Abstract
Background
The oral PARP inhibitor olaparib has shown efficacy in patients with BRCA-mutated cancer. This Phase I, open-label, three-part study (Parts A–C) in patients with advanced solid tumours evaluated the effect of food on the pharmacokinetics (PK) of olaparib when administered in tablet formulation.
Methods
PK data were obtained in Part A using a two-treatment period crossover design; single-dose olaparib 300 mg (two 150 mg tablets) was administered in two prandial states: fasted and fed. In Part B, patients received olaparib tablets (300 mg bid) for 5 days under fasting conditions; in Part C, patients were allowed continued access to olaparib. Safety was assessed throughout, with data reported for Parts A and B.
Results
A total of 60 and 56 patients were evaluable for safety and PK analyses, respectively; 57 patients entered Part B. Rate of olaparib absorption was slower in the presence of food (t max delayed by 2.5 h), resulting in a statistically significant ~21 % decrease in peak plasma exposure (C max) [ratio of geometric means (90 % CI), 0.79 (0.72, 0.86)] but only a marginal increase in olaparib absorption (AUC0–∞) [ratio of geometric means (90 % CI), 1.08 (1.01, 1.16)]. The point estimate and 90 % CI for the AUC0–∞ treatment ratio were within pre-defined bioequivalence limits (0.80–1.25). Adverse event data were consistent with the known safety profile of olaparib.
Conclusions
Results of this study showed that a high-fat meal decreases the rate of absorption and peak exposure to olaparib 300 mg tablets, although in the absence of an effect on the extent of olaparib absorption.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The oral poly(ADP-ribose) polymerase (PARP) inhibitor, olaparib (Lynparza™), blocks base excision repair by binding PARP at sites of DNA damage, leading to synthetic lethality, most notably in tumour cells with deficiencies in homologous recombination repair, such as BRCA1/2 mutations [1, 2]. Olaparib is the most studied PARP inhibitor [3], with results from several Phase II studies showing that olaparib (capsule formulation) monotherapy demonstrated antitumour activity in patients with breast and ovarian cancer who harboured germline BRCA1/2 (gBRCA1/2) mutations [4–8]. In patients with platinum-sensitive recurrent serous ovarian cancer (PSR SOC), maintenance monotherapy with olaparib (capsule formulation) significantly prolonged progression-free survival (PFS) versus placebo [9]. Subsequent analysis of this patient population has shown that patients with a BRCA mutation (BRCAm) receive greater treatment benefit [10].
Olaparib can be administered as either a capsule or a tablet formulation. The capsule formulation of olaparib has been shown to be well tolerated at doses ≤400 mg twice daily (bid) [4–6, 9, 11]. In December 2014, the European Commission (EC) granted marketing authorization for olaparib capsules (400 mg bid) as the first therapy for the maintenance treatment of adult patients with platinum-sensitive relapsed BRCA-mutated (germline and/or somatic) high-grade serous epithelial ovarian, fallopian tube, or primary peritoneal cancer who are in complete response or partial response to platinum-based chemotherapy. In addition, the US Food and Drug Administration (FDA) approved olaparib capsules (400 mg bid) as the first monotherapy for patients with deleterious or suspected deleterious germline BRCA-mutated (gBRCAm) advanced ovarian cancer, as detected by a FDA-approved companion diagnostic test (BRACAnalysis CDx™), who have been treated with three or more prior lines of chemotherapy. In order to receive a 400 mg bid dose of the olaparib capsule formulation, patients are required to take 8 × 50 mg large capsules (size 0) twice a day. A tablet formulation has, therefore, been developed to facilitate delivery of olaparib doses in fewer, smaller units. However, the capsule and tablet formulations are not bioequivalent [12]. Results of a Phase I trial identified 300 mg (bid) olaparib as the recommended tablet dose for Phase III studies in BRCA-mutated ovarian cancer, including the maintenance therapy setting [13, 14]. For any oral therapy, it is important to evaluate the effect of food on the pharmacokinetics (PK) of the drug. This Phase I study evaluated the effect of food on the PK of olaparib following oral dosing of the 300 mg tablet formulation in patients with advanced solid tumours.
