Abstract
Background
Some therapeutic agents in oncology can be causally associated with specific cardiovascular events including QT/QTc interval prolongation. We investigated the effect of multiple dosing of the oral poly (ADP-ribose)-polymerase (PARP) inhibitor, olaparib (tablet formulation) on QT/QTc interval.
Methods
Two phase I, open-label, three-part studies (NCT01921140 [study 4] and NCT01900028 [study 7]) were conducted in adults with refractory/resistant advanced solid tumours. In both studies, parts A and B assessed the QT/QTc interval effects of single-dose oral olaparib 100 (study 4) or 300 (study 7) mg and multiple-dose olaparib 300 mg bid for 5 days, respectively, while part C evaluated continued access to olaparib for additional safety analyses. An ANCOVA model tested the primary objective of multiple-dose effects of olaparib on QT interval corrected using Fridericia’s formula (QTcF).
Results
Data from 119 and 109 patients were pooled from parts A and B, respectively, for QT/QTc analysis. At pre-dose and up to 12 h post-dose, the upper limits of the 90 % confidence intervals (CIs) for the difference in QTcF least squares means after olaparib multiple dosing versus control (day −1) were <10 ms, suggesting a lack of clinically relevant effect on cardiac repolarization. A slight shortening of QTcF was observed at most time points versus control. QTcF results for the individual studies and single-dose olaparib paralleled the primary multiple-dose pooled analysis, with upper limits of the 90 % CIs < 10 ms.
Conclusion
Olaparib tablets administered as multiple or single doses had no clinically significant effect on QT/QTc interval.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Olaparib (Lynparza™) is an oral poly (ADP-ribose)-polymerase (PARP) inhibitor that, by virtue of its mechanism of action, prevents single-strand DNA repair and leads to double-strand DNA breaks during replication [1, 2]. As a result, olaparib has been evaluated for targeted treatment of tumours with homologous recombination repair deficiencies such as mutations to the BRCA1 and BRCA2 genes. The antitumour efficacy of olaparib has been demonstrated in phase II trials of patients with ovarian and breast cancer [3–5]. Maintenance therapy with olaparib capsules at a dose of 400 mg twice daily (bid) was observed to significantly prolong progression-free survival (PFS; 8.4 vs. 4.8 months for placebo; P < 0.001) in patients with platinum-sensitive recurrent serous ovarian cancer, with those patients harbouring a BRCA mutation deriving the greatest benefit from olaparib treatment (11.2 vs. 4.3 months; P < 0.001) [6, 7]. To receive the recommended 400 mg bid dose of olaparib capsules, patients are required to take 16 × 50 mg large capsules per day, and consequently, patient compliance may be compromised. A tablet formulation has, therefore, been developed to deliver a therapeutic dose in fewer and smaller units. A recommended tablet dose of 300 mg bid has been determined in a phase I trial for administration in phase III clinical trials [8, 9].
Some novel oncology agents targeting critical pathways for cancer cell survival and growth have been shown to lead to cardiovascular (CV) events, including QT/QTc prolongation (delayed cardiac repolarization), hypertension and congestive heart failure [10, 11]. Prolongation of the QT/QTc interval can lead to the development of cardiac arrhythmias, most seriously torsade de pointes [12]. The QT interval is a measure of the duration of the electrical depolarization and repolarization of the heart ventricles and is evaluated from the beginning of the QRS complex (combination of the Q wave, R wave and S wave representing ventricular depolarization) to the offset of the T wave on the surface electrocardiogram (ECG) [12]. As the QT interval is dependent on heart rate, a correction factor is applied and is known as the QTc interval (QT interval corrected for heart rate), which serves as a surrogate marker for the risk of torsade de pointes [12].
The International Conference on Harmonisation (ICH) E14 guidelines have provided recommendations to enable routine evaluation of the effects of drugs on QT/QTc interval within the design of clinical studies [12]. However, while laudable, there remain concerns regarding the implementation of a ‘thorough QT/QTc analysis’ in a dedicated study (as recommended by the ICH) in oncology for drugs with toxicity levels that preclude use in healthy volunteers [13]. In these cases, alternative approaches to the thorough QT/QTc study have been proposed that take into account safety and ethical issues [13].
Preclinical studies with olaparib have not shown any QT/QTc liability or identified a risk of other adverse ECG or CV effects (unpublished data). Moreover, clinical trials of olaparib to date do not indicate any clinically important changes from baseline in ECG measurements following treatment with olaparib [3–7]. QT/QTc interval analysis was conducted in two clinical pharmacology studies (ClinicalTrials.gov NCT01921140 [referred to as study 4] and NCT01900028 [referred to as study 7]) in order to provide a more thorough analysis of the effect of multiple dosing of olaparib tablet formulation on QT/QTc interval. Herein, multiple-dose and supporting single-dose results are reported for individual studies, as well as data from a pooled analysis of both studies.
