction of TNF-alpha, uPA, IL-8 and MCP-1 by doxorubicin in human lung carcinoma cells. Cancer Chemother Pharmacol 52:391–398
Ning Y, Manegold PC, Hong YK, Zhang W, Pohl A, Lurje G, Winder T, Yang D, LaBonte MJ, Wilson PM et al (2011) Interleukin-8 is associated with proliferation, migration, angiogenesis and chemosensitivity in vitro and in vivo in colon cancer cell line models. Int J Cancer 128:2038–2049
Orditura M, De Vita F, Catalano G, Infusino S, Lieto E, Martinelli E, Morgillo F, Castellano P, Pignatelli C, Galizia G (2002) Elevated serum levels of interleukin-8 in advanced non-small cell lung cancer patients: relationship with prognosis. J Interferon Cytokine Res 22:1129–1135
Park SY, Han J, Kim JB, Yang MG, Kim YJ, Lim HJ, An SY, Kim JH (2014) Interleukin-8 is related to poor chemotherapeutic response and tumourigenicity in hepatocellular carcinoma. Eur J Cancer 50:341–350
Qu J, Zhao L, Zhang PZ, Wang J, Xu N, Mi WJ, Jiang XW, Zhang CM, Qu J (2015) MicroRNA-195 chemosensitizes colon cancer cells to the chemotherapeutic drug doxorubicin by targeting the first binding site of BCL2L2 mRNA. J Cell Physiol 230:535–545
Rial NS, Lazennec G, Prasad AR, Krouse RS, Lance P, Gerner EW (2009) Regulation of deoxycholate induction of CXCL8 by the adenomatous polyposis coli gene in colorectal cancer. Int J Cancer 124:2270–2280
Schneikert J, Grohmann A, Behrens J (2007) Truncated APC regulates the transcriptional activity of beta-catenin in a cell cycle dependent manner. Hum Mol Genet 16:199–209
Scotto KW (2003) Transcriptional regulation of ABC drug transporters. Oncogene 22:7496–7511
Shao N, Chen LH, Ye RY, Lin Y, Wang SM (2013) The depletion of interleukin-8 causes cell cycle arrest and increases the efficacy of docetaxel in breast cancer cells. Biochem Biophys Res Commun 431:535–541
Shi Z, Yang WM, Chen LP, Yang DH, Zhou Q, Zhu J, Chen JJ, Huang RC, Chen ZS, Huang RP (2012) Enhanced chemosensitization in multidrug-resistant human breast cancer cells by inhibition of IL-6 and IL-8 production. Breast Cancer Res Treat 135:737–747
Sui H, Fan ZZ, Li Q (2012) Signal transduction pathways and transcriptional mechanisms of ABCB1/Pgp-mediated multiple drug resistance in human cancer cells. J Int Med Res 40:426–435
VanKlompenberg MK, Bedalov CO, Soto KF, Prosperi JR (2015) APC selectively mediates response to chemotherapeutic agents in breast cancer. BMC Cancer 15:457
VanKlompenberg MK, Leyden E, Arnason AH, Zhang JT, Stefanski CD, Prosperi JR (2017) APC loss in breast cancer leads to doxorubicin resistance via STAT3 activation. Oncotarget 8:102868–102879
Wang Y, Qu Y, Niu XL, Sun WJ, Zhang XL, Li LZ (2011) Autocrine production of interleukin-8 confers cisplatin and paclitaxel resistance in ovarian cancer cells. Cytokine 56:365–375
Waugh DJ, Wilson C (2008) The interleukin-8 pathway in cancer. Clin Cancer Res 14:6735–6741
Wu JT, Kral JG (2005) The NF-kappaB/IkappaB signaling system: a molecular target in breast cancer therapy. J Surg Res 123:158–169
Wu YM, Chen ZJ, Liu H, Wei WD, Lu LL, Yang XL, Liang WT, Liu T, Liu HL, Du J, Wang HS (2015) Inhibition of ERRalpha suppresses epithelial mesenchymal transition of triple negative breast cancer cells by directly targeting fibronectin. Oncotarget 6:25588–25601
Xi G, Hayes E, Lewis R, Ichi S, Mania-Farnell B, Shim K, Takao T, Allender E, Mayanil CS, Tomita T (2016) CD133 and DNA-PK regulate MDR1 via the PI3K- or Akt-NF-kappaB pathway in multidrug-resistant glioblastoma cells in vitro. Oncogene 35:241–250
Yu M, Ocana A, Tannock IF (2013) Reversal of ATP-binding cassette drug transporter activity to modulate chemoresistance: why has it failed to provide clinical benefit? Cancer Metastasis Rev 32:211–227
Zhang ZP, Wang Y, Xu SH, Yu YN, Hussain A, Shen YY, Guo SR (2017) Photothermal gold nanocages filled with temperature sensitive tetradecanol and encapsulated with glutathione responsive polycurcumin for controlled DOX delivery to maximize anti-MDR tumor effects. J Mater Chem B 5:5464–5472
Funding
This work was supported by the Natural Science Foundation of Zhejiang Province (No. LQ17H160016) and the Zhejiang Provincial Medical and Healthy Science and Technology Projects (No. 2015KYA029).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Du, J., He, Y., Li, P. et al. IL-8 regulates the doxorubicin resistance of colorectal cancer cells via modulation of multidrug resistance 1 (MDR1). Cancer Chemother Pharmacol 81, 1111–1119 (2018). https://doi.org/10.1007/s00280-018-3584-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-018-3584-x