Methods
Study design
This was a Phase I, open-label, randomized, two-period, crossover study conducted in adult patients with refractory/resistant advanced solid tumours (ClinicalTrials.gov NCT01921140). The study consisted of three parts (Parts A–C). Part A assessed the effect of food on the PK of olaparib and the effect of olaparib on cardiac repolarization (assessed by change in QT interval) following a single oral dose of olaparib tablets (300 mg); Part B determined the effect of olaparib on cardiac repolarization following multiple oral dosing of olaparib tablets (300 mg bid) under fasting conditions, and Part C (ongoing) allowed patients continued access to olaparib tablets (300 mg bid) to provide additional long-term safety data. We report here on the results of the effect of food on olaparib PK in Part A, and safety assessments in Parts A and B. QT analyses from Parts A and B will be reported separately (manuscript in preparation), and Part C multiple-dose safety data will be reported once completed. The institutional review boards or independent ethics committees of all investigational sites approved the protocol. The study was performed in accordance with the Declaration of Helsinki, Good Clinical Practice, and the AstraZeneca policy on Bioethics [15].
Part A was a randomized, open-label, two-treatment period crossover design. Each patient received a single oral dose of olaparib 300 mg (two 150 mg tablets) in each of two prandial states: once in the overnight fasted state and once immediately following a high-fat meal at breakfast time. Doses were separated with a 5–≤14 day washout. For the fasted treatment state, patients were fasted from ≥10 h before olaparib dosing and until 4 h post-dose. Water was allowed except for 1 h before and after olaparib administration. For the fed treatment state, patients were fasted from ≥10 h before receiving a high-fat meal which was to be eaten, in full, within 30 min (patients were still considered evaluable if they had consumed at least 75 % of the meal within 45 min). Olaparib was administered 30 min after the start of the meal (or a maximum of 45 min if the meal was not completed), after which patients were fasted until 4 h post-dose. Water was allowed except for 1 h before and after olaparib administration. In accordance with the US Food and Drug Administration (FDA) guidance (Food and Drug Administration 2002) [16], the high-fat meal had a total calorie content of approximately 800–1000 kcal, with approximately 50 % of the calorie content derived from fat. The meal derived approximately 150, 250, and 500–600 kcal from protein, carbohydrate, and fat, respectively.
Part B was an open-label design in which patients received olaparib tablets 300 mg bid for 5 days. Patients underwent a 5- to 14-day washout period between the last dose of olaparib in Part A and day-1 of Part B. Patients received olaparib under fasted conditions (from 1 h prior to 2 h after dosing) except on the day 5 morning dose, where they remained fasting for 4 h post-dose.
Patient selection
Eligible patients were aged ≥18 years and had a confirmed (histologically or, where appropriate, cytologically) malignant solid tumour refractory or resistant to standard therapy and for which no suitable standard therapy exists. Healthy volunteers could not be used for this study as preclinical toxicology data (unpublished) preclude the use of olaparib in this population.
Patients needed to be able to eat a high-fat meal within a 30-min period and have had no changes to their concomitant medication in the 2 weeks prior to the start of olaparib treatment. Patients also needed to have a life expectancy of ≥16 weeks, an Eastern Cooperative Oncology Group (ECOG) performance status ≤2, and have adequate organ and bone marrow function measured within 28 days prior to administration of olaparib. Patients were excluded if they were unable to swallow orally administered medication, if they had disorders likely to interfere with absorption of olaparib, and if they had a previous gastrointestinal resection. Concomitant medication with inhibitors or inducers of CYP3A4 was not permitted.
Study objectives
The primary objective was to evaluate the effect of food on the PK of olaparib following oral dosing of the tablet formulation in patients with advanced solid tumours. Secondary objectives included assessment of the safety and tolerability of olaparib tablets.
Food effect pharmacokinetic assessment
In Part A, blood samples were taken for olaparib analysis pre-dose and at 0.25, 0.5, 1, 1.5, 2, 3, 4, 6, 8, 12, 24, 48, and 72 h post-dose. Blood samples were centrifuged at 4 °C for 10 min and at 1500 g within 30 min of collection, to provide plasma samples for olaparib analysis. These were stored at ≤−20 °C and transported to Covance (Harrogate, UK), where concentrations of olaparib were determined by solid-phase extraction and analysis using reversed-phase high-performance liquid chromatography (HPLC) with turbo ion spray tandem mass spectrometric (MS)/MS detection (positive ion mode) [17].
