Abstract
Purpose
S-1 has systemic activity for locally advanced pancreatic cancer (LAPC). Here, the efficacy and safety of induction gemcitabine (GEM) and S-1 (GS) followed by chemoradiotherapy (CRT) and systemic chemotherapy using S-1 for LAPC were assessed.
Methods
The treatment consisted of four cycles of induction GS (S-1 60, 80, or 100 mg/day based on body surface area for 14 days every 3 weeks plus GEM 1000 mg/m2 on days 8 and 15), followed by S-1 (80, 100, or 120 mg/day based on body surface area on days 1–14 and 22–35) and concurrent radiotherapy (50.4 Gy in 28 fractions). Maintenance chemotherapy with S-1 was started 1–4 weeks after CRT until disease progression or unacceptable toxicity was observed. The primary endpoint was 1-year survival.
Results
A total of 30 patients with LAPC were enrolled. The median survival and progression-free survival were 21.3 and 12.7 months, respectively. Overall survival rates at 1, 2, 3, and 4 years were 73.3, 36.7, 23.3, and 16.7%, respectively. The median survival of 23 patients who received CRT was 22.9 months, with a 3-year survival rate of 30.4%. The two most common grade 3 or 4 adverse events during induction GS were neutropenia (63.3%) and biliary tract infection (20%). Toxicities during CRT or maintenance chemotherapy were generally mild.
Conclusions
This regimen was feasible and highly active resulting in encouraging survival in patients with LAPC. Further investigations are warranted to elucidate the effectiveness of this treatment strategy in future studies.
Clinical trials information: UMIN000006332.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chemoradiotherapy (CRT) is regarded as a therapeutic option for locally advanced pancreatic cancer (LAPC) [1,2,3]. However, the efficacy of the conventional CRT using 5-FU as a radiosensitizer remains poor with a reported median survival of approximately 10 months [4]. This local treatment is clearly inadequate for tumor control, given the fact that distant metastasis is the main cause of treatment failure [4]. Hence, the addition of effective systemic chemotherapy is crucial to improve patient survival.
S-1 is an oral fluoropyrimidine preparation with demonstrated systemic anti-tumor effects against pancreatic cancer. In a phase III study of gemcitabine (GEM) plus S-1 (GS), S-1 alone, or GEM alone for metastatic or LAPC (the GEST study), S-1 showed non-inferiority to GEM in overall survival (OS) with a higher response rate [5]. Furthermore, OS with S-1 was shown to be superior to that with GEM alone in a phase III study of adjuvant chemotherapy for resected pancreatic cancer (the JASPAC-01 study) [6].
S-1 has systemic activity for LAPC; thus, the combination therapy of S-1 and radiotherapy (RT) is expected to convey both systemic and local anti-tumor effects. Phase II studies have shown promising efficacy of S-1 and concurrent RT followed by maintenance chemotherapy with S-1 for LAPC [median survival time (MST) = 14.3–16.8 months] [7,8,9].
In the present study, induction GEM and S-1 (GS) was administered before S-1 and RT to intensify systemic anti-tumor effects and to select the patients who would benefit from local treatment. Induction chemotherapy before CRT is regarded as a therapeutic option to exclude patients who develop rapidly progressive distant metastases, although no high-level evidence supports this treatment strategy [10]. GS was superior with regard to progression-free survival (PFS) compared with GEM alone in randomised phase II studies for metastatic or LAPC [11,12,13]. Although GS did not improve OS compared to GEM alone in the GEST study, sub-group analyses showed that GS significantly improved OS in patients with LAPC [hazard ratio (HR) = 0.67; 95% confidence interval (CI) 0.46–0.99] [5]. Furthermore, compared to administration of GEM alone, GS improved ORR, PFS, and OS in patients with LAPC in a pooled analysis of three randomised controlled studies [14].
The aim of the current study was to assess the efficacy and safety of induction GS followed by CRT and systemic chemotherapy using S-1 in patients with LAPC.
