Abstract
Purpose
Acute kidney injury (AKI) is a common and serious adverse effect of cisplatin-based chemotherapy. However, traditional markers of kidney function, such as serum creatinine, are suboptimal, because they are not sensitive measures of proximal tubular injury. We aimed to determine whether the new urinary biomarkers such as kidney injury molecule-1 (KIM-1), monocyte chemotactic protein-1 (MCP-1), and neutrophil gelatinase-associated lipocalin (NGAL) could detect cisplatin-induced AKI in lung cancer patients in comparison with the conventional urinary proteins such as N-acetyl-β-d-glucosaminidase (NAG) and β2-microglobulin.
Methods
We measured KIM-1, MCP-1, NGAL, NAG, and β2-microglobulin concentrations in urine samples from 11 lung cancer patients, which were collected the day before cisplatin administration and on days 3, 7, and 14. Subsequently, we evaluated these biomarkers by comparing their concentrations in 30 AKI positive (+) and 12 AKI negative (−) samples and performing receiver operating characteristic (ROC) curve analyses.
Results
The urinary levels normalized with urine creatinine of KIM-1 and MCP-1, but not NGAL, NAG, and β2-microglobulin in AKI (+) samples were significantly higher than those in AKI (−) samples. In addition, ROC curve analyses revealed that KIM-1 and MCP-1, but not NGAL, could detect AKI with high accuracy (area under the curve [AUC] = 0.858, 0.850, and 0.608, respectively). The combination of KIM-1 and MCP-1 outperformed either biomarker alone (AUC = 0.871).
Conclusions
Urinary KIM-1 and MCP-1, either alone or in combination, may represent biomarkers of cisplatin-induced AKI in lung cancer patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cisplatin is a widely used anticancer drug for several types of solid tumors, such as bladder, cervical, head and neck, esophageal, and lung cancers [1]. However, nephrotoxicity, which is a dose-limiting adverse effect, is a serious problem. About 20 % of patients treated with high doses of cisplatin have peak serum creatinine (Scr) levels greater than 2.0 mg/dL, which are associated with mortality rates of 30 % or more [2, 3]. Although this toxicity is transient in most patients and can be mitigated by other treatments, such as prehydration or concomitant osmotic diuresis [2], long-term treatment with cisplatin requires careful monitoring of kidney function.
In November 2010, the standard dosage of cisplatin for lung cancer treatment in Kyoto University Hospital was increased from 60 to 80 mg/m2. This is of concern not only due to the dose-dependent nephrotoxicity of cisplatin but also because our previous research on a rat model of acute kidney injury (AKI) showed that proximal tubular injuries are not always associated with significant changes in the Scr levels [4]. Although renal biopsy is the gold standard for diagnosing AKI, not all cancer patients treated with cisplatin can undergo this procedure. Therefore, there is a need for noninvasive biomarkers of cisplatin-induced AKI.
Traditional serum markers of kidney function, such as Scr and blood urea nitrogen (BUN), are suboptimal because they only reflect changes in the glomerular filtration rate [3], which is a nonspecific measure of proximal tubular injury that is usually apparent only after significant kidney damage [5]. As a result, serum biomarkers of kidney function may not be adequate to accurately detect AKI. Other noninvasive urinary biomarkers, such as kidney injury molecule-1 (KIM-1) [6, 7] and neutrophil gelatinase-associated lipocalin (NGAL) [8, 9], may be more useful indicators of proximal tubular injury or AKI. KIM-1 is a type-1 cell membrane glycoprotein up-regulated in dedifferentiated proximal tubule epithelial cells [10]. Its ectodomain was shed and could be quantitated in the urine following kidney injury in a rodent model of cisplatin-induced AKI [6]. On the other hand, NGAL expression is induced in epithelial cells upon inflammation or malignancy. The expression of NGAL has been shown to be up-regulated in the kidney proximal tubule cells and urine in a murine model following ischemic or cisplatin-induced AKI [11]. In addition, recently we showed that the monocyte chemotactic protein-1 (MCP-1) levels are significantly increased in proximal tubular epithelial cells and urine following cisplatin-induced AKI in rats [12]. MCP-1 is a proinflammatory chemokine that plays a role in the recruitment of monocytes to the sites of injury and infection. Similar to NGAL, its expression levels are also up-regulated in the kidney proximal tubule cells following ischemic injury [13]. Therefore, we here investigated whether KIM-1, NGAL, and MCP-1, either individually or in combination, can detect cisplatin-induced nephrotoxicity in lung cancer patients in comparison with two conventional urinary proteins N-acetyl-β-d-glucosaminidase (NAG) and β2-microglobulin.
