Abstract
Purpose
The solute carrier family 29 (equilibrative nucleoside transporter), member 1 (SLC29A1) is known to be involved in the transportation and resistance of the nucleoside analog cytosine arabinoside (AraC), one of the most effective drugs in the treatment of acute myeloid leukemia (AML).
Methods
In vitro functional analysis in AML cells and genetic association study were performed.
Results
Our functional analysis of SLC29A1 on anticancer effects of AraC showed that cytotoxic effects of AraC in AML cell lines were decreased by the reduction of SLC29A1 expression (P < 0.05). To investigate whether SLC29A1 polymorphisms could affect the achievement of complete remission (CR) in AML, we genotyped a total of six common single nucleotide polymorphisms on SLC29A1 in 103 AML patients, including 17 successes and 86 failures in CR. As a result, rs3734703 in 3’-untranslated region was significantly associated with CR even after correction for multiple testing (Fisher’s exact test, P = 0.008; P corr = 0.04). A haplotype, ht3 (A–G–G–T–C–A; frequency = 0.294 in success group; frequency = 0.120 in failure group), also revealed a significant association with CR (P = 0.01; simulated P sim = 0.02).
Conclusions
Although further replication in larger subjects and further functional evaluations are required, our results suggest the contribution of SLC29A1 to cytotoxic effects of AraC. In addition, genetic variations of SLC29A1 could be a potential marker for the achievement of CR of cancers of white blood cells including AML.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Patients with acute myeloid leukemia (AML), a rare and heterogeneous cancer, are generally treated with cytosine arabinoside (1-beta-d-arabinofuranosylcytosine; cytarabine; AraC, which is one of the most widely used drugs in the treatment of AML) or AraC-combined chemotherapy [1, 2]. Generally, AML patients have shown a poor 5-year survival (in particular, 13.9 % for older patients over the age of 50 years) after the initial diagnosis [3]. In addition, the outcome of complete remission (CR) of AraC-based treatment in AML is still unsatisfactory, and clinical resistance to AraC and its adverse side effects have been observed [3–5]. However, the causes of resistance to AraC and eventual treatment failure in hematologic cancers such as AML remain unclear.
The solute carrier family 29 (equilibrative nucleoside transporter), member 1 (SLC29A1, or ENT1) plays a crucial role in the cellular uptake of anticancer nucleoside agents as well as physiologic nucleosides. For instance, a higher expression of SLC29A1 has been observed to be less sensitive to 5-fluorouracil (5-FU), a nucleoside analog used in the treatment of cancer, in pancreatic cancer, suggesting that the highly expressed SLC29A1 may interrupt the 5-FU function that blocks synthesis of pyrimidine thymidine [6]. In addition, a significant association of SLC29A1 expression with sensitivity to gemcitabine and its uptake into mantle cell lymphoma has been found [7]. These results suggest that SLC29A1 may play an important role in the sensitivity to anticancer drugs.
Although it is known that SLC29A1 transports about 80 % of AraC into leukemic cells [8, 9], the chemotherapeutic efficacy of AraC varies between patients [10]. Among the identified mechanisms of AraC resistance in leukemic cells, the altered SLC29A1 has been suggested to be a responsible factor for the resistance [11]. Many studies have also reported that functional abnormalities in SLC29A1 are associated with resistance and sensitivity to AraC in AML [4, 12, 13]. However, only limited studies have recently suggested potential contributions of SLC29A1 genetic variants, rs3734703 and rs693955, to the clinical outcomes in AML patients [14, 15]. In addition, there are almost no studies which investigated the association between SLC29A1 polymorphisms and remission in AML, except for one recent report [16].
Therefore, this study performed functional analysis of SLC29A1 on anticancer effects of AraC in AML cells and genetic association analysis of SLC29A1 single nucleotide polymorphisms (SNPs) with the achievement of CR in AML patients during the induction and subsequent consolidation chemotherapies.