Methods
Study designs
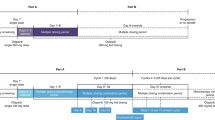
This report focuses on the QT/QTc data from the two studies; pharmacokinetic (PK) and other safety data have been reported separately [14, 15]. Both studies comprised an initial, multi-period, single-dose PK phase [part A; to address the primary objective of each study (i.e. food effect and drug–drug interactions)] followed by a multiple-dose phase (part B), which comprised the primary period for QT/QTc assessments. Across the studies, recruitment of approximately 48 patients was planned to ensure that at least 42 evaluable patients completed the study, thus resulting in a sample size of 80–100 evaluable patients. Study designs have been described previously [14, 15].
The protocol for the respective studies was reviewed and approved by the ethics committees of the participating study sites. Both studies were performed in accordance with the Declaration of Helsinki, Good Clinical Practice, and the AstraZeneca Policy on Bioethics [16]. All patients provided written informed consent prior to study entry.
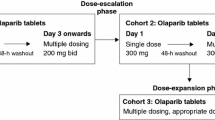
Study 4 (ClinicalTrials.gov NCT01921140; AstraZeneca study number D0816C00004) was a three-part (parts A–C) phase I, open-label, randomized, two-period crossover study in adult patients with refractory/resistant advanced solid tumours. Part A determined the effect of food on the PK of olaparib, and the effect of olaparib on QT/QTc interval was evaluated following administration of a single oral dose of olaparib tablets (300 mg [2 × 150 mg]). The effect of olaparib on QT/QTc interval following multiple oral dosing of olaparib tablets (300 mg bid) for 5 days was determined in part B, while part C allowed patients continued access to olaparib tablets to provide additional safety analyses.
Study 7 (ClinicalTrials.gov NCT01900028; AstraZeneca study number D0816C00007) was a three-part (parts A–C) phase I, open-label study in adult patients with refractory/resistant advanced solid tumours. Part A assessed the effect of itraconazole on the PK parameters of olaparib, and the effect of olaparib on QT/QTc interval following a single oral dose of olaparib tablet formulation (100 mg). A lower 100 mg single dose of olaparib was chosen to mitigate against potential risks associated with higher olaparib exposure because of the anticipated inhibitory effect of itraconazole. Part B determined the effect of olaparib on QT/QTc interval following multiple oral dosing of olaparib tablets (300 mg bid), and part C allowed patients continued access to olaparib tablets to allow for additional safety analyses.
During part B in both studies, patients received open-label treatment with olaparib tablets at 300 mg bid for 5 days after a washout period of at least 5 days (7 days in study 7), and no more than 14 days after the last dose in part A and day −1 of part B. During part B, patients self-administered olaparib in a fasted state (no food was ingested between 1 h prior to and 2 h after olaparib administration) on an outpatient basis except for the day 4 evening and day 5 morning doses, which were given at the clinic during study assessments. The day 5 morning olaparib dose was administered in an overnight-fasted state, with fasted conditions maintained until 4 h post-dose.
Patient selection
A detailed description of specific patient eligibility criteria has been previously provided for studies 4 and 7 [14, 15]. Briefly, male or female patients aged ≥18 years with solid tumours that were refractory or resistant to standard therapy, a life expectancy of at least 16 weeks, and an Eastern Cooperative Oncology Group (ECOG) performance status ≤2 were eligible for inclusion in either study. In addition, patients were required to have normal organ and bone marrow function up to 28 days before study treatment administration.
Patients with mean QTcF > 470 ms at screening, a familial history of long QT syndrome, heart failure, left ventricular dysfunction or hypokalemia were excluded from study entry, as were those taking calcium channel blockers or concomitant medications known to prolong the QT interval. Other concomitant medications were permitted if the regimen was deemed stable as defined by no change in medication or dose within 2 or 4 weeks prior to study treatment initiation in study 4 or study 7, respectively.
