Abstract
Purpose
To explore the levels of thioguanine incorporated into DNA (DNA-TG), and erythrocyte levels of 6-thioguanine nucleotides (Ery-TGN) and methylated metabolites (Ery-MeMP) during 6-mercaptopurine (6MP)/Methotrexate (MTX) therapy of childhood acute lymphoblastic leukemia (ALL) and the relation to inosine triphosphatase (ITPA) and thiopurine methyltransferase (TPMT) gene variants.
Methods
Blood samples were drawn during 6MP/MTX maintenance therapy from 132 children treated for ALL at Rigshospitalet, Copenhagen. The samples were analysed for thiopurine metabolites and compared to TPMT (rs1800460 and rs1142345) and ITPA (rs1127354) genotypes.
Results
Median DNA-TG (mDNA-TG) levels were higher in TPMT and ITPA low-activity patients as compared to wildtype patients (TPMTLA 549 vs. 364 fmol/µg DNA, p = 0.007, ITPALA 465 vs. 387 fmol/µg DNA, p = 0.04). mDNA-TG levels were positively correlated to median Ery-TGN (mEry-TGN)(rs = 0.37, p = 0.001), but plateaued at higher mEry-TGN levels. DNA-TG indices (mDNA-TG/mEry-TGN) were 42% higher in TPMTWT patients as compared to TPMTLA patients but no difference in DNA-TG indices was observed between ITPAWT and ITPALA patients (median 1.7 vs. 1.6 fmol/µg DNA/ nmol/mmol Hb, p = 0.81). DNA-TG indices increased with median Ery-MeMP (mEry-MeMP) levels (rs = 0.25, p = 0.001).
Conclusions
TPMT and ITPA genotypes significantly influence the metabolism of 6MP. DNA-TG may prove to be a more relevant pharmacokinetic parameter for monitoring 6MP treatment intensity than cytosolic metabolites. Prospective trials are needed to evaluate the usefulness of DNA-TGN for individual dose adjustments in childhood ALL maintenance therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Genetic variation is of particular importance in drugs with a narrow therapeutic index, such as thiopurines, which are the most essential drugs in the maintenance treatment of childhood acute lymphoblastic leukemia (ALL). To date, there is only one widely accepted clinical guideline for dose modification based on gene status which is thiopurine dosing based on thiopurine methyltransferase (TPMT) (rs1800460 and rs1142345) genotype. Other gene variants such as nudix hydrolase 15 (NUDT15) (rs1168553232 and rs186364861) [1,2,3,4,5,6,7] and inosine triphosphatase (ITPA) (rs1127354) have been associated with 6-MP treatment-related toxicity [8,9,10,11,12].
6MP is a pro-drug and its cytotoxicity depends on conversion to 6-thioguanine nucleotides (TGN) which can be incorporated into DNA (DNA-TG). DNA-TG may mismatch during DNA replication, which activates the mismatch repair system. Continuous DNA-TG mismatching will eventually lead to apoptosis or mutations in the DNA copy strand [13]. TPMT methylates 6MP and thereby reduces the amount of drug available for TGN formation [14, 15]. However, the methylated metabolite methyl-thioinosine monophosphate (Me-TIMP) is a potent inhibitor of the purine de novo synthesis, resulting in a lower level of endogenous purines for incorporation into DNA, and a relatively higher level of thiogunanine nucleotides (TGN), which in the end enhances the incorporation of TGN into DNA [16, 17].
ITPA catalyzes the hydrolysis of thioinosine triphosphate (TITP) to thioinosine monophosphate (TIMP). Through this loop, TITP is reconverted to TIMP, which can then increase TGN levels. NUDT15 is hypothesized to dephosphorylate the thiopurine active metabolite TGTP to TGMP, thus preventing the incorporation into DNA and reducing the desired cytotoxic effect of 6MP (Fig. 1).