Methods
Cells and cell culture
The human acute myeloid leukemia cell lines, HEL and KG-1, were obtained from the American Type Culture Collection (Rockville, MD, USA) and maintained in sodium pyruvate, vitamins, and l-glutamine contained RPMI-1640 medium (Gibco-BRL, Gaithersburg, MD, USA) with 10 % heat-inactivated fetal bovine serum, penicillin (100 U/mL), and streptomycin (100 g/mL) (GIBCO, Grand Island, NY, USA). Cells were cultured and maintained in a humidified atmosphere of 5 % CO2 and 95 % air at 37 °C in an incubator. All experiments were performed in the logarithmic growth phase of each cell line.
Reagents
Analog cytosine arabinoside was purchased from Sigma (St Louis, MO, USA). AraC was dissolved in saline solution and stored at 4 °C freezer as a stock solution. Cell proliferation assay was performed using Cell Counting Kit-8 (CCK-8, Dojindo Laboratories, Kumamoto, Japan), which was stored at 4 °C freezer. All solutions were protected from light.
Cell proliferation assay
100 µL of cell suspension was seeded into 96-well culture plates at 1 × 104 cells per well and then incubated for 24 h. Reagents were added to each well in the plate, and cells were incubated for the indicated time period. Then, 10 µL of the CCK-8 solution was added to each well for another 4 h. The absorbance of each well was measured in a microplate reader (Becton–Dickinson Labware, Le Pont de Claix, France) at 450 nm. Means and standard deviations were generated from three independent experiments. Absorbance values were normalized to the values obtained from the control group to determine the value for % of survival. Values are the mean ± SD.
Gene expression regulation using plasmid vector transfection
Plasmid vectors containing shRNA sequence for target genes were obtained from OriGene Technologies, Inc. (Rockville, MD, USA). The SLC29A1 and targeted shRNA sequence were used. One set of shRNA constructs contains four different sequences of each shRNA expression vectors in the pGFP-V-RS plasmid. Plasmids were amplified with transformation method using DH5α competent cell strain. In the transfection study, a uni-dose mixture of different shRNA vectors for the same gene was used. The Amaxa® Nucleofector® Device and solution were used for electroporation method (Walkersville, MD, USA) using about 1 × 106 cells at one experiment according to the manufacturer’s protocol.
Reverse transcription polymerase chain reaction (RT-PCR)
Total RNA was extracted from appropriate cells using TRIsol reagent (Gibco-BRL, Invitrogen, Carlsbad, CA, USA), and its concentration was measured by ND-1000 Spectrophotometer (NanoDrop Technologies, Inc. Wilmington, DE, USA). About 1 μg RNA per sample was reversely transcribed with SuperScript reverse transcriptase (Invitrogen, Carlsbad, CA, USA) using random hexamers. The cDNA was synthesized by incubation at 45 °C for 1 h and then inactivated by heating at 95 °C for 5 min. Using cDNA, PCR was performed in a total volume of 20 μL for 95 °C for 5 min, 30 cycles at 95 °C for 1 min, 48 °C for 30 s, and 72 °C for 1 min, and finally 10 min at 72 °C. The Beta-actin was used as an internal control. PCR products were performed using electrophoresis in 1 % agarose gel and analyzed by ethidium bromide staining.
Study patients
A total of 103 AML patients (60 men and 43 women), with the median age of 50.4 years (range 16–76 years), were recruited from Seoul National University Hospital in Korea. Patients were divided into subtypes according to the French–American–British (FAB) classification: M0 (n = 2), M1 (n = 14), M2 (n = 47), M4 (n = 33), M5 (n = 5), and M7 (n = 2). This study excluded patients with acute promyelocytic leukemia (M3 FAB subtype). The criteria for cytogenetic clone and karyotype were based on the guidelines of the International System for Human Cytogenetic Nomenclature [17]. All 103 AML patients received standard induction chemotherapy (idarubicin 12 mg/m2/day intravenously for days 1–3 and AraC 200 mg/m2/day intravenously for days 1–7), with a modified dose of idarubicin to two-thirds of the total dose in patients older than 66 years of age. Two more cycles of high-dose AraC (3 g/m2 per day intravenously twice a day on D1, 3, 5) were performed for consolidation therapies. In this study, the success of CR means the achievement of CR sustained during the follow-up period, whereas no success of CR means a failure of attaining CR after induction chemotherapy or relapse any time during the courses of the treatment. The study protocol was approved by the Institutional Review Board of Seoul National University Hospital, and all patients provided written informed consent.