Materials and methods
Patients and study design
We enrolled 11 primary lung cancer patients treated with cisplatin-based chemotherapy at Kyoto University Hospital between June 2011 and June 2012. The administration schedules of chemotherapy and supportive therapy are shown in Fig. 1. None of the patients received magnesium supplementation.
Detail schedules of the chemotherapy regimens used. Detailed administration schedules of each regimen, VNR/CDDP or ETP/CDDP, for our lung cancer patients are shown. As the supportive therapy, maintenance fluid (Soldem 3A®) and saline are injected along with antiemetics (palonosetron and dexamethasone sodium phosphate). At the first day of chemotherapy, the patients received extensive continuous infusion of approximately 3000 mL. The standard dosages of VNR and ETP are 25 and 100 mg/m2, respectively. CDDP cisplatin, VNR vinorelbine, ETP etoposide
This study was conducted in accordance with the Declaration of Helsinki and was approved by the Kyoto University Graduate School and Faculty of Medicine Ethics Committee. All patients provided written informed consent.
Data collection and diagnostic criteria for acute kidney injury
Clinical information, treatments, and laboratory data were obtained from the patients’ electronic medical records. Considering that extensive continuous hydration (3000 mL/24 h) is provided with the administration of cisplatin, cisplatin-induced renal impairment was diagnosed in patients with an increase in the BUN level of more than 20 mg/dL and/or the Scr level of 50 % in comparison with the baseline. Patients whose BUN level at the administration of cisplatin was higher than 20 mg/dL were excluded. Urine samples were classified as either AKI positive (+) or negative (−).
Urine collection and biomarker analysis
We collected urine samples on the day before cisplatin administration (day 0) and subsequently on days 3, 7, and 14. Urine samples were collected into tubes with protease inhibitor cocktail tablets (Complete, Mini; Roche Diagnostics, Mannheim, Germany).
We measured the KIM-1 concentrations using Luminex xMAP microspheres with polyclonal antibodies raised against the ectodomain of human KIM-1, as described previously [14]. To measure the MCP-1 and NGAL concentrations, we used the Human CCL2/MCP-1 DuoSet and Human Lipocalin-2/NGAL DuoSet enzyme-linked immunosorbent assay kits (R&D Systems Inc., Minneapolis, MN), respectively, according to the manufacturer’s instructions. Briefly, a 96-well microplate was coated with capture antibodies and then blocked with 1 % bovine serum albumin in phosphate-buffered saline. Subsequently, 100-µL samples were incubated in the blocked wells for 2 h, followed by incubation with biotinylated detection antibodies for 2 h and streptavidin-conjugated horseradish peroxidase (HRP) for 20 min at room temperature. Finally, MCP-1 and NGAL were detected by adding HRP substrate and measuring the optical density at 450 nm.
The concentrations of NAG and β2-microglobulin, which are tubular injury markers, were measured by using commercial kits: the NAG test Shionogi (Shionogi Co., Osaka, Japan) and beta-2 Microglobulin Human SimpleStep ELISA™ Kit (Abcam, Cambridge, UK), according to the manufacturers’ instructions.
The levels of all biomarkers were normalized to the urinary creatinine concentration, which was measured by using an assay kit (LabAssay™ Creatinine; Wako Pure Chemical Industries, Osaka, Japan).
Statistical analyses
To evaluate the diagnostic accuracy of KIM-1, MCP-1, and NGAL, we calculated the area under the receiver operating characteristic (AUC-ROC) curve using SPSS version 18.0 (SPSS Inc., Chicago, IL). Differences were compared using the Mann–Whitney U test, and p values less than 0.05 were considered statistically significant. These analyses were performed using Prism version 5.0 (GraphPad, San Diego, CA).
Results
Patient characteristics
The patient characteristics are shown in Table 1. The mean (standard deviation [SD]) age of the patients in this study was 65.9 (10.1) years (range 49–77 years). All patients had stage III lung cancer. The baseline mean (SD) levels of Scr and BUN were 0.75 (0.26) mg/dL and 14.3 (3.9) mg/dL, respectively. Seven patients were treated with 80 mg/m2 cisplatin, while the remaining patients were administered lower doses due to reduced kidney function. The mean total cisplatin dosage was 108.1 (20.1) mg. Ten out of the 11 patients received cisplatin combined with vinorelbine (VNR).