Materials and methods
Eligibility
The eligibility criteria for enrollment in this study were as follows: cytologically or histologically confirmed, locally advanced adenocarcinoma of the pancreas; no distant metastases; no evidence of gastroduodenal invasion or obstruction; measurable lesion according to the Response Evaluation Criteria in Solid Tumors (version 1.1); 20–79 years of age; no prior chemotherapy or RT; Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0 or 1; written informed consent; and adequate organ function [leukocytes ≥3500/mm3, neutrophils ≥2000/mm3, platelets ≥100,000/mm3, hemoglobin ≥9.0 g/dL, normal serum creatinine and creatinine clearance ≥ 50 mL/min, serum aspartate transaminase (AST) ≤120 IU/L, serum alanine transaminase (ALT) ≤ 120 IU/L, and serum bilirubin ≤2.0 or ≤3.0 mg/dL after biliary drainage in patients with obstructive jaundice]. Locally advanced disease was defined as tumor invasion of the superior mesenteric artery, celiac axis, hepatic artery, or bilateral stenosis of the portal vein or superior mesenteric vein.
Exclusion criteria were as follows: severe concurrent disease; interstitial pneumonia; mental disorder; concomitant malignancy; moderate or severe diarrhea, hemorrhagic colitis or peptic ulcer; severe drug hypersensitivity; pregnant or lactating females; regular use of phenytoin, warfarin, or frucitocin; receiving systemic steroid therapy; and active infection.
This prospective phase II study was performed in compliance with the guidelines of the Declaration of Helsinki. The study protocol was approved by the institutional ethical review board of Chiba Cancer Center and all patients submitted written informed consent before participating in the study. This study was registered at the UMIN Clinical Trials Registry (UMIN000006332).
Treatment
Induction GS
Patients received four cycles of induction GS (oral S-1 twice a day after meals from the evening of day 1 to the morning of day 15 every 3 weeks and GEM at 1000 mg/m2 on days 8 and 15). The daily S-1 dose was established according to the body surface area (BSA) as follows: BSA <1.25 m2, 60 mg/day; 1.25 m2 ≤ BSA < 1.50 m2, 80 mg/day; and 1.50 m2 ≤BSA, 100 mg/day. Dose modifications were made according to the predefined criteria.
S-1 and RT
Patients who did not develop distant metastases after induction GS received 50.4 Gy of RT in 28 fractions concurrent with oral S-1 twice a day on days 1–14 and 22–35. S-1 and concurrent radiotherapy were started with at least a 2-week washout period after the completion of induction GS. The daily S-1 dose was determined according to BSA as follows: BSA <1.25 m2, 80 mg/day; 1.25 m2 ≤ BSA < 1.50 m2, 100 mg/day; and 1.50 m2 ≤BSA, and 120 mg/day. Dose modifications were performed according to the predefined criteria.
RT consisted of 50.4 Gy in 28 daily fractions of 1.8 Gy over 5.5 weeks using 10 MV photons. RT was prescribed with involved-field irradiation not including prophylactic nodal irradiation, as reported previously [7, 15]. Histograms of the dose distribution and dose volume were created using a three-dimensional treatment planning system. The gross tumor volume (GTV) was defined as the primary tumor and metastatic lymph nodes detected on CT. The clinical target volume (CTV) was defined as GTV plus a 0.5 cm margin, and the planning target volume (PTV) was defined as CTV plus a 1–1.5 cm margin for daily setup variation and respiratory movement.
Maintenance chemotherapy with S-1
Maintenance chemotherapy with S-1 (for 14 days every 3 weeks) was started within 1–4 weeks after CRT. Treatment was continued until disease progression or unacceptable toxicity.
Pre-treatment and follow-up evaluation
Baseline evaluation included a medical history and physical examination, blood examination, CT scans of the abdomen with intravenous contrast, and CT scan of the chest. Tumor stage was assessed according to the Union for International Cancer Control (UICC) TMN classification (7th edition).
Physical examination, complete blood cell counts, and serum biochemistry tests were conducted once a week during induction GS and CRT and at least once every 3 weeks during maintenance therapy. Adverse events were evaluated according to the National Cancer Institute Common Toxicity Criteria, version 4.0.
CT was performed every two cycles during induction GS and then at the completion of RT. During maintenance therapy, CT was performed every 2 months. Tumor response was evaluated using RECIST version 1.1.