Simplified scheme of the metabolism of 6MP and the enzymes involved. 6MP 6-mercaptopurine, HPGRT hypoxanthine–guanine phosphoribosyltransferase, TIMP Thioinosine monophosphate, IMPDH inosine monophosphate dehydrogenase, GMPS guanosine monophosphate synthase, TGMP thioguanosine monophosphate, TGDP thioguanosine diphosphate, TGTP thioguanosine triphosphate, ITPA inosine triphosphate pyrophosphatase, TPMT thiopurine methyltransferase, MeMP methylmercaptopurine, XO xanthine oxidase, TIDP thioinosine diphosphate, TITP thioinosine triphosphate, NUDT15 Nudix hydrolase 15. Deoxy forms ignored. Illustration made in Illustator
SNPs in the TPMT gene result in approximately 90% of Caucasians being homozygous for high TPMT activity (TPMTWT), 10% carry one low-activity allele (TPMTLA), and 1 in 300 carries two low-activity alleles and are TPMT-deficient (TPMTDE) [7]. The frequency of ITPALA alleles is 5–7% in Caucasians and confers a reduction in enzyme activity by approximately 75 and 100% among heterozygous and homozygous individuals, respectively [18, 19]. ITPA and NUDT15 genetic variations are both most common in the Asian population and have both proved to significantly affect thiopurine toxicity [3, 7, 11, 20,21,22;7;11;20,21,22].
In a single-institution study, we explored the effect of TPMT and ITPA genotypes on the levels of the end point metabolite DNA-TG and its relation to levels of Ery-TGN and Ery-MeMP in 132 children with ALL undergoing 6MP/MTX maintenance treatment.
Patients and methods
Childhood ALL patients diagnosed at the University Hospital, Rigshospitalet, before December 31st, 2011 and treated according to the ALL2000 or ALL2008 protocols were eligible for this study. Infants and patients allocated to the high-risk treatment (HR) were excluded, as their treatment deviate substantially from standard risk (SR) and intermediate risk (IR) patients [23, 24]. Baseline parameters of the 132 patients who met the inclusion criteria and for which blood samples were available are shown in Table 1. Children diagnosed with ALL in the Nordic and Baltic countries are treated according to the common Nordic Society of Pediatric Hematology and Oncology (NOPHO) protocols, which are updated approximately every eighth year. In the NOPHO ALL2000 protocol, maintenance therapy was initiated at week 17 for SR patients and week 30 for IR patients; and in the ALL2008 protocol, at week 20 and 22, respectively. Regardless of risk group, the initial dose of oral 6MP was 75; 50 and 1–10 mg/m2/24 h for TPMTWT, TPMTLA and TPMTDE patients. No dose adjustments according to ITPA variants were done. The starting dose of oral MTX was 20 mg/m2/week. 6MP and MTX doses were subsequently adjusted by a target white blood cell count (WBC) of 1.5–3.5 × 109/l for patients treated according to the NOPHO ALL2000 protocol and 1.5–3.0 × 109/l for patients treated according to the NOPHO ALL2008 protocol. During the first year of maintenance therapy, treatment intensifications were given at 4-week intervals, with alternating high-dose MTX (5 g/m2/24 h with Leucovurin rescue) or Vincristine (2.0 mg/m2)/Dexamethasone (6 mg/m2/day for five days), until five high-dose MTX infusions had been given. The second maintenance therapy phase (oral 6MP/MTX only, except for age-adjusted, intrathecal MTX at 8-week interval for IR patients) was initiated at week 56/70 (SR/IR, ALL 2000), or at week 58/66 (IR/IR, ALL2008). Treatment was discontinued 130 weeks after diagnosis in both protocols.
From January 2001 to January 2012, blood samples were collected from all patients treated at the Department of Pediatrics and Adolescent Medicine, The University Hospital Rigshospitalet, Copenhagen after approval by the Ethical Committee of Copenhagen (no. H-2-2010-002) and informed consent by the parents, according to the Declaration of Helsinki. For DNA-TGN analysis, 2.095 blood samples collected during maintenance therapy were available with a median of 23 samples per patient. Five patients had only one sample taken. Determination of Ery-TGN and Ery-MeMP concentrations had been performed in 2,636 samples, and 2,743 samples, respectively. All children diagnosed with ALL and treated according to the ALL2000 and ALL2008 protocols are routinely genotyped for TPMT polymorphisms (rs1800460 and rs1142345). As a part of this study, all patients were genotyped for ITPA polymorphisms (rs1127354) with TaqMan technology. ITPA genotypes were verified by independent analyses by a collaborating research group at Linköping University [25].