Electrocardiogram assessments
Digital ECG (dECG) collection was conducted using a flash card-based, digital 12-lead ECG Holter system with extractions of triplicate 10-s ECGs at each time point. QT/QTc intervals were based on the mean of triplicate dECG readings (patients rested for at least 10 min before triplicate extractions). These dECG data were time-matched for collection in part A on baseline/control day −1 and on day 1 in the olaparib fed and fasted treatment arms in study 4, or on day 1 with olaparib alone and day 9 with olaparib co-administered with itraconazole in study 7 at the following time points: pre-dose, 0.5, 1, 1.5, 2, 3, 4, 6, 8, 12 and 24 h after olaparib dosing. In both studies, dECG data were extracted at similar time-matched intervals on day −1 (baseline/control) and day 5 during multiple-dose evaluation in part B.
The continuous Holter recordings were sent to a central ECG laboratory (eResearch Technology [ERT], Peterborough, UK), where the triplicate dECG extraction and analysis were performed. A single assessor fully blinded to time, treatment and sequence read the ECGs from one patient centrally. Any clinically significant abnormal ECG findings were recorded as adverse events (AEs).
Pharmacokinetic assessment
Blood samples were taken to investigate the relationship between QT/QTc interval and olaparib concentration at each ECG monitoring period up to 24 h post-dose in parts A and B of studies 4 and 7. In addition, PK samples were collected 48 (day 3) and 72 (day 4) h post-dose in part A of both studies. After collection, samples were processed and shipped to Covance (Harrogate, UK) for analysis.
Statistical analyses
Digital ECG data were analysed by study and as a pooled dataset using the QT analysis set, which was defined as all patients who had at least one evaluable time-matched QT/QTc interval value at a scheduled time point in part A or part B. The safety analysis set comprised all patients who received at least one dose of olaparib.
ECG evaluations comprised the QT interval and QT corrected using Fridericia’s correction (QTcF). Secondary analysis was performed based on individually corrected QT (QTcI). Heart rate, PR interval (the time from the onset of the P wave to the start of the QRS complex) and QRS interval data were also summarized.
The primary objective was to investigate the effect of multiple doses of olaparib on QTcF interval, with supportive analyses to investigate the effect of single doses on QTcF. Effects of single and multiple doses of olaparib on QTcI intervals were also evaluated as supportive analyses. In addition, a modelled exposure–QT response analysis was performed to assess the relationship between change from baseline in QTc (ΔQTc) and olaparib concentrations. This relationship was then used to predict the magnitude of QTc prolongation that could be expected allowing for the range of maximum plasma concentrations achieved with the 300 mg bid tablet dose.
Analysis of effect of olaparib on QTc
An ANCOVA model with factors for effect of study, treatment (multiple-dose olaparib/control) and time, interaction terms between study and time, and between treatment and time, and a random effect for patient within study was fitted to part B pooled data to assess the change in time-matched QTcF from control (day −1; ∆QTcF). This analysis is similar to that detailed in the ICH E14 guidelines for the clinical evaluation of QT/QTc data, but accounts for the fact that the pre-dose (time zero) measurements in part B were made following 4 days of dosing on olaparib [12]. Therefore, for the multiple-dose setting, the analysis did not include baseline (i.e. the ‘on treatment’ time zero measurement) as a correction factor but instead included it as an additional time point in the model. The ANCOVA model was used to assess the effect of multiple doses of olaparib on the QTcF by calculating the difference in least squares (LS) means (olaparib QTcF interval LS mean at time t minus control QTcF interval LS mean at time t; i.e. change from time-matched control at day −1 of part B) and two-sided 90 % CIs. Validity of the ANCOVA model was assessed by checking distributional assumptions of the data using a residual plot and a quantile‒quantile plot. Lack of a clinically relevant effect on cardiac repolarization, as detected by QTcF prolongation, was concluded if the upper bound of the 90 % CI around the mean effect was <10 ms for the contrast multiple-dose olaparib versus control for every time point. Similarly, lack of a clinically relevant effect on QTcF shortening was concluded if the lower bound of the 90 % CI was not less than 10 ms below zero for all time points.
QTcF assumes that the slope of the relationship between QT and RR (duration of a heart beat) on a natural logarithm scale is 1/3, i.e. QTcF = (QT/RR)1/3. Deviation from 1/3 can introduce bias in the estimates of treatment effect. If QTcF was not found to be the most appropriate correction for the data, it was planned to analyse the secondary variable QTcI using the same model as for the primary analysis with QTcF to check the consistency of the results. QTcI was calculated using the formula QTcI = QT/RRβi, where βi is the individual-specific correction factor assessed via linear regression models.