Genotyping of SNPs and statistical analysis
A total of six common SLC29A1 SNPs were selected based on minor allele frequency (MAF) > 0.05 and linkage disequilibrium (LD) using the genotype data of Asian populations (Chinese and Japanese) from the 1000 Genomes Project (http://browser.1000genomes.org/index.html). All SNPs were successfully genotyped in 103 study subjects using TaqMan assay based on the ABI prism 7900HT sequence detection system (Applied Biosystems, Carlsbad, CA, USA). Statistical analyses were performed using Fisher’s exact test and the HaploStats program in R [18], adjusting for age and sex as covariates. To correct for multiple testing errors, we used the SNPSpD program (http://gump.qimr.edu.au/general/daleN/SNPSpD/).
Results
Functional analysis of SLC29A1 on anticancer effects of AraC
To evaluate whether the decrease of SLC29A1 expression affects anticancer effects of AraC or not, we transfected shRNA of SLC29A1 into AML cell lines (HEL and KG-1) and the anticancer effects of AraC were examined. AraC showed cytotoxic effects on these cells, and HEL cells were more sensitive to the cytotoxic effect of AraC than KG-1 cells (Supplementary Fig. 1). Reduction of SLC29A1 was detected by green fluorescence protein (GFP) that was contained in shRNA vectors and RT-PCR in cell lines (Fig. 1a, b). When expression of SLC29A1 was inhibited by shRNA, cytotoxic effects of AraC in both of HEL and KG-1 cells were reduced by approximately 20 % (P = 0.028) and 12 % (P = 0.017), respectively, compared with parental cells (Fig. 1c).
Effect of SLC29A1 shRNA on the resistance against AraC in AML cells. a GFP expression in AML cells (HEL and KG-1) transfected GFP tagged expression vector. Cells are transfected with the 1 μg of shRNA contained plasmid vector that targets against SLC29A1. After transfection (48 h), cells are treated with AraC at 10−8 M concentration. b Detection of shRNA-mediated downregulation of SLC29A1 expression by RT-PCR in HEL and KG-1 cell lines. All RNA expression values are normalized with internal control of Beta-actin (B-actin). c Cell viability is determined by MTT assay method using CCK-8 cell proliferation kit. Data are expressed as mean ± SE
SNPs, LDs, and haplotypes of SLC29A1
To investigate genetic associations of SLC29A1 with CR in AML, a total of six SNPs of SLC29A1 on chromosome 6p21.1 were selected under the criteria in the Methods section and genotyped (Fig. 2a). Pair-wise comparisons revealed that six common SLC29A1 SNPs showed a tight LD block in 103 Korean AML patients (17 successes and 86 failures in CR), and five major haplotypes with frequencies over 0.05 were inferred (Fig. 2b, c). For haplotype association analysis, only these common haplotypes were used.
Physical map, LD, and haplotypes of SLC29A1. a Schematic map of SLC29A1 and its SNPs investigated in this study. Black blocks indicate coding exons; white blocks indicate 5′-untranslated region (UTR) and 3′-UTR. The MAF of SNP is indicated in the bracket. b LD plot of SLC29A1. Number in block represents the value of LD coefficient |D’|. c Haplotypes of SLC29A1. Only common haplotypes (frequency > 0.05) are shown and considered for further analyses
Association analysis of SLC29A1 SNPs and haplotypes with CR in AML
As a result, four SNPs were observed to have significances in the association with CR in AML under the Fisher’s exact test (minimum P = 0.008, Table 1). Among these statistically significant SNPs, rs3734703 in 3′-untranslated region (3′UTR) retained a significant signal even after correction for multiple testing (P corr = 0.04), showing a higher MAF in remission success group (MAF = 0.324) than in failure group (MAF = 0.194). In addition, one haplotype (ht3, A–G–G–T–C–A), containing the minor “A” allele of the significant rs3734703, showed a significant association with CR in AML (P = 0.01; simulated P sim = 0.02, Table 1), with about 2.5-fold higher rate of frequency in remission success group than in failure group.