PK parameters were determined using standard, non-compartmental analyses. The PK parameters determined for olaparib in each treatment period were maximum plasma concentration (C max), area under the plasma concentration time curve from zero (pre-dose) to infinity (AUC0–∞), AUC from zero to time of last quantifiable sample (AUC0–t ), time to maximum plasma concentration (t max), terminal half-life (t ½), terminal rate constant (λ z), apparent clearance (CL/F), and apparent volume of distribution (V z/F). PK computations were performed using Phoenix™ for WinNonlin version 6.3.
Safety assessment
Patients were monitored for adverse events (AEs) throughout the study periods—data are presented here for the safety assessment in Parts A and B of the trial. AEs were graded according to the National Cancer Institute Common Terminology Criteria (NCI-CTC) version 4. All serious AEs (SAEs) and AEs related to treatment were followed to resolution. Clinical laboratory, vital signs, and physical examination parameters were also evaluated. All safety analyses were descriptive and presented for the safety analysis set, defined as all patients who received at least one dose of olaparib.
Statistical analyses
The study sample size was based on providing an estimate of the difference between olaparib PK parameters in the fed and fasted states. Power calculations showed that if this difference was minimal, 42 evaluable patients (21 per sequence) would provide 90 % power to show whether the 90 % confidence intervals (CIs) for the food effect (ratio of geometric least-squares means of AUC0–∞ or C max in the fed state to the fasted state) would be entirely within the bioequivalence boundaries of 0.8–1.25, i.e. would rule out a 20 % change in the log-transformed AUC0–∞ or C max. Recruitment of approximately 48 patients was therefore planned to ensure 42 evaluable patients completed the study. The PK analysis set included all patients who received at least one dose of olaparib and had evaluable PK profiles in either the fed or fasted state. The safety analysis set includes all patients who entered the study and received at least one dose of olaparib.
Following log-transformation, the PK parameters AUC0–∞ and C max were analysed separately using a mixed-effect analysis of variance model allowing for treatment (food condition: fasted or high-fat meal), sequence and treatment period; patient within sequence was treated as a random effect in the model. Point estimates and adjusted 90 % CIs for the difference in treatment (fasted or high-fat meal) were constructed and then exponentially back-transformed to provide point and CI estimates for the ratio of interest (i.e. C max or AUC0–∞ of olaparib following the high-fat meal to C max or AUC0–∞ of olaparib in the fasted state). No effect of food on the PK of olaparib was concluded if the two-sided 90 % CIs for the ratios of AUC0–∞ and C max were within the range of 0.80–1.25. A Hodges–Lehmann median estimator of difference and 90 % CI were calculated for t max.
All summaries and statistical analyses were performed using SAS® version 8.1.
Results
A total of 80 patients were enrolled (provided informed consent) at 11 sites in four countries: Belgium (three sites), Denmark (two sites), the Netherlands (three sites), and the UK (three sites). Of these, 60 patients were randomized (18 patients did not fulfil eligibility criteria; one patient withdrew due to an AE, and one patient decided not to continue with the study) and completed Part A of the study; 60 and 56 patients were included in the safety (Parts A and B) and PK populations, respectively. Four patients were excluded from the PK population: n = 3, due to previous gastric surgery; n = 1, due to incomplete PK sampling during the fasted period and not fulfilling the meal consumption criteria as defined in the protocol.
Of the 56 patients in the PK population, a total of 55 and 54 patient profiles were evaluable for the PK analysis from the fasted and fed arms, respectively (PK parameters could not be determined for a small number of patients as a result of incomplete sampling). All patients randomized to treatment completed Part A, with 57 patients entering Part B (of the three patients who did not enter Part B; one died as a result of progressive disease (PD); one developed rapid PD; and one became ineligible to continue into Part B). One patient discontinued Part B because of PD.
The demographics and patient characteristics of the randomized patients are shown in Table 1. The majority of patients (96.7 %) had an Eastern Cooperative Oncology group (ECOG) performance status ≤1. The most common cancers were ovary, breast, lung, and colorectal (Table 1).