In a supportive analysis of the effect of single dosing of olaparib on QTc, an ANCOVA model using similar factors to the primary analysis was fitted to pooled data from part A (data were pooled for: the fasted arm in study 4 and the olaparib-alone arm in study 7; and the fed arm in study 4 and olaparib co-administered with itraconazole in study 7) and compared with control. As the pre-dose (time zero) measurements on each dosing day in part A were taken prior to any dosing with olaparib or after the appropriate washout period, this analysis assessed the effect on change from pre-dose in QTcF and additionally included a covariate for the pre-dose (time zero) measurement (i.e. was baseline-corrected).
Modelling of relationship between ∆QTc and olaparib concentration
For both QTcF and QTcI, day −1 (control)-corrected ∆QTc (∆∆QTc) was used as the endpoint for PK/PD (pharmacodynamic) modelling of the potential relationship between ∆QTc interval and olaparib concentration, according to models based on ICH E14 guidance [17, 18]; gender was used as a covariate in the analysis. Modelling was performed using the software package NONMEM, version 7.2 (ICON, Hanover, MD, USA), with the choice of model guided by exploratory graphical analysis of the data (linear mixed-effect model, nonlinear mixed-effect model of E max type and log-linear model).
The linear mixed-effect model was defined according to the equation:
where θ 1 and θ 2 are the population mean intercept and slope, respectively, η 1 and η 2 are the IIV of θ 1 and θ 2, respectively, Conc is the olaparib concentration, and ε is the residual error. η 1, η 2 and ε are random variables and are normally distributed with a mean of 0 and a variance of ω 21 , ω 22 and σ 2, respectively (i.e. η 1 ~ N[0, ω 21 ], η 2 ~ N[0, ω 22 ] and ε ~ N[0, σ 22 ]).
The nonlinear, mixed-effect model of the E max type was defined as follows:
where θ 1 is population mean intercept, θ 2 is maximum ∆∆QTc interval prolongation (E max), and θ 3 is the concentration to achieve half of E max. Variables η 1, η 2 and η 3 are the IIV of θ 1, θ 2 and θ 3, respectively, and ε is the residual error. η 1, η 2, η 3 and ε are random variables and are normally distributed with a mean of 0 and a variance of ω 21 , ω 22 , ω 23 and σ 2, respectively (i.e. η 1 ~ N[0, ω 21 ], η 2 ~ N[0, ω 22 ], η 3 ~ N[0, ω 23 ] and ε ~ N[0, σ 22 ]).
Other models were also explored, including the log-linear model, which was defined as follows:
where θ 1 and θ 2 are the population mean intercept and slope, respectively, η 1 and η 2 are the IIV of θ 1 and θ 2, respectively, and ε is the residual error.
For validation, the final model was required to fulfil certain acceptance criteria: the estimation and covariance step terminated without error messages; 95 % CIs of each estimated parameter do not contain their null value; significant digits average 3.0 or higher; all gradients at the last iteration are reasonably small; and the correlation between model parameters is less than 0.95. A battery of diagnostic plots were also generated for validation, to ensure that models performed adequately.
A statistically significant estimate of the slope (θ 2) from the linear mixed model or the maximum change in QTc interval prolongation from an E max or sigmoid E max model indicated potential drug-related QTc interval prolongation. A potential QTc interval prolongation with regulatory concern for a linear relationship for QT study is defined as the predicted interval at the mean maximum plasma concentration (C max) of olaparib dose given in the three different parts of studies 4 and 7:
where SE (θ 2) is the standard error of the slope (θ 2) and C max,mean is the mean maximum drug concentration [18]. The C max,mean of olaparib was used in both equations. The concentration‒QTc relationship was used to predict the magnitude of QTc prolongation.
Results
Patient characteristics
In studies 4 and 7, 60 and 59 patients received olaparib and completed part A, respectively. Overall, 56 and 53 patients completed part B in studies 4 and 7, respectively. A total of 119 and 109 patients were included in the pooled QT/QTc analysis for parts A and B, respectively.
Patient demographics and baseline characteristics were comparable between studies and are summarized in Table 1. In both studies, patients were over 60 years of age, on average, and were mainly female and Caucasian. In approximately one-third of the patient population in studies 4 and 7, the ovary was the primary tumour site.
Important protocol deviations with potential to affect part A single-dose and part B multiple-dose QT assessment periods were documented in three and six patients in study 4, respectively, and five and nine patients in study 7, respectively. In these patients (more than one violation may have occurred per patient), protocol violations comprised previous gastric surgery or banding (n = 4), ingestion of disallowed medication (n = 8), vomiting post-olaparib dose (n = 1), not taking study treatment according to the protocol (n = 4) and incorrect enrolment of a patient with a QTcF reading >470 ms at screening (n = 1).