Statistical analysis
The primary endpoint was 1-year survival on an intention-to-treat basis. The secondary endpoints included OS, PFS, ORR, and adverse events. The sample size was determined using the Southwest Oncology Group One Arm Survival program (https://stattools.crab.org/). We assumed an expected 1-year survival rate of 60% and a threshold rate of 40% with a power of 85% and a one-sided alpha level of 0.05, with an accrual time of 48 months and follow-up of 12 months. The planned sample size was set at 30.
Time-related parameters were calculated using the Kaplan–Meier method from the initiation of induction chemotherapy. Statistical analyses were performed using EZR ver. 1.33 software (https://cran.r-project.org/web/packages/RcmdrPlugin.EZR/index.html) [16], a graphical user interface for R (The R Foundation for Statistical Computing, version 3.3.1).
Results
Patient characteristics
A total of 30 patients were enrolled in this study between September 2011 and November 2013. The baseline characteristics of the patients are listed in Table 1. Sixty-three percent of the patients had an ECOG PS of 0, with a median age of 67.5 years. The median tumor size was 41.5 mm (range 30–53 mm). Eighty-three percent of patients had T4 disease. The median serum CA 19-9 concentration was 206 U/mL.
Treatment
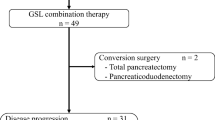
Doses of GEM or S-1 were reduced because of toxicity in 23 patients (76.7%) during induction GS. Treatment was terminated in seven patients because of adverse events (n = 3), disease progression (n = 2), withdrawal of consent (n = 1), or surgical resection after a significant response (n = 1) (Fig. 1). The remaining 23 patients received S-1 and concurrent RT. Eight patients started with reduced doses of S-1 because of toxicity observed during induction GS. No patient required a dose reduction of RT. The median PTV was 195 cm3 (range 99–662). At the completion of S-1 and RT, two patients terminated treatment because of disease progression. Twenty-one patients received maintenance chemotherapy with S-1 with a median of 11 cycles (range 1–33 cycles).
Safety
All patients (n = 30) were assessed for toxicity. As shown in Table 2, Grade 3/4 toxicities were more frequent during induction GS. The most prevalent grade 3/4 adverse events were neutropenia (63.3%) and biliary tract infection (20%). Biliary tract infection and hepatic infection were mainly due to biliary stent occlusion. One patient developed interstitial pneumonitis, probably due to GEM, but recovered with steroid therapy. One patient died suddenly during the induction chemotherapy phase, possibly because of the aspiration caused by obstruction of the gastric outlet due to tumor invasion into the duodenum.
Only mild toxicities were observed during S-1 and RT or maintenance chemotherapy with S-1. Grade 3/4 non-hematological adverse events, other than biliary tract infection, were uncommon.
One patient developed acute cholecystitis with minor perforation 32 months after treatment initiation, mainly due to repeated biliary infections associated with stent occlusion. This patient recovered after surgical treatment.
Response
For the best overall response in all treated patients (n = 30), 10 (33%) achieved a partial response and 19 (63%) had stable disease. Among the 23 patients who received CRT, induction GS showed a significant reduction in tumor size. (median size, from 41 to 32 mm; Wilcoxon signed-rank test, p = 0.0004).
Three patients underwent surgery with curative intent and achieved R0 resection. One patient underwent surgical resection after 21 cycles of maintenance chemotherapy with S-1 and was confirmed to have a pathological complete response (CR). Another patient underwent surgical resection after four cycles of induction GS. However, this patient experienced local recurrence 11 months after surgery. The other patient underwent surgical resection 41 months after treatment initiation.
Survival
The median follow-up time for censored cases was 48.5 months (range 41.9–55.9 months). For all treated patients (n = 30), OS rates at 1, 2, 3, and 4 years were 73.3% (95% CI 53.7–85.7%), 36.7% (95% CI 20.1–53.4%), 23.3% (95% CI 10.3–39.4%), and 16.7% (95% CI 6.1–31.8%), respectively. The median durations of OS and PFS were 21.3 (95% CI 14.3–24.9) months and 12.7 (95% CI 9.6–15.5) months, respectively (Fig. 2).
For 23 patients who did not develop distant metastases and received CRT, OS rates at 1, 2, 3, and 4 years were 82.6% (95% CI 60.1–93.1%), 43.5% (95% CI 23.3–62.1%), 30.4% (95% CI 13.5–49.3%), and 21.7% (95% CI 7.9–39.9%), respectively. The median OS was 22.9 months (95% CI 15.3–35.5 months), while the median OS was 11.8 months (95% CI 2.5–24 months) for 7 patients who did not receive planned CRT (Logrank test, p = 0.0199) (Fig. 2).