DNA-TG quantification
DNA-TG was quantified by derivatizing approximately 2 µg of whole blood DNA with chloroacetaldehyde in a phosphate buffer at 100 °C for 3 hours to produce etheno(ε)-TG and ε-guanine. The samples were cleaned on a strong cation exchanger (Strata-X-C, 30 mg, Phenomenex) and dried under flowing N2 (40 °C). After resuspension in 200 µl 0.1% formic acid (FA)/95% acetonitrile ε-TG and ε-guanine were quantified by hydrophilic interaction liquid chromatography (HILIC) on an aquity tandem mass spectrometry, as described by Jacobsen et al. [26].
Statistics
Data analyses were performed using SPSS version 22. Where indicated, arithmetic means (prefix m) of DNA-TG, Ery-MeMP and Ery-TGN were calculated for each patient based on all the samples available during maintenance therapy. DNA-TG indices were calculated as mDNA-TG/mEry-TGN. Correlation of continuous variables was assessed by Spearman’s rank correlation coefficient (rs). 6MP metabolite levels across genotypes were compared with Mann–Whitney U tests. Distribution of gender, risk group and genotype were assessed by Chi-square test. P values < 0.05 were regarded statistically significant.
Results
Patients’ baseline parameters are presented in Table 1. DNA for ITPA genotyping was not available for 17 patients, but ITPA allele frequencies for the remaining 115 patients were in Hardy–Weinberg equilibrium (p = 0.89). Furthermore, distributions of patients with TPMT WT, LA and DE genotypes were in line with reported frequencies among Caucasians [27] and as expected the G460A and A719G variants were in strong disequilibrium. None of the included patients carried low-activity alleles for both ITPA and TPMT.
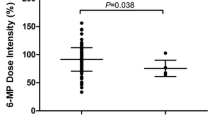
TPMTLA patients had significantly higher levels of mEry-TGN (median 491 vs. 232 nmol/mmol Hb, p = 0.001) (Fig. 2b; Table 2) and mDNA-TG (median 549 vs. 364 fmol/µg DNA, p = 0.007) (Fig. 2a; Table 2), but significantly lower levels of mEry-MeMP (median 6193 vs. 17,499 nmol/mmol Hb, p = 0.001) (Fig. 2c; Table 2) and DNA-TG indices (median 1.2 vs. 1.7 (fmol TG/µg DNA)/(nmol TG/mmol Hb), p = 0.007) (Fig. 2d; Table 2), when compared to TPMTWT patients.
We found significantly higher levels of mDNA-TG (median 465 vs. 387 fmol/µg DNA, p = 0.04) in ITPALA as compared to ITPAWT patients (Fig. 2.a, Table 2). However, ITPALA patients did not differ significantly from ITPAWT patients with respect to mEry-TGN (median 305 vs. 260 nmol/ mmol Hb, p = 0.086) (Fig. 2b; Table 2), mEry-MeMP (median 20,866 vs. 15,902 nmol/mmol Hb, p = 0.12) (Fig. 2c; Table 2), and DNA-TG indices (median 1.7 vs. 1.6 fmol/µg DNA/ nmol/mmol Hb, p = 0.81) (Fig. 2c; Table 2).
Gender, age, and number of samples available per patient were not significantly associated with metabolite levels. SR patients had higher levels of mEry-MeMP (17,759 vs. 14,286 nmol/mmol Hb, p = 0.021) and DNA-TG indices (1.8 vs. 1.5, p = 0.041), when compared to IR patients. Risk group was not significantly associated with mDNA-TG (p = 0.138) or mEry-TGN levels (p = 0.69) (Table 2).
Levels of mDNA-TG were positively correlated with mEry-TGN (rs = 0.37, p = 0.001) (Fig. 3). In addition, mEry-MeMP levels were positively correlated to DNA-TG indices (rs = 0.33, p = 0.001) (Fig. 4).