No evidence of an effect of multiple doses of olaparib on QTc
For all time points, the upper limit of the 90 % CI was <10 ms, indicating the absence of a clinically relevant effect of olaparib on cardiac repolarization. The LS mean QTcF interval and change from control at each ECG assessment time point for the pooled data are reported in Fig. 1 and Table 2.
There was a slight shortening of QTcF at all time points, except at 4 h post-dose, where there was no change compared with control. However, the lower limits of the 90 % CI were >−10 ms for all time points. In order to test the assumption that the treatment effect was similar in both studies, the interaction between study and treatment was assessed. A statistically significant interaction between treatment and study was observed at the 5 % level for multiple-dose data (P = 0.0098), and the primary analysis was, therefore, repeated for each individual study separately to check for consistency of results. Results of the separate study analyses of QTcF data were consistent with the primary data (Tables 4, 5 in “Appendix”), with the upper limits of the 90 % CIs noted as <10 ms for all time points. Results of sensitivity analyses excluding patients with important protocol deviations were consistent with the primary QTcF analysis for both the combined and individual studies.
The relationship between ln(QT) and ln(RR) was investigated. The median of the slopes from the linear regression models of QT was found to be greater than 1/3; therefore, a secondary analysis of QTcI was performed. This analysis of QTcI confirmed the absence of a clinically relevant effect of multiple-dose olaparib on the primary analysis of cardiac repolarization, with the upper limits of the 90 % CI being <10 ms in all cases (Fig. 2).
No evidence of an effect of single doses of olaparib tablets on QTc
The effect of single-dose olaparib on the QTcF interval was assessed as a supportive analysis for two pooled populations (the fasted arm in study 4 combined with the olaparib-alone arm in study 7 and the fed arm in study 4 combined with olaparib co-administered with itraconazole in study 7) compared with control. For both pooled populations, there was no evidence of an effect of olaparib on QTc; the upper limit of the 90 % CI was < 10 ms for all time points.
Sensitivity analyses excluding patients with important protocol deviations that could have potentially affected the dECG data paralleled the primary QTcF analysis for the pooled populations. Moreover, as for multiple-dose data, secondary analysis of QTcI was performed. In all cases, the upper limit of the two-sided 90 % CI was <10 ms, indicating the absence of a clinically relevant effect of single- or multiple-dose olaparib on cardiac repolarization.
Concentration–QTc analysis
Findings from the pooled analysis and individual studies 4 and 7 showed that there was no obvious correlation between olaparib plasma concentration and ΔΔQTcF (Fig. 3).
Results of the pooled analysis showed that the relationship of the change from baseline in ΔΔQTcF with olaparib plasma concentration was best described by a linear model with a coefficient of 0.238 (95 % CI 0.033, 0.445) ms·(µg/mL)‒1 and an intercept of −1.25 (95 % CI −2.32, −0.18) ms. Furthermore, findings from pooled analysis data for change from baseline in ΔΔQTcI with olaparib plasma concentration were best described by the baseline value and a log-linear model of the olaparib plasma concentration with a value of 0.86 (95 % CI 0.18, 1.54) ms·(µg/mL)‒1 and an intercept of −2.18 (95 % CI −3.59, −0.77) ms. The pooled analysis included a food effect study (study 4). One of the findings of the pooled analysis was that food was likely to have altered the baseline of the PK/PD model; a negative baseline value was found in the model likely due to the impact of food, suggesting a difference in baseline values between the fasted and fed groups.
Predictions of the magnitude of effect of olaparib, allowing for the range of maximum plasma concentrations achieved following the 300 mg bid tablet dose, indicated no clinical impact of olaparib on ΔΔQTcF or ΔΔQTcI (Table 3).
Additional QT data
One patient (0.9 %) in the single olaparib dose fasted/alone analysis (combined data from studies 4 and 7) and one patient (1.0 %) in the multiple-dose (combined data) part B analysis recorded an absolute QTcF value of >480 ms. No patients recorded an absolute QTcF of >500 ms.
No patients in the multiple-dose assessments had an increase in QTcI of >30 ms, and no patients had increases in QTcF of >60 ms compared with time-matched measurements on day −1 (control).
Olaparib did not have any clinically relevant effects on PR and QRS interval or heart rate, as assessed by central tendency by time point analyses and the analysis of outliers (data not shown).