Patterns of initial disease progression
At the time of analysis, 3 (10%) patients were still alive without disease progression, with a survival duration of 41.9–55.9 months. Among the three patients, two were treated with CRT without additional resection.
Distant metastasis was noted in 11 (37%) patients, local progression in 10 (33%), and both in 4 (13%) (Table 3).
Discussion
The majority of patients with LAPC eventually develop distant metastasis during the course of treatment [17, 18]. The treatment regimen used in this study comprised continuous systemic chemotherapy throughout the entire treatment period combined with local treatment with RT in selected patients. The estimated 1-year survival rate of 73.3% (95% CI 53.7–85.7%) obtained in the current study is promising, and the study met its primary endpoint. Furthermore, the median survival (21.3 months) and long-term outcomes (2-year survival of 36.7%, 3-year survival of 23.3%, and 4-year survival of 16.7%) were also encouraging when compared with those of other prospective studies for LAPC (Table 4) [4, 7, 18,19,20,21,22,23,24]. Although a direct comparison across studies is difficult because of the difference in patient backgrounds, our regimen appears to be favorable in PFS, which may have resulted in the longer OS.
The favorable outcomes achieved in this study may partly be due to the long-term feasibility of this regimen. Although induction GS is often associated with grade 3/4 neutropenia or infection, toxicity of CRT, or maintenance chemotherapy with S-1 was generally mild. To decrease radiation toxicity and deliver sufficient systemic chemotherapy, we adopted involved-field irradiation without prophylactic nodal irradiation. Furthermore, in patients who received RT, tumor size was significantly reduced after induction GS (median size, from 41 to 32 mm). In LAPC, ORR was greater for GS compared to that for GEM alone in a pooled analysis of three randomised controlled studies [14]. Reduction in tumor size may have contributed to the mild toxicity profile of RT after induction GS via reduction of PTV (median PTV, 195 cm3). Large PTV (≥500 cm3) is reportedly an important factor for severe acute intestinal toxicity of RT for LAPC [25]. Most patients in the current study received systemic chemotherapy even after CRT.
In the current study, induction GS appeared to play an important role in better patient selection. The outcome of 23 (76.7%) patients treated with additional CRT was very encouraging. The median survival period of 22.9 months was comparable to that in patients who underwent surgery after neo-adjuvant therapy for initially unresectable pancreatic cancer. Gillen et al. reported that one-third of patients with initially unresectable LAPC would be expected to have resectable tumors after neo-adjuvant therapy, and the MST was 20.5 months following resection [26]. In the current study, long-term outcomes of patients treated with CRT were also favorable (2-year survival of 43.5%, 3-year survival of 30.4%, and 4-year survival of 21.7%). In addition, one patient treated with CRT experienced pathologic CR. The role of additional CRT after systemic chemotherapy, especially after GEM or GEM plus erlotinib, remains controversial in LAPC, as shown in the LAP-07 study [27]. However, these findings in the current study suggest that CRT may confer a survival benefit in some populations.
The analyses of patterns of initial disease progression indicated that more potent therapies are crucial for both systemic and local tumor control. Recent evidence in metastatic pancreatic cancer suggests that FOLFIRINOX or GEM plus nab-paclitaxel regimen may be promising options for LAPC [28, 29]. Future studies should assess the role of these regimens with or without CRT for LAPC.
There were a few limitations to the current study that should be addressed. The sample size was relatively small, and the study was conducted in a single institute. It cannot be ruled out that the better outcomes observed in the current study were due to better patient selection. However, similar results have been reported in a prior phase II study on induction GS followed by S-1 and concurrent RT [30]. In that study, 32 patients were treated with induction GS (different protocol from ours) followed by 50.4 Gy of intensity-modulated RT and maintenance therapy with S-1. The study showed a favorable ORR of 53.1%, 1-year survival of 75% and MST of 15.9 months. These results warrant further investigation of this regimen in a future study.