In silico analyses for potential functions of rs3734703
To predict a potential function of the significantly associated rs3734703 in the 3′UTR, in silico analyses were performed. Intriguingly, in the UTRScan (http://itbtools.ba.itb.cnr.it/utrscan) as a program estimating the regulatory motifs in the untranslated regions of eukaryotic mRNAs, a sequence region including rs3734703 was predicted to be a potential regulatory element of upstream open reading frame (uORF), a motif involved in the interference with expression of its downstream primary ORF (Supplementary Table 1). In addition, using the expression quantitative trait (eQTL) browser (http://eqtl.uchicago.edu/cgi-bin/gbrowse/eqtl/), this rs3734703 was observed to act as a potential cis-acting variant for SLC29A1 (ENSG00000112759) expression, with an eQTL score of 0.874 (Supplementary Fig. 2).
Discussion
The cytotoxicity of AraC results from a combination of DNA polymerase inhibition and incorporation of AraC into DNA, in competition with deoxycytidine triphosphate [19]. AraC is considered as a primary antimetabolite treated for hematologic malignancies and is therefore used as one of the most effective drugs for the treatment of AML. However, resistance to drugs, including AraC, is a major cause of chemotherapeutic failure among AML patients [20, 21]. Chromosomal abnormalities in pediatric AML have been reported to be related to AraC resistance [22]. In addition, candidate gene approaches have identified genetic variants associated with the resistance to AraC and its treatment outcome [23, 24]. These previous results suggest that abnormality of genes in the pharmacokinetic pathway of AraC may also be a potential cause of AraC resistance. Actually, based on our functional in vitro experiment in AML cells, reduced SLC29A1 expression was observed to result in the resistant effect on AML cells. Although 7 + 3 (7 days of AraC; 3 days of idarubicin) is quite a standard regimen as an induction treatment for most subtypes of AML, the single most important agent in almost all AML subtypes except M3 AML is AraC throughout the courses of induction and consolidation treatment. Therefore, this study focused on AraC and related polymorphisms.
In our prior tests of karyotype and mutations for the study subjects, no associations with event-free survival and overall survival were observed. Therefore, to investigate other genetic effects on outcome in AML, this association study between genetic polymorphisms of candidate genes and CR in AML was performed. This study found the effect of SLC29A1 genetic variants on the outcome of CR in AML patients. Considering that SLC29A1, as an equilibrative nucleoside transporter, is responsible for ~80 % AraC influx in leukemic cells [8, 9], our preliminary findings suggest that SLC29A1 polymorphisms, including rs3734703 and the haplotype ht3, may be potential markers to identify patients more likely to have enhanced response to AraC-based chemotherapy in white blood cell cancers such as AML.
Recently, under the suggestion that SNP analysis could be an important tool for developing tailor-made treatments for AML patients who benefit by receiving consolidation of high-dose AraC therapy, SLC29A1 rs693955 has been observed to be associated with a shorter time to relapse [14]. Another study has also shown that rs9394992 and rs324148 of SLC29A1 might be potential prognostic factors to overall survival and disease-free survival in Chinese AML patients [16], supporting our result of the potential effect of SLC29A1 genetic variants on CR in AML. However, MAFs of the two SNPs were opposite between Chinese and Korean patients. Considering different genetic distributions among ethnic population groups, further researches of SLC29A1 SNPs may provide useful information to pharmacogenetics for drug responses in cancers of white blood cells including AML.