Median Ery-TGN vs. median DNA-TG. The relationship between mEry-TGN and mDNA-TG. Median DNA-TG levels show a trend to reach plateau at high Ery-TGN levels. Spearman’s rank correlation coefficient (rs) was calculated. p values < 0.05 were regarded statistically significant. Circle: ITPAWT/TPMTWT patients, square: ITPAWT/TPMTLA patients, triangle: ITPALA/TPMTWT patients, diamond: ITPALA/TPMTLA patient. Illustration made in GraphPad Prizm
Mean Ery-MeMP vs. DNA-TG index in relation to TPMT and ITPA genotype. Mean Ery-MeMP levels were significantly correlated to DNA-TG index indicating that with high levels of mEry-MeMP there was a significant increase in the relative incorporation of 6TGN into DNA. Spearman’s rank correlation coefficient (rs) was calculated. p values < 0.05 were regarded statistically significant. Circle: ITPAWT/TPMTWT patients, square: ITPAWT/TPMTLA patients, triangle: ITPALA/TPMTWT patients, diamond: ITPALA/TPMTLA patient. Illustration made in GraphPad Prizm
Discussion
It is well established that TPMTLA patients experience higher Ery-TGN levels and lower relapse rates as compared to TPMTWT patients, and some groups treat with reduced initial 6-MP doses to prevent unnecessary toxicity [28,29,30,31]. However, 6MP dose adjustments are guided by white blood cell count (WBC) aiming for WBC of 1.5 −3.0 × 109 L [32] to reach equitoxic treatment [33,34,35], and it remains uncertain if patients benefit from this upfront dose reduction.
Findings indicate room for improvement to better tailor treatment with 6MP. DNA-TG is the principal cytotoxic metabolite, and we find significantly higher levels in TPMTLA patients as compared to TPMTWT patients. Contradictory to our findings, Ebbesen et al. found similar median levels of mDNA-TG in TPMTLA and TPMTWT patients, but non-significantly more TPMTLA patients had very high DNA-TG levels. The diverse findings may reflect the higher number of samples in the current study. In addition, our center extensively focusses on maintenance treatment, and the treatment intensity may overall have been higher than in the cohort of Ebbesen et al. [36].
The trend of DNA-TG levels to reach a plateau at high Ery-TGN levels indicates that with increasing Ery-TGN levels, proportionally less will be incorporated into DNA. This finding is in accordance with a previous study by Hedeland et al. [16]. This limit of incorporation may explain why TPMTLA patients have and tolerate higher levels of Ery-TGN without unacceptable myelosuppression, not least since they also have lower levels of methylated 6MP metabolites and thus less inhibition of purine de novo synthesis.
To achieve a more adequate understanding of the 6MP metabolism, we explored the impact of the enzyme ITPA, as recent studies have indicated that a SNP in the gene-encoding ITPA is associated with adverse drug reactions in thiopurine treatment [37, 38]. Moreover, ITPALA has been associated with high levels of methylated 6MP metabolites [10, 39], higher risk of fever and neutropenia [10, 20, 40], hepatotoxicity [12, 20, 41], and possibly an increased risk of relapse [9, 42].
We found significantly higher levels of mDNA-TG, but only moderate, non-significant higher mEry-MeMP levels in ITPALA patients as compared to ITPAWT patients. Methylated thiopurine metabolites has previously been shown to be significantly correlated to the incorporation of TGN into DNA [43]. ITPALA patients may reconvert less TITP to TIMP resulting in higher levels of MeTITP. We speculate that these will add to the pools of methylated metabolites, inhibiting the purine de novo synthesis and leading to lower levels of endogenous purines to compete with TGN to be incorporated into DNA, as also suggested by Stocco et al. [8, 10]. Still, the difference in mDNA-TG levels observed for the ITPA genotype in this study is not explained by neither mEry-TGN levels nor mEry-MeMP levels alone. We speculate that the explanation could be found in the trends of ITPALA patients having higher levels of mEry-TGN and mEry-MeMP metabolites; on their own the trends are too small to reach statistical significance, but their joint effect may result in the higher levels of mDNA-TG observed in ITPALA patients.
As outlined above, this present study supports that polymorphisms in the genes encoding the enzymes TPMT and ITPA significantly modifies the metabolism of 6MP during maintenance treatment of childhood ALL resulting in higher levels of mDNA-TG in both TPMTLA and ITPALA patients.
A shortcoming of this study is that free cytosol 6TGN and methylated metabolite levels are measured in erythrocytes as a surrogate for nucleated cells, including lymphoblasts. This may not accurately reflect the metabolic status of the latter target population, as clinical studies have shown major differences between Ery-TGN and leukocyte cytosol 6TGN levels in the same blood samples [44;45]. This study is also limited by the fact that individual treatment doses and hematological parameters were not collected. The TPMTLA patients are treated with reduced doses as compared to TPMTWT patients to reach equitoxic treatment; but our results indicate that this may not be achieved.