Effect of food on olaparib pharmacokinetics
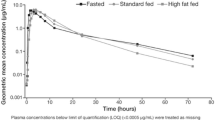
The derived PK parameters for olaparib following dosing of the tablet formulation in the fasted and fed states during Part A are shown in Table 2, and the olaparib plasma concentration versus time profiles are shown in Fig. 1. The rate of olaparib absorption was slower in the presence of food (t max delayed by 2.5 h, 90 % CI 2.07–2.96) (Fig. 1, Table 2). Point estimates for the treatment ratio for C max showed that there was a statistically significant ~21 % decrease in olaparib peak plasma exposure (C max) following a high-fat meal (treatment ratio: 0.79; 90 % CI, 0.72, 0.86), an effect likely because of the observed reduction in the rate of olaparib absorption (Table 3).
Point estimates for the treatment ratio for AUC0–∞ indicated a small (8 %) increase in the extent of olaparib exposure when administered with food, which was of borderline statistical significance (treatment ratio: 1.08; 90 % CI 1.01, 1.16). However, both the point estimates and 90 % CIs for the treatment ratios for AUC0–∞ were within the pre-defined bioequivalence range of 0.80–1.25 (Table 3), indicating that the presence of food did not alter the extent of olaparib absorption. No effect of food was observed on the terminal elimination half-life, clearance, or volume of distribution (Table 2).
There was no obvious impact of food on inter-patient variability in olaparib PK, with percentage geometric coefficients of variation (%GCV) for geometric mean AUC0–∞ and C max values being within the range of 55–57 % and 35–41 %, respectively, independent of the prandial state.
Safety
A total of 52 (86.7 %) and 41 (68.3 %) patients reported at least one AE of any grade in Parts A and B, respectively; AEs of gastrointestinal origin (nausea, vomiting, diarrhoea, abdominal pain, and dyspepsia) of grade ≤2 were the most common (Table 4).
There were no obvious differences observed between the AEs reported under fed/fasted conditions; however, nausea and vomiting were reported more frequently during the fasted period (18.3 vs. 8.3 % for nausea and 16.7 vs. 5.0 % for vomiting).
AEs of grade 3 were reported in six patients in Part A (three patients in each treatment condition; each of the following events was reported in one patient: ascites, constipation, nausea, vomiting, fatigue, and malignant pleural effusion) and one patient in Part B (nausea and decreased appetite). One serious AE (malignant pleural effusion, grade 3) was reported in Part A, which was not considered by the investigator to be related to olaparib. No serious AEs were reported in Part B, and there were no AEs that led to olaparib discontinuation in either part of the study.
Two deaths related to the disease under investigation/disease progression occurred during the study (one during Part A with a primary cause of death of haemoptysis and one during Part B with a primary cause of death of PD); neither death was related to study treatment nor reported as an AE. No safety issues were identified based on ECG findings, vital signs, physical examinations, and laboratory parameters.
Discussion
It is important to understand the effects of concomitant food intake on the PK and safety profile of oral medications. This open-label, Phase I trial was conducted to allow appropriate patient recommendations to be made regarding administration of olaparib tablets with food.
Findings from the PK analysis demonstrated that food decreases peak exposures to olaparib (C max) and slows the rate of absorption (t max), but does not alter the extent of olaparib absorption (AUC0–∞). Both the point estimates and 90 % CIs for the treatment ratios for AUC lie entirely within the pre-defined bioequivalence range of 0.80–1.25, and it can, therefore, be concluded that there was no effect of food on olaparib AUC. However, given the observed statistically significant decrease in C max despite no effect on AUC0–∞, an effect of food on the PK of olaparib tablets cannot be ruled out. Preclinical data obtained from studies in mice suggest that efficacy of olaparib was associated with plasma concentrations being maintained above the IC90 for PARP inhibition for the majority of the dosing interval (unpublished data). It would therefore appear that following treatment with olaparib, maintaining the inhibition of PARP is of most importance in driving efficacy, and on that basis we do not believe that the observed difference in C max in the current study will be clinically relevant. The decrease in C max in the presence of food is small, and with no alteration on the extent of olaparib absorption, the effect of food on the efficacy of olaparib following dosing of the tablet formulation is considered to be minimal. Phase III trials of olaparib 300 mg tablets in patients with BRCA-mutated ovarian cancer (clinicaltrial.gov NCT01844986 and NCT01874353) allow patients to take olaparib tablets with a light snack (a piece of buttered toast or two plain biscuits).