Safety
Adverse events experienced by patients participating in studies 4 and 7 have been reported in detail previously, and no new safety findings were observed [14, 15]. Therefore, only CV AEs are included here. One CV AE was reported in a 60-year-old male from study 4 who experienced Common Terminology Criteria for Adverse Events version 3.0 (CTCAE) grade 1 tachycardia during the fasted state. Four patients reported five CV AEs in study 7, which were CTCAE grade 1 atrioventricular block and grade 1 sinus bradycardia in one patient, grade 3 atrial fibrillation (n = 1), grade 1 tachycardia (n = 1), and grade 3 cardiac failure (n = 1). The event of cardiac failure led to study discontinuation.
Discussion
The potential for non-antiarrhythmic drugs to induce QT/QTc interval prolongation has led to the development of international harmonized recommendations supporting thorough QT/QTc analyses of novel targeted agents [12, 13]. Due to ethical issues in conducting a thorough QT/QTc study of some oncology agents in healthy patient populations, modifications in study design have been proposed [13]. Accordingly, two three-part clinical pharmacology studies were conducted in patients with refractory/resistant advanced solid tumours to assess the effect of multiple-dose olaparib tablet formulation on QT/QTc interval, with single-dose assessments undertaken as supportive analyses.
As with other oncology studies, a thorough QT/QTc analysis, defined by the ICH E14 guidance [12], was not feasible owing to safety and ethical reasons. While the design of studies 4 and 7 may have been subject to some methodological limitations, our studies were designed to rigorously investigate the QT interval (including sensitivity analyses, time window-matched dECG assessments and data analysis in a central ECG laboratory) with results analysed separately and pooled to provide a total dataset of over 100 patients.
Previously published PK data showed that food reduced olaparib exposure, whereas the antifungal agent, itraconazole, increased olaparib exposure [14, 15]. A previous study specifically evaluating the effects of food on QT reported that food itself may shorten the QTc interval and PR intervals for at least 4 h after ingestion [19]. Studies have also reported prolongation effects of itraconazole on QT interval, although these reports are few in number and largely reflect the potential for itraconazole to affect serum concentrations of agents known to directly affect QT prolongation [20–23]. For these reasons, to eliminate any potential food or concomitant drug exposure effects in the present study, the primary analysis of the potential effect of olaparib on QT interval was conducted when olaparib was taken in the fasted state and following a washout period of at least 5 days (7 days for patients in the itraconazole study).
Overall, the primary analyses and sensitivity analysis of the pooled multiple-dose olaparib data indicate that multiple dosing of olaparib at the dosage utilized in phase III monotherapy trials (300 mg bid) does not exert a clinically significant effect on cardiac repolarization. Findings from the individual studies were consistent with that shown for the pooled analysis. Data from the supportive single-dose analyses may have been potentially confounded by the effect of food, itraconazole, or the different single doses of olaparib (100 and 300 mg) in the individual studies. Regardless of these potential confounders, the QT results from the single-dose analyses supported the primary analysis for multiple-dose olaparib. Thus, across all analyses, the upper limit of the two-sided 90 % CI around the mean treatment effect was < 10 ms, indicating a lack of clinically relevant effects of olaparib on cardiac repolarization.
Shortening of QTcF was observed pre-dose in the absence of olaparib exposure, suggestive of normal variation in heart rate on the QT/QTc interval or diurnal variation of the QT/QTc interval due to the influence of the autonomic system (occurring independently of heart rate), as previously proposed in a study that included patients with fitted pacemakers who had normally innervated hearts [24]. In these patients, pronounced diurnal variation in the QT interval was noted over 24 h of evaluation, with QT intervals shortening during the day and lengthening in the evening to result in an average difference of 23 ms between waking and sleeping hours [24]. By time-matching the QT assessments in the present studies, the potential confounding effects of such diurnal variation were controlled for.
In both studies, dECG evaluations showed no clinically relevant effect of olaparib on PR and QRS intervals or heart rate in either the single (part A)- or multiple (part B)-dose periods of the studies. These observations are consistent with previous phase II studies of patients with ovarian or breast cancer, which did not indicate any clinically important changes from baseline in ECG measurements following olaparib treatment [3–5].
The concentration–effect model was used to support the primary statistical analyses and allow the potential confounding influence of dose, food or itraconazole to be addressed. Results from the pooled data used in the concentration–effect model to determine the relationship between increasing olaparib plasma concentrations and ΔΔQTcF indicated that only a small change in QTcF occurred for every µg/mL increment in olaparib plasma concentration. Similar findings were noted for the effect of increasing olaparib plasma concentrations on ΔΔQTcI. The finding that food altered the baseline of the model is in agreement with the literature regarding shortening of the QT interval after food administration [19]. From the predicted relationships identified in the concentration–effect models, the maximum range of olaparib drug plasma concentration reached with multiple dosing would not be expected to cause prolongation of the QTc interval of a magnitude that would be of clinical concern or that would cross the threshold of regulatory concern, as set out in the ICH E14 guidelines [12].