In conclusion, our findings support that measurements of DNA-TG may prove to be a more relevant parameter for monitoring 6MP treatment intensity, since it combines the effects of TGN and MeMP levels.
References
Chiengthong K, Ittiwut C, Muensri S, Sophonphan J, Sosothikul D, Seksan P, et al (2016) NUDT15 c.415C > T increases risk of 6-mercaptopurine induced myelosuppression during maintenance therapy in children with acute lymphoblastic leukemia. Haematologica 101(1):e24–e26
Liang DC, Yang CP, Liu HC, Jaing TH, Chen SH, Hung IJ et al (2016) NUDT15 gene polymorphism related to mercaptopurine intolerance in Taiwan Chinese children with acute lymphoblastic leukemia. Pharmacogenomics J 16(6):536–539
Moriyama T, Nishii R, Perez-Andreu V, Yang W, Klussmann FA, Zhao X et al (2016) NUDT15 polymorphisms alter thiopurine metabolism and hematopoietic toxicity. Nat Genet 48(4):367–373
Moriyama T, Nishii R, Lin TN, Kihira K, Toyoda H, Jacob N et al (2017) The effects of inherited NUDT15 polymorphisms on thiopurine active metabolites in Japanese children with acute lymphoblastic leukemia. Pharmacogenet Genomics 27(6):236–239
Suzuki H, Fukushima H, Suzuki R, Hosaka S, Yamaki Y, Kobayashi C et al (2016) Genotyping NUDT15 can predict the dose reduction of 6-MP for children with acute lymphoblastic leukemia especially at a preschool age. J Hum Genet 61(9):797–801
Tanaka Y, Kato M, Hasegawa D, Urayama KY, Nakadate H, Kondoh K et al (2015) Susceptibility to 6-MP toxicity conferred by a NUDT15 variant in Japanese children with acute lymphoblastic leukaemia. Br J Haematol 171(1):109 – 15
Yang JJ, Landier W, Yang W, Liu C, Hageman L, Cheng C et al (2015) Inherited NUDT15 variant is a genetic determinant of mercaptopurine intolerance in children with acute lymphoblastic leukemia. J Clin Oncol 33(11):1235–1242
Bierau J, Lindhout M, Bakker JA (2007) Pharmacogenetic significance of inosine triphosphatase. Pharmacogenomics 8(9):1221–1228
Kim H, Kang HJ, Kim HJ, Jang MK, Kim NH, Oh Y et al (2012) Pharmacogenetic analysis of pediatric patients with acute lymphoblastic leukemia: a possible association between survival rate and ITPA polymorphism. PLoS One 7(9):e45558
Stocco G, Crews KR, Evans WE (2010) Genetic polymorphism of inosine-triphosphate-pyrophosphatase influences mercaptopurine metabolism and toxicity during treatment of acute lymphoblastic leukemia individualized for thiopurine-S-methyl-transferase status. Expert Opin Drug Saf 9(1):23–37
Stocco G, Franca R, Londero M, Decorti G (2013) ITPA genetic polymorphism is possibly associated with survival rate in Korean children with acute lymphoblastic leukemia. Pharmacogenomics 14(3):237–238
Tanaka Y, Manabe A, Nakadate H, Kondoh K, Nakamura K, Koh K et al (2012) The activity of the inosine triphosphate pyrophosphatase affects toxicity of 6-mercaptopurine during maintenance therapy for acute lymphoblastic leukemia in Japanese children. Leuk Res 36(5):560–564
Swann PF, Waters TR, Moulton DC, Xu YZ, Zheng Q, Edwards M et al (1996) Role of postreplicative DNA mismatch repair in the cytotoxic action of thioguanine. Science 273(5278):1109–1111
Karran P, Attard N (2008) Thiopurines in current medical practice: molecular mechanisms and contributions to therapy-related cancer. Nat Rev Cancer 8(1):24–36
Lilleyman JS, Lennard L (1994) Mercaptopurine metabolism and risk of relapse in childhood lymphoblastic leukaemia. Lancet 343(8907):1188–1190
Hedeland RL, Hvidt K, Nersting J, Rosthoj S, Dalhoff K, Lausen B et al (2010) DNA incorporation of 6-thioguanine nucleotides during maintenance therapy of childhood acute lymphoblastic leukaemia and non-Hodgkin lymphoma. Cancer Chemother Pharmacol 66(3):485–491