In conclusion, the current study demonstrated that induction GS followed by CRT and systemic chemotherapy using S-1 was feasible and highly active with encouraging survival in patients with LAPC. The rationale lies in continuous systemic chemotherapy throughout the entire treatment period combined with local treatment with RT in selected patients. We consider that induction GS played an important role in better patient selection and reduction of tumor size before RT. Further investigations are warranted to elucidate the effectiveness of this treatment strategy in future studies.
References
Moertel CG, Childs DS Jr, Reitemeier RJ et al (1969) Combined 5-fluorouracil and supervoltage radiation therapy of locally unresectable gastrointestinal cancer. Lancet 2:865–867
Moertel CG, Frytak S, Hahn RG et al (1981) Therapy of locally unresectable pancreatic carcinoma: a randomized comparison of high dose (6000 rads) radiation alone, moderate dose radiation (4000 rads + 5-fluorouracil), and high dose radiation + 5-fluorouracil: the Gastrointestinal Tumor Study Group. Cancer 48:1705–1710
Gastrointestinal Tumor Study Group (1988) Treatment of locally unresectable carcinoma of the pancreas: comparison of combined-modality therapy (chemotherapy plus radiotherapy) to chemotherapy alone. J Natl Cancer Inst 80:751–755
Ishii H, Okada S, Tokuuye K et al (1997) Protracted 5-fluorouracil infusion with concurrent radiotherapy as a treatment for locally advanced pancreatic carcinoma. Cancer 79:1516–1520
Ueno H, Ioka T, Ikeda M et al (2013) Randomized phase III study of gemcitabine plus S-1, S-1 alone, or gemcitabine alone in patients with locally advanced and metastatic pancreatic cancer in Japan and Taiwan: GEST Study. J Clin Oncol 31:1640–1648
Uesaka K, Boku N, Fukutomi A et al (2016) Adjuvant chemotherapy of S-1 versus gemcitabine for resected pancreatic cancer: a phase 3, open-label, randomised, non-inferiority trial (JASPAC 01). Lancet 388:248–257
Sudo K, Yamaguchi T, Ishihara T et al (2011) Phase II study of oral S-1 and concurrent radiotherapy in patients with unresectable locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys 80:119–125
Ikeda M, Ioka T, Ito Y et al (2013) A multicenter phase II trial of S-1 with concurrent radiation therapy for locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys 85:163–169
Shinchi H, Maemura K, Mataki Y et al (2012) A phase II study of oral S-1 with concurrent radiotherapy followed by chemotherapy with S-1 alone for locally advanced pancreatic cancer. J Hepatobiliary Pancreat Sci 19:152–158
Huguet F, André T, Hammel P et al (2007) Impact of chemoradiotherapy after disease control with chemotherapy in locally advanced pancreatic adenocarcinoma in GERCOR phase II and III studies. J Clin Oncol 25:326–331
Sudo K, Ishihara T, Hirata N et al (2014) Randomized controlled study of gemcitabine plus S-1 combination chemotherapy versus gemcitabine for unresectable pancreatic cancer. Cancer Chemother Pharmacol 73:389–396
Nakai Y, Isayama H, Sasaki T et al (2012) A multicentre randomized phase II trial of gemcitabine alone vs gemcitabine and S-1 combination therapy in advanced pancreatic cancer: GEMSAP study. Br J Cancer 106:1934–1939
Ozaka M, Matsumura Y, Ishii H et al (2012) Randomized phase II study of gemcitabine and S-1 combination versus gemcitabine alone in the treatment of unresectable advanced pancreatic cancer (Japan Clinical Cancer Research Organization PC-01 study). Cancer Chemother Pharmacol 69:1197–1204
Yanagimoto H, Ishii H, Nakai Y et al (2014) Improved survival with combined gemcitabine and S-1 for locally advanced pancreatic cancer: pooled analysis of three randomized studies. J Hepatobiliary Pancreat Sci 21:761–766
Kawakami H, Uno T, Isobe K et al (2005) Toxicities and effects of involved-field irradiation with concurrent cisplatin for unresectable carcinoma of the pancreas. Int J Radiat Oncol Biol Phys 62:1357–1362
Kanda Y (2013) Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant 48:452–458
Peixoto RD, Speers C, McGahan CE et al (2015) Prognostic factors and sites of metastasis in unresectable locally advanced pancreatic cancer. Cancer Med 8:1171–1177