In conclusion, the results from these two clinical pharmacology studies conducted in patients with refractory/resistant advanced solid tumours demonstrate that olaparib (when given as tablet formulation) had no clinically relevant effect on QT/QTc interval when administered as either a single dose or as multiple doses at the 300 mg bid dosage being utilized in phase III monotherapy trials in platinum-sensitive relapsed ovarian cancer and other solid tumour types.
References
Evers B, Drost R, Schut E, de Bruin M, van der Burg E, Derksen PW, Holstege H, Liu X, van Drunen E, Beverloo HB, Smith GC, Martin NM, Lau A, O’Connor MJ, Jonkers J (2008) Selective inhibition of BRCA2-deficient mammary tumor cell growth by AZD2281 and cisplatin. Clin Cancer Res 14:3916–3925
Rottenberg S, Jaspers JE, Kersbergen A, van der Burg E, Nygren AO, Zander SA, Derksen PW, de Bruin M, Zevenhoven J, Lau A, Boulter R, Cranston A, O’Connor MJ, Martin NM, Borst P, Jonkers J (2008) High sensitivity of BRCA1-deficient mammary tumors to the PARP inhibitor AZD2281 alone and in combination with platinum drugs. Proc Natl Acad Sci USA 105:17079–17084
Audeh MW, Carmichael J, Penson RT, Friedlander M, Powell B, Bell-McGuinn KM, Scott C, Weitzel JN, Oaknin A, Loman N, Lu K, Schmutzler RK, Matulonis U, Wickens M, Tutt A (2010) Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer: a proof-of-concept trial. Lancet 376:245–251
Tutt A, Robson M, Garber JE, Domchek SM, Audeh MW, Weitzel JN, Friedlander M, Arun B, Loman N, Schmutzler RK, Wardley A, Mitchell G, Earl H, Wickens M, Carmichael J (2010) Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: a proof-of-concept trial. Lancet 376:235–244
Gelmon KA, Tischkowitz M, Mackay H, Swenerton K, Robidoux A, Tonkin K, Hirte H, Huntsman D, Clemons M, Gilks B, Yerushalmi R, MacPherson E, Carmichael J, Oza A (2011) Olaparib in patients with recurrent high-grade serous or poorly differentiated ovarian carcinoma or triple-negative breast cancer: a phase 2, multicentre, open-label, non-randomised study. Lancet Oncol 12:852–861
Ledermann J, Harter P, Gourley C, Friedlander M, Vergote I, Rustin G, Scott C, Meier W, Shapira Frommer R, Safra T, Matei D, MacPherson E, Watkins C, Carmichael J, Matulonis U (2012) Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer. N Engl J Med 366:1382–1392
Ledermann J, Harter P, Gourley C, Friedlander M, Vergote I, Rustin G, Scott CL, Meier W, Shapira-Frommer R, Safra T, Matei D, Fielding A, Spencer S, Dougherty B, Orr M, Hodgson D, Barrett JC, Matulonis U (2014) Olaparib maintenance therapy in patients with platinum-sensitive relapsed serous ovarian cancer: a preplanned retrospective analysis of outcomes by BRCA status in a randomised phase 2 trial. Lancet Oncol 15:852–861
Mateo J, Friedlander M, Sessa C, Leunen K, Nicum S, Gourley C, Fielding A, Bowen K, Kaye S, Molife LR (2013) Administration of continuous/intermittent olaparib in ovarian cancer patients with a germline BRCA1/2 mutation to determine an optimal dosing schedule for the tablet formulation. Eur J Cancer 49(Suppl 2):abst 801
Molife LR, Mateo J, McGoldrick T, Krebs M, Drew Y, Banerjee SN, Nicum S, Ranson M, Rustin GJ, Sessa C, Plummer R, Leunen K, Friedlander M, Swaisland H, Burke W, McCormack P, Pemberton K, Tchakov I, Kaye SB, Gourley C (2012) Safety and efficacy results from two randomized expansions of a phase I study of a tablet formulation of the PARP inhibitor, olaparib, in ovarian and breast cancer patients with BRCA1/2 mutations. J Clin Oncol 30(15S):abst 3048
Hedhli N, Russell KS (2011) Cardiotoxicity of molecularly targeted agents. Curr Cardiol Rev 7:221–233
Bagnes C, Panchuk PN, Recondo G (2010) Antineoplastic chemotherapy induced QTc prolongation. Curr Drug Saf 5:93–96
ICH Harmonised Tripartite Guideline. The clinical evaluation of QT/QTc interval prolongation and proarrhythmic potential for non-antiarrhythmic drugs E14. 2005. http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E14/E14_Guideline.pdf