Krynetski EY, Krynetskaia NF, Yanishevski Y, Evans WE (1995) Methylation of mercaptopurine, thioguanine, and their nucleotide metabolites by heterologously expressed human thiopurine S-methyltransferase. Mol Pharmacol 47(6):1141–1147
Marsh S, King CR, Ahluwalia R, McLeod HL (2004) Distribution of ITPA P32T alleles in multiple world populations. J Hum Genet 49(10):579–581
Mohandas T, Sparkes RS, Passage MB, Sparkes MC, Miles JH, Kaback MM (1980) Regional mapping of ADA and ITP on human chromosome 20: cytogenetic and somatic cell studies in an X/20 translocation. Cytogenet Cell Genet 26(1):28–35
Azimi F, Mortazavi Y, Alavi S, Khalili M, Ramazani A (2015 Oct) Frequency of ITPA gene polymorphisms in Iranian patients with acute lymphoblastic leukemia and prediction of its myelosuppressive effects. Leuk Res 39(10):1048–1054
Marinaki AM, Duley JA, Arenas M, Ansari A, Sumi S, Lewis CM et al (2004) Mutation in the ITPA gene predicts intolerance to azathioprine. Nucleosides Nucleotides Nucleic Acids 23(8–9):1393–1397
Stocco G, Franca R, Verzegnassi F, Londero M, Rabusin M, Decorti G (2012) Multilocus genotypes of relevance for drug metabolizing enzymes and therapy with thiopurines in patients with acute lymphoblastic leukemia. Front Genet 3:309
Toft N, Schmiegelow K, Klausen TW, Birgens H (2012) Adult acute lymphoblastic leukaemia in Denmark. A national population-based retrospective study on acute lymphoblastic leukaemia in Denmark 1998–2008. Br J Haematol 157(1):97–104
Toft N, Birgens H, Abrahamsson J, Bernell P, Griskevicius L, Hallbook H et al (2013) Risk group assignment differs for children and adults 1–45 year with acute lymphoblastic leukemia treated by the NOPHO ALL-2008 protocol. Eur J Haematol 90(5):404–412
Hindorf U, Lindqvist M, Peterson C, Soderkvist P, Strom M, Hjortswang H et al (2006 Oct) Pharmacogenetics during standardised initiation of thiopurine treatment in inflammatory bowel disease. Gut 55(10):1423–1431
Jacobsen JH, Schmiegelow K, Nersting J (2012) Liquid chromatography-tandem mass spectrometry quantification of 6-thioguanine in DNA using endogenous guanine as internal standard. J Chromatogr B Analyt Technol Biomed Life Sci 881–882:115–118
Wang L, Weinshilboum R (2006) Thiopurine S-methyltransferase pharmacogenetics: insights, challenges and future directions. Oncogene 25(11):1629–1638
Davidsen ML, Dalhoff K, Schmiegelow K (2008) Pharmacogenetics influence treatment efficacy in childhood acute lymphoblastic leukemia. J Pediatr Hematol Oncol 30(11):831–849
Farfan MJ, Salas C, Canales C, Silva F, Villarroel M, Kopp K et al. Prevalence of TPMT and ITPA gene polymorphisms and effect on mercaptopurine dosage in Chilean children with acute lymphoblastic leukemia. BMC Cancer 2014 Apr 28;14:299
Fotoohi AK, Coulthard SA, Albertioni F (2010) Thiopurines: factors influencing toxicity and response. Biochem Pharmacol 79(9):1211–1220
Relling MV, Gardner EE, Sandborn WJ, Schmiegelow K, Pui CH, Yee SW et al (2013) Clinical pharmacogenetics implementation consortium guidelines for thiopurine methyltransferase genotype and thiopurine dosing: 2013 update. Clin Pharmacol Ther 93(4):324–325
Schmiegelow K, Pulczynska MK (1990) Maintenance chemotherapy for childhood acute lymphoblastic leukemia: should dosage be guided by white blood cell counts? Am J Pediatr Hematol Oncol 12(4):462–467