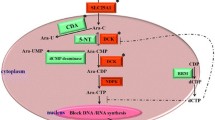
Functional abnormalities in human SLC29A1 (such as loss-of-function mutations, downregulated gene expression and low promoter activity) have been implicated in the resistance and sensitivity to AraC as well as reduced cellular uptake of AraC in AML [4, 11–13, 25]. A recent genome-wide association study and its follow-up meta-analysis showed a significant association between intronic rs186556 of SLC29A1 and AraC sensitivity in lymphoblastoid cell lines [26]. In addition, functional haplotypes in promoter regulatory region of SLC29A1 have been observed to have potential roles in the gene expression and altered AraC chemosensitivity [27]. Furthermore, AML patients with SLC29A1 deficiency were predicted to have a decreased survival and an increased rate of relapse [4]. These lines of evidence and our result suggest that SLC29A1 variants and haplotypes may affect the attainment of CR and alter the uptake activity in AML patients. As presented in Fig. 3, a possible explanation is that genetic variations of SLC29A1 (e.g., rs3734703) may lead to increased uptake activity of AraC, resulting in increased apoptosis and CR in AML.
Simplified schematic model for genetic association between SLC29A1 and remission in AML. a In wild-type condition, a moderate exchange activity of SLC29A1 is maintained. b Dysfunctional SLC29A1 derived from genetic variation (e.g., rs3734703) can increase uptake activity of SLC29A1, which lead to apoptosis and increasing success in complete remission in AML
In this study, high frequency for the major “C” allele (in other words, low MAF) of rs3734703 in 3′UTR showed a poor response to AraC-based therapy. Variants in the 3′UTR usually have a role in the expression of the gene by altering its stability and/or secondary structure [28]. Although further functional evaluations are required, it is a possible explanation that the genetic effect of rs3734703 on transcriptional regulations, such as stability and secondary structure of mRNAs, may lead to an expression change of the gene product and accompanying different outcome in CR in AML.
Among the haplotypes, only the ht3 (A–G–G–T–C–A) showed a significant association with CR in AML (P = 0.01) even after corrections for simulated statistics (P sim = 0.02, Table 1). The ht3 was the unique haplotype containing the minor “A” allele of the significant rs3734703, with about 2.5-fold frequency rate in remission success group compared with failure group. Although there are several study limitations, such as no additional function studies and insufficient sample size, this ht3 haplotype of SLC29A1 could be a potential genetic marker that is associated with CR in AML.
Conclusions
Although further replication and evaluation studies are needed, our results suggest that SLC29A1 may have a role in the outcome of CR in AML, with providing potential information to identify patients more likely to achieve remission in AML.
References
Harousseau JL, Reiffers J, Hurteloup P, Milpied N, Guy H, Rigal-Huguet F, Facon T, Dufour P, Ifrah N (1989) Treatment of relapsed acute myeloid leukemia with idarubicin and intermediate-dose cytarabine. J Clin Oncol 7:45–49
Nazha A, Kantarjian H, Ravandi F, Huang X, Choi S, Garcia-Manero G, Jabbour E, Borthakur G, Kadia T, Konopleva M, Cortes J, Ferrajoli A, Kornblau S, Daver N, Pemmaraju N, Andreeff M, Estrov Z, Du M, Brandt M, Faderl S (2013) Clofarabine, idarubicin, and cytarabine (CIA) as frontline therapy for patients ≤60 years with newly diagnosed acute myeloid leukemia. Am J Hematol 88:961–966