Safety data from Parts A and B were consistent with the known safety profile of olaparib [4–6, 9, 11], with the majority of AEs reported being of mild or moderate severity. For all treatment conditions, and for single and multiple dosing, the most frequently reported AEs were of gastrointestinal origin.
In conclusion, the results of this study showed that a high-fat meal decreases the rate of absorption and peak exposure to olaparib 300 mg tablets although in the absence of an effect on the extent of olaparib absorption.
References
Evers B, Drost R, Schut E, de Bruin M, van der Burg E, Derksen PW, Holstege H, Liu X, van Drunen E, Beverloo HB, Smith GC, Martin NM, Lau A, O’Connor MJ, Jonkers J (2008) Selective inhibition of BRCA2-deficient mammary tumor cell growth by AZD2281 and cisplatin. Clin Cancer Res 14:3916–3925
Rottenberg S, Jaspers JE, Kersbergen A, van der Burg E, Nygren AO, Zander SA, Derksen PW, de Bruin M, Zevenhoven J, Lau A, Boulter R, Cranston A, O’Connor MJ, Martin NM, Borst P, Jonkers J (2008) High sensitivity of BRCA1-deficient mammary tumors to the PARP inhibitor AZD2281 alone and in combination with platinum drugs. Proc Natl Acad Sci USA 105:17079–17084
Marchetti C, Imperiale L, Gasparri ML, Palaia I, Pignata S, Boni T, Bellati F, Benedetti PP (2012) Olaparib, PARP1 inhibitor in ovarian cancer. Expert Opin Investig Drugs 21:1575–1584
Audeh MW, Carmichael J, Penson RT, Friedlander M, Powell B, Bell-McGuinn KM, Scott C, Weitzel JN, Oaknin A, Loman N, Lu K, Schmutzler RK, Matulonis U, Wickens M, Tutt A (2010) Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer: a proof-of-concept trial. Lancet 376:245–251
Tutt A, Robson M, Garber JE, Domchek SM, Audeh MW, Weitzel JN, Friedlander M, Arun B, Loman N, Schmutzler RK, Wardley A, Mitchell G, Earl H, Wickens M, Carmichael J (2010) Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: a proof-of-concept trial. Lancet 376:235–244
Gelmon KA, Tischkowitz M, Mackay H, Swenerton K, Robidoux A, Tonkin K, Hirte H, Huntsman D, Clemons M, Gilks B, Yerushalmi R, MacPherson E, Carmichael J, Oza A (2011) Olaparib in patients with recurrent high-grade serous or poorly differentiated ovarian carcinoma or triple-negative breast cancer: a phase 2, multicentre, open-label, non-randomised study. Lancet Oncol 12:852–861
Kaufman B, Shapira-Frommer R, Schmutzler RK, Audeh MW, Friedlander M, Balmaña J, Mitchell G, Fried G, Stemmer SM, Hubert A, Rosengarten O, Steiner M, Loman N, Bowen K, Fielding A, Domchek SM (2014) Olaparib monotherapy in patients with advanced cancer and a germ-line BRCA1/2 mutation. J Clin Oncol. doi:10.1200/JCO.2014.56.2728 (Nov 3 [Epub ahead of print])
Oza A, Cibula D, Benzaquen AO, Poole C, Mathijssen RHJ, Sonke GS, Colombo N, Spacek J, Vuylsteke P, Hirte H, Mahner S, Plante M, Schmalfeldt B, Mackay H, Rowbottom J, Lowe E, Dougherty B, Barrett JC, Friedlander M (2015) Olaparib combined with chemotherapy for recurrent platinum-sensitive ovarian cancer: a randomised phase 2 trial. Lancet Oncol 16:87–97
Ledermann J, Harter P, Gourley C, Friedlander M, Vergote I, Rustin G, Scott C, Meier W, Shapira Frommer R, Safra T, Matei D, MacPherson E, Watkins C, Carmichael J, Matulonis U (2012) Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer. N Engl J Med 366:1382–1392
Ledermann JA, Harter P, Gourley C, Friedlander M, Vergote I, Rustin G, Scott C, Meier W, Shapira-Frommer R, Safra T, Matei D, Fielding A, Spencer S, Dougherty B, Orr M, Hodgson D, Barrett J, Matulonis U (2014) Randomized trial of olaparib maintenance therapy in patients with platinum-sensitive relapsed serous ovarian cancer: a preplanned retrospective analysis by BRCA mutation status. Lancet Oncol 15:852–861