Rock EP, Finkle J, Fingert HJ, Booth BP, Garnett CE, Grant S, Justice RL, Kovacs RJ, Kowey PR, Rodriguez I, Sanhai WR, Strnadova C, Targum SL, Tsong Y, Uhl K, Stockbridge N (2009) Assessing proarrhythmic potential of drugs when optimal studies are infeasible. Am Heart J 157(827–36):836
Plummer R, Swaisland H, Leunen K, van Herpen CM, Jerusalem G, De Grève J, Lolkema MP, Soetekouw P, Mau-Sorensen M, Nielsen D, Spicer J, Fielding A, So K, Bannister W, Molife LR (2015) Olaparib tablet formulation: effect of food on the pharmacokinetics after oral dosing in patients with advanced solid tumours. Cancer Chemother Pharmacol 76:723–729
Plummer R, Verheul H, Rottey S, Leunen K, Jerusalem G, Rolfo C, Nielsen D, Molife R, Kristeleit R, de Vos-Geelen J, Mau-Sørensen M, Soetekouw P, van Herpen C, Swaisland H, Fielding A, So K, Bannister W, Dirix L (2015) Effect of itraconazole and rifampin on the pharmacokinetics of olaparib tablet formulation in patients with advanced solid tumours: a Phase 1 open label study. J Clin Oncol 33(Suppl):abst 2565
AstraZeneca. Global Policy: Bioethics. 2015. Available at: https://www.astrazeneca.com/sustainability/responsible-research.html
Piotrovsky V (2005) Pharmacokinetic-pharmacodynamic modeling in the data analysis and interpretation of drug-induced QT/QTc prolongation. AAPS J 7:E609–E624
Garnett CE, Beasley N, Bhattaram VA, Jadhav PR, Madabushi R, Stockbridge N, Tornøe CW, Wang Y, Zhu H, Gobburu JV (2008) Concentration–QT relationships play a key role in the evaluation of proarrhythmic risk during regulatory review. J Clin Pharmacol 48:13–18
Taubel J, Wong AH, Naseem A, Ferber G, Camm AJ (2012) Shortening of the QT interval after food can be used to demonstrate assay sensitivity in thorough QT studies. J Clin Pharmacol 52:1558–1565
Iribarren C, Round AD, Peng JA, Lu M, Zaroff JG, Holve TJ, Prasad A, Stang P (2013) Validation of a population-based method to assess drug-induced alterations in the QT interval: a self-controlled crossover study. Pharmacoepidemiol Drug Saf 22:1222–1232
Bedford TA, Rowbotham DJ (1996) Cisapride. Drug interactions of clinical significance. Drug Saf 15:167–175
Honig PK, Wortham DC, Hull R, Zamani K, Smith JE, Cantilena LR (1993) Itraconazole affects single-dose terfenadine pharmacokinetics and cardiac repolarization pharmacodynamics. J Clin Pharmacol 33:1201–1206
Cruccu V, Pedretti D, Confalonieri F (1995) A case of pulmonary aspergillosis effectively treated with itraconazole. Possible interaction of the antimycotic agent with hydroquinidine. Clin Ter 146:383–389
Bexton RS, Vallin HO, Camm AJ (1986) Diurnal variation of the QT interval–influence of the autonomic nervous system. Br Heart J 55:253–258
Acknowledgments
The authors would like to thank the patients that participated in the study. This study was sponsored by AstraZeneca. Medical writing assistance was provided by Claire Routley PhD from Mudskipper Business Ltd, funded by AstraZeneca.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
H.S., K.S., S.G., M.-A.F., C.D. and A.F. are/were employees of AstraZeneca. H.S., S.G., C.D. and A.F. own stock in AstraZeneca. RP has received honoraria from AstraZeneca for a consulting role. WB is a contractor for AstraZeneca.
Additional information
Helen Swaisland and Marc-Antoine Fabre were employees of AstraZeneca when the study was conducted.
Appendix
Appendix
Rights and permissions
About this article
Cite this article
Swaisland, H., Plummer, R., So, K. et al. Olaparib does not cause clinically relevant QT/QTc interval prolongation in patients with advanced solid tumours: results from two phase I studies. Cancer Chemother Pharmacol 78, 775–784 (2016). https://doi.org/10.1007/s00280-016-3124-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-016-3124-5