Faderl S, Wetzler M, Rizzieri D, Schiller G, Jagasia M, Stuart R, Ganguly S, Avigan D, Craig M, Collins R, Maris M, Kovacsovics T, Goldberg S, Seiter K, Hari P, Greiner J, Vey N, Recher C, Ravandi F, Wang ES, Vasconcelles M, Huebner D, Kantarjian HM (2012) Clofarabine plus cytarabine compared with cytarabine alone in older patients with relapsed or refractory acute myelogenous leukemia: results from the CLASSIC I trial. J Clin Oncol 30:2492–2499
Galmarini CM, Thomas X, Calvo F, Rousselot P, El Jafaari A, Cros E, Dumontet C (2002) Potential mechanisms of resistance to cytarabine in AML patients. Leuk Res 26:621–629
Ishikawa F, Yoshida S, Saito Y, Hijikata A, Kitamura H, Tanaka S, Nakamura R, Tanaka T, Tomiyama H, Saito N, Fukata M, Miyamoto T, Lyons B, Ohshima K, Uchida N, Taniguchi S, Ohara O, Akashi K, Harada M, Shultz LD (2007) Chemotherapy-resistant human AML stem cells home to and engraft within the bone-marrow endosteal region. Nat Biotechnol 25:1315–1321
Tsujie M, Nakamori S, Nakahira S, Takahashi Y, Hayashi N, Okami J, Nagano H, Dono K, Umeshita K, Sakon M, Monden M (2007) Human equilibrative nucleoside transporter 1, as a predictor of 5-fluorouracil resistance in human pancreatic cancer. Anticancer Res 27:2241–2249
Marce S, Molina-Arcas M, Villamor N, Casado FJ, Campo E, Pastor-Anglada M, Colomer D (2006) Expression of human equilibrative nucleoside transporter 1 (hENT1) and its correlation with gemcitabine uptake and cytotoxicity in mantle cell lymphoma. Haematologica 91:895–902
Clarke ML, Mackey JR, Baldwin SA, Young JD, Cass CE (2002) The role of membrane transporters in cellular resistance to anticancer nucleoside drugs. Cancer Treat Res 112:27–47
Reese ND, Schiller GJ (2013) High-dose cytarabine (HD araC) in the treatment of leukemias: a review. Curr Hematol Malig Rep 8:141–148
Lamba JK (2009) Genetic factors influencing cytarabine therapy. Pharmacogenomics 10:1657–1674
Cai J, Damaraju VL, Groulx N, Mowles D, Peng Y, Robins MJ, Cass CE, Gros P (2008) Two distinct molecular mechanisms underlying cytarabine resistance in human leukemic cells. Cancer Res 68:2349–2357
Hubeek I, Stam RW, Peters GJ, Broekhuizen R, Meijerink JP, van Wering ER, Gibson BE, Creutzig U, Zwaan CM, Cloos J, Kuik DJ, Pieters R, Kaspers GJ (2005) The human equilibrative nucleoside transporter 1 mediates in vitro cytarabine sensitivity in childhood acute myeloid leukaemia. Br J Cancer 93:1388–1394
Macanas-Pirard P, Leisewitz A, Broekhuizen R, Cautivo K, Barriga FM, Leisewitz F, Gidi V, Riquelme E, Montecinos VP, Swett P, Besa P, Ramirez P, Ocqueteau M, Kalergis AM, Holt M, Rettig M, DiPersio JF, Nervi B (2012) Bone marrow stromal cells modulate mouse ENT1 activity and protect leukemia cells from cytarabine induced apoptosis. PLoS One 7:e37203
Amaki J, Onizuka M, Ohmachi K, Aoyama Y, Hara R, Ichiki A, Kawai H, Sato A, Miyamoto M, Toyosaki M, Machida S, Kojima M, Shirasugi Y, Kawada H, Ogawa Y, Ando K (2015) Single nucleotide polymorphisms of cytarabine metabolic genes influence clinical outcome in acute myeloid leukemia patients receiving high-dose cytarabine therapy. Int J Hematol 101:543–553
Kim KI, Huh IS, Kim IW, Park T, Ahn KS, Yoon SS, Yoon JH, Oh JM (2013) Combined interaction of multi-locus genetic polymorphisms in cytarabine arabinoside metabolic pathway on clinical outcomes in adult acute myeloid leukaemia (AML) patients. Eur J Cancer 49:403–410
Wan H, Zhu J, Chen F, Xiao F, Huang H, Han X, Zhong L, Zhong H, Xu L, Ni B, Zhong J (2014) SLC29A1 single nucleotide polymorphisms as independent prognostic predictors for survival of patients with acute myeloid leukemia: an in vitro study. J Exp Clin Cancer Res 33:90