Schmiegelow K, Nersting J, Nielsen SN, Heyman M, Wesenberg F, Kristinsson J et al (2016 Dec) Maintenance therapy of childhood acute lymphoblastic leukemia revisited-Should drug doses be adjusted by white blood cell, neutrophil, or lymphocyte counts? Pediatr Blood Cancer 63(12):2104–2111
Schmiegelow K, Pulczynska MK (1990 Jan) White-cell counts in childhood acute lymphoblastic leukemia. Eur J Haematol 44(1):72–74
Van den Bossche J, Devreese K, Malfait R, Van d V, Wauters A, Neeis H et al (2002 Jan) Reference intervals for a complete blood count determined on different automated haematology analysers: Abx Pentra 120 Retic, Coulter Gen-S, Sysmex SE 9500, Abbott Cell Dyn 4000 and Bayer Advia 120. Clin Chem Lab Med 40(1):69–73
Ebbesen MS, Nersting J, Jacobsen JH, Frandsen TL, Vettenranta K, Abramsson J et al (2013 Jun) Incorporation of 6-thioguanine nucleotides into DNA during maintenance therapy of childhood acute lymphoblastic leukemia—the influence of thiopurine methyltransferase genotypes. J Clin Pharmacol 53(6):670–674
Marinaki AM, Ansari A, Duley JA, Arenas M, Sumi S, Lewis CM et al (2004) Adverse drug reactions to azathioprine therapy are associated with polymorphism in the gene encoding inosine triphosphate pyrophosphatase (ITPase). Pharmacogenetics 14(3):181–187
Zelinkova Z, Derijks LJ, Stokkers PC, Vogels EW, van Kampen AH, Curvers WL et al (2006) Inosine triphosphate pyrophosphatase and thiopurine s-methyltransferase genotypes relationship to azathioprine-induced myelosuppression. Clin Gastroenterol Hepatol 4(1):44–49
Adam de BT, Fakhoury M, Medard Y, Azougagh S, Zhang D, Yakouben K et al (2011) Determinants of mercaptopurine toxicity in paediatric acute lymphoblastic leukemia maintenance therapy. Br J Clin Pharmacol 71(4):575–584
Hareedy MS, El Desoky ES, Woillard JB, Thabet RH, Ali AM, Marquet P et al (2015) Genetic variants in 6-mercaptopurine pathway as potential factors of hematological toxicity in acute lymphoblastic leukemia patients. Pharmacogenomics 16(10):1119–1134
Wan Rosalina WR, Teh LK, Mohamad N, Nasir A, Yusoff R, Baba AA et al (2012) Polymorphism of ITPA 94C > A and risk of adverse effects among patients with acute lymphoblastic leukaemia treated with 6-mercaptopurine. J Clin Pharm Ther 37(2):237–241
Smid A, Karas-Kuzelicki N, Milek M, Jazbec J, Mlinaric-Rascan I (2014) Association of ITPA genotype with event-free survival and relapse rates in children with acute lymphoblastic leukemia undergoing maintenance therapy. PLoS One 9(10):e109551
Duley JA, Florin TH (2005 Oct) Thiopurine therapies: problems, complexities, and progress with monitoring thioguanine nucleotides. Ther Drug Monit 27(5):647–654
Bergan S, Bentdal O, Sodal G, Brun A, Rugstad HE, Stokke O (1997 Oct) Patterns of azathioprine metabolites in neutrophils, lymphocytes, reticulocytes, and erythrocytes: relevance to toxicity and monitoring in recipients of renal allografts. Ther Drug Monit 19(5):502–509
Lancaster DL, Lennard L, Rowland K, Vora AJ, Lilleyman JS (1998 Jul) Thioguanine versus mercaptopurine for therapy of childhood lymphoblastic leukaemia: a comparison of haematological toxicity and drug metabolite concentrations. Br J Haematol 102(2):439–443
Acknowledgements
This work is supported by the Danish Cancer Society and the Danish Childhood Cancer Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors of this article declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Gerbek, T., Ebbesen, M., Nersting, J. et al. Role of TPMT and ITPA variants in mercaptopurine disposition. Cancer Chemother Pharmacol 81, 579–586 (2018). https://doi.org/10.1007/s00280-018-3525-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-018-3525-8