Okusaka T, Ito Y, Ueno H et al (2004) Phase II study of radiotherapy combined with gemcitabine for locally advanced pancreatic cancer. Br J Cancer 91:673–677
Saif MW, Black G, Roy S et al (2007) Phase II study of capecitabine with concomitant radiotherapy for patients with locally advanced pancreatic cancer: up-regulation of thymidine phosphorylase. Cancer J 13:247–256
Shibuya K, Oya N, Fujii T et al (2011) Phase II study of radiation therapy combined with weekly low-dose gemcitabine for locally advanced, unresectable pancreatic cancer. Am J Clin Oncol 34:115–119
Moureau-Zabotto L, Phelip JM, Afchain P et al (2008) Concomitant administration of weekly oxaliplatin, fluorouracil continuous infusion, and radiotherapy after 2 months of gemcitabine and oxaliplatin induction in patients with locally advanced pancreatic cancer: a Groupe Coordinateur Multidisciplinaire en Oncologie phase II study. J Clin Oncol 26:1080–1085
Crane CH, Varadhachary GR, Yordy JS et al (2011) Phase II trial of cetuximab, gemcitabine, and oxaliplatin followed by chemoradiation with cetuximab for locally advanced (T4) pancreatic adenocarcinoma: correlation of Smad4(Dpc4) immunostaining with pattern of disease progression. J Clin Oncol 29:3037–3043
Ch’ang HJ, Lin YL, Wang HP et al (2011) Induction chemotherapy with gemcitabine, oxaliplatin, and 5-fluorouracil/leucovorin followed by concomitant chemoradiotherapy in patients with locally advanced pancreatic cancer: a Taiwan cooperative oncology group phase II study. Int J Radiat Oncol Biol Phys 81:749–757
Goldstein D, Spry N, Cummins MM et al (2012) The GOFURTGO Study: AGITG phase II study of fixed dose rate gemcitabine-oxaliplatin integrated with concomitant 5FU and 3-D conformal radiotherapy for the treatment of localised pancreatic cancer. Br J Cancer 106:61–69
Ito Y, Okusaka T, Kagami Y et al (2006) Evaluation of acute intestinal toxicity in relation to the volume of irradiated small bowel in patients treated with concurrent weekly gemcitabine and radiotherapy for locally advanced pancreatic cancer. Anticancer Res 26:3755–3759
Gillen S, Schuster T, Meyer Zum Büschenfelde C et al (2010) Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLoS Med 7:e1000267
Hammel P, Huguet F, Van Laethem JL et al (2016) Effect of chemoradiotherapy vs chemotherapy on survival in patients with locally advanced pancreatic cancer controlled after 4 months of gemcitabine with or without erlotinib: The LAP07 Randomized Clinical Trial. JAMA 315:1844–1853
Conroy T, Desseigne F, Ychou M et al (2011) FOLFIRINOX vs gemcitabine for metastatic pancreatic cancer. N Engl J Med 364:1817–1825
Von Hoff DD, Ervin T, Arena FP et al (2013) Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 369:1691–1703
Ke QH, Zhou SQ, Yang JY et al (2014) S-1 plus gemcitabine chemotherapy followed by concurrent radiotherapy and maintenance therapy with S-1 for unresectable pancreatic cancer. World J Gastroenterol 20:13987–13992
Acknowledgements
The authors would like to thank Enago (http://www.enago.jp) for the English language review.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Taketo Yamaguchi received research funding from Oncotherapy Science, Inc, Merck Serono Co., Ltd., Taiho Pharmaceutical Co., Ltd., Zeria Pharmaceutical Co., Ltd., NanoCarrier Co., Ltd., and Baxalta Japan Limited, outside the submitted work. Other authors declare no conflict of interest.
Funding
This research did not receive any specific grant from funding agencies.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. All study participants provided written informed consent and the study design was approved by the ethical review board of Chiba Cancer Center
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Sudo, K., Hara, R., Nakamura, K. et al. Phase II study of induction gemcitabine and S-1 followed by chemoradiotherapy and systemic chemotherapy using S-1 for locally advanced pancreatic cancer. Cancer Chemother Pharmacol 80, 195–202 (2017). https://doi.org/10.1007/s00280-017-3350-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-017-3350-5