Schoch C, Schnittger S, Kern W, Dugas M, Hiddemann W, Haferlach T (2003) Acute myeloid leukemia with recurring chromosome abnormalities as defined by the WHO-classification: incidence of subgroups, additional genetic abnormalities, FAB subtypes and age distribution in an unselected series of 1897 patients with acute myeloid leukemia. Haematologica 88:351–352
Schaid DJ, Rowland CM, Tines DE, Jacobson RM, Poland GA (2002) Score tests for association between traits and haplotypes when linkage phase is ambiguous. Am J Hum Genet 70:425–434
Kufe DW, Major PP, Egan EM, Beardsley GP (1980) Correlation of cytotoxicity with incorporation of ara-C into DNA. J Biol Chem 255:8997
Galmarini CM, Thomas X, Calvo F, Rousselot P, Rabilloud M, El Jaffari A, Cros E, Dumontet C (2002) In vivo mechanisms of resistance to cytarabine in acute myeloid leukaemia. Br J Haematol 117:860–868
Styczynski J (2007) Drug resistance in childhood acute myeloid leukemia. Curr Pharm Biotechnol 8:59–75
Zwaan CM, Kaspers GJ, Pieters R, Hahlen K, Huismans DR, Zimmermann M, Harbott J, Slater RM, Creutzig U, Veerman AJ (2002) Cellular drug resistance in childhood acute myeloid leukemia is related to chromosomal abnormalities. Blood 100:3352–3360
Falk IJ, Fyrberg A, Paul E, Nahi H, Hermanson M, Rosenquist R, Hoglund M, Palmqvist L, Stockelberg D, Wei Y, Green H, Lotfi K (2013) Decreased survival in normal karyotype AML with single-nucleotide polymorphisms in genes encoding the AraC metabolizing enzymes cytidine deaminase and 5’-nucleotidase. Am J Hematol 88:1001–1006
Hartford CM, Duan S, Delaney SM, Mi S, Kistner EO, Lamba JK, Huang RS, Dolan ME (2009) Population-specific genetic variants important in susceptibility to cytarabine arabinoside cytotoxicity. Blood 113:2145–2153
Jin G, Matsushita H, Asai S, Tsukamoto H, Ono R, Nosaka T, Yahata T, Takahashi S, Miyachi H (2009) FLT3-ITD induces ara-C resistance in myeloid leukemic cells through the repression of the ENT1 expression. Biochem Biophys Res Commun 390:1001–1006
Gamazon ER, Lamba JK, Pounds S, Stark AL, Wheeler HE, Cao X, Im HK, Mitra AK, Rubnitz JE, Ribeiro RC, Raimondi S, Campana D, Crews KR, Wong SS, Welsh M, Hulur I, Gorsic L, Hartford CM, Zhang W, Cox NJ, Dolan ME (2013) Comprehensive genetic analysis of cytarabine sensitivity in a cell-based model identifies polymorphisms associated with outcome in AML patients. Blood 121:4366–4376
Myers SN, Goyal RK, Roy JD, Fairfull LD, Wilson JW, Ferrell RE (2006) Functional single nucleotide polymorphism haplotypes in the human equilibrative nucleoside transporter 1. Pharmacogenet Genom 16:315–320
Chen JM, Ferec C, Cooper DN (2006) A systematic analysis of disease-associated variants in the 3’ regulatory regions of human protein-coding genes II: the importance of mRNA secondary structure in assessing the functionality of 3’UTR variants. Hum Genet 120:301–333
Funding
This study was supported by Grant 03-2010-0080 from the SNUH Research Fund; the grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health and Welfare, Republic of Korea (Grant number: HI14C2399); the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology (2011-0008846).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
There are no conflicts of interest.
Research involving human participants
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Jeong-Hyun Kim and Chansu Lee have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kim, JH., Lee, C., Cheong, H.S. et al. SLC29A1 (ENT1) polymorphisms and outcome of complete remission in acute myeloid leukemia. Cancer Chemother Pharmacol 78, 533–540 (2016). https://doi.org/10.1007/s00280-016-3103-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-016-3103-x