Fong PC, Boss DS, Yap TA, Tutt A, Wu P, Mergui-Roelvink M, Mortimer P, Swaisland H, Lau A, O’Connor MJ, Ashworth A, Carmichael J, Kaye SB, Schellens JHM, de Bono JS (2009) Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med 361:123–134
Molife R, Kaye S, Forster M, Ransom M, Greystoke A, Middleton M, Pwint T, McCormack P, Swailand H, Carmichael J (2010) A phase I study to determine the comparative bioavailability of two different oral formulations of the PARP inhibitor, olaparib (AZD2281), in patients with advanced solid tumors. J Clin Oncol 28(7S):abst 2599
Mateo J, Friedlander M, Sessa C, Leunen K, Nicum S, Gourley C, Fielding A, Bowen K, Kaye S, Molife LR (2013) Administration of continuous/intermittent olaparib in ovarian cancer patients with a germline BRCA1/2 mutation to determine an optimal dosing schedule for the tablet formulation. Eur J Cancer 49(2 Suppl):abst 801
Molife LR, Mateo J, McGoldrick T, Krebs M, Drew Y, Banerjee SN, Nicum S, Ranson M, Rustin GJ, Sessa C, Plummer R, Leunen K, Friedlander M, Swaisland H, Burke W, McCormack P, Pemberton K, Tchakov I, Kaye SB, Gourley C (2012) Safety and efficacy results from two randomized expansions of a phase I study of a tablet formulation of the PARP inhibitor, olaparib, in ovarian and breast cancer patients with BRCA1/2 mutations. J Clin Oncol 30(15S):abst 3048
AstraZeneca. Global policy: bioethics. 2015. http://www.astrazeneca.com/Responsibility/Code-policies-standards/Our-global-policies
U.S. Department of Health and Human Services. Food and Drug Administration Center for Drug Evaluation and Research (CDER) (2002) Guidance for industry: food-effect bioavailability and fed bioequivalence studies. FDA
Rolfo C, Swaisland H, Leunen K, Rutten A, Soetekouw P, Slater S, Verheul HM, Fielding A, So K, Bannister W, Dean E (2015) Effect of food on the pharmacokinetics of olaparib after oral dosing of the capsule formulation in patients with advanced solid tumors. Adv Ther 32:510–522
Acknowledgments
The authors would like to thank all patients who consented to participate in this study. We also acknowledge Gil Morrison (Covance) for analysis of the PK data, and Quintiles for conducting the study and associated data management activities. This study was sponsored by AstraZeneca. Medical writing assistance was provided by Claire Routley Ph.D. from Mudskipper Business Ltd, funded by AstraZeneca. The UK Centres participating in this research receive funding from Cancer Research UK (CRUK) and the Department of Health as Experimental Cancer Medicine Centres.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
RP has received institutional remuneration from AstraZeneca and has received funding from AstraZeneca for chairing an advisory board. GJ has received remuneration from Novartis, Celgene, and Roche, funding from Novartis, MSD, and Roche, and has consulted/had an advisory role for Novartis and Celgene. LRM has received funding from AstraZeneca. JDG has received funding from AstraZeneca for attendance at a national advisory board. AF is an employee of, and owns stock in, AstraZeneca. HS was formally an employee of AstraZeneca and owns stock in AstraZeneca. KS is a contractor for AstraZeneca. KL, CvH, DN, ML, JS, MMS, PS, and WB have no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
HS was an employee of AstraZeneca while the study was conducted.
Rights and permissions
About this article
Cite this article
Plummer, R., Swaisland, H., Leunen, K. et al. Olaparib tablet formulation: effect of food on the pharmacokinetics after oral dosing in patients with advanced solid tumours. Cancer Chemother Pharmacol 76, 723–729 (2015). https://doi.org/10.1007/s00280-015-2836-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-015-2836-2