Urinary and serum biomarkers of kidney function exhibit time-dependent changes during cisplatin-induced acute kidney injury
Surprisingly, all but one patient met the diagnostic criteria for AKI throughout the study. We observed time-dependent changes in the levels of serum and urinary biomarkers during cisplatin treatment. Figure 2 shows an example of these changes in a 49-year-old male patient with stage IIIA non-small cell lung adenocarcinoma treated with a combination of vinorelbine (20 mg/m2, 31 mg/body), cisplatin (80 mg/m2, 125 mg/body), and radiation. On day 3 after cisplatin treatment, AKI was diagnosed, because the BUN level exceeded 20 mg/dL. Between the cisplatin treatment initiation (day 0) and day 7, the Scr and BUN (Fig. 2a) and urinary KIM-1 and MCP-1 levels increased relative to the baseline, whereas the urinary NGAL levels decreased (Fig. 2b).
Changes in biomarker levels in a representative lung cancer patient with cisplatin-induced acute kidney injury. Time-dependent changes in the levels of serum and urinary biomarkers during cisplatin-based chemotherapy in a 49-year-old male patient with stage IIIA non-small cell lung adenocarcinoma. a Changes in serum creatinine (Scr; white circle) and blood urea nitrogen (BUN; black circle). Since BUN is greater than 20 mg/dL on day 3, this patient is diagnosed with acute kidney injury (AKI). b Changes in urinary levels of kidney injury molecule-1 (KIM-1; white circle), neutrophil gelatinase-associated lipocalin (NGAL; white triangle), and monocyte chemotactic protein-1 (MCP-1; black circle)
Urinary levels of kidney injury molecule-1 or monocyte chemotactic protein-1 can detect cisplatin-induced acute kidney injury
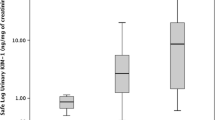
To determine whether the urinary levels of KIM-1, MCP-1, and NGAL can be used to detect cisplatin-induced AKI, we compared the levels of these biomarkers in 30 AKI (+) and 12 AKI (−) urine samples. First, we compared the absolute concentrations of urinary KIM-1, MCP-1, and NGAL, as shown in SF1. The concentrations of KIM-1 were significantly higher in AKI (+) samples than in AKI (−) samples (p < 0.01), while the MCP-1 and NGAL concentrations in the urine did not differ between the two groups. Next, to consider the inter-individual differences of urine samples, the concentrations of these markers normalized to urinary creatinine concentration were compared between AKI (−) samples and AKI (+) samples (Fig. 3). The urinary KIM-1 and MCP-1 levels in AKI (+) samples were significantly higher than in AKI (−) samples (p < 0.01; Fig. 3a, b). However, the NGAL concentrations did not significantly differ between AKI (+) and AKI (−) samples (Fig. 3c). These results suggested that the urinary levels of KIM-1 or MCP-1 could detect cisplatin-induced AKI. In addition, we measured the urinary concentrations of NAG and β2-microglobulin, as tubular toxicity markers (SF1d, e and Fig. 3d, e). There were no significant differences observed between the two groups, with or without normalization to the urinary creatinine concentrations.
Differences in urinary levels of kidney function biomarkers in lung cancer patients with or without acute kidney injury. Differences in the urinary levels of kidney injury molecule-1 (KIM-1) (a), monocyte chemotactic protein-1 (MCP-1) (b), neutrophil gelatinase-associated lipocalin (NGAL) (c), N-acetyl-β-d-glucosaminidase (NAG) (d), and β2-microglobulin (e) in acute kidney injury (AKI) positive (+) and AKI negative (−) samples from lung cancer patients treated with cisplatin. The biomarker concentrations are normalized to the urinary creatinine concentration. Statistical analyses are performed using the Mann–Whitney U test. **p < 0.01 versus AKI (−). Horizontal bar indicates the median value
Further, we compared the Scr levels to the concentrations of KIM-1, MCP-1, and NGAL (SF2). However, there was no correlation between the Scr level and any of the urinary biomarkers.
Receiver operating characteristic (ROC) curve analyses of urinary biomarkers
To confirm the above findings, we performed ROC curve analyses (Fig. 4). The AUC-ROCs of KIM-1, MCP-1, and NGAL were 0.858 (p < 0.01), 0.850 (p < 0.01), and 0.608 (p > 0.05), respectively (Table 2), supporting the conclusion that urinary KIM-1 or MCP-1 can accurately detect cisplatin-induced AKI in lung cancer patients. In addition, the cutoff values of KIM-1, MCP-1, and NGAL were 2.45, 0.26, and 17.2 ng/mg creatinine, respectively (Table 2).
Receiver operating characteristic curve analyses of urinary biomarkers of acute kidney injury. Receiver operating characteristic (ROC) curves demonstrating the sensitivity and specificity of kidney injury molecule-1 (KIM-1) (a), monocyte chemotactic protein-1 (MCP-1) (b), neutrophil gelatinase-associated lipocalin (NGAL) (c), and the combination of KIM-1 and MCP-1 (d) with respect to the definition of acute kidney injury (AKI) by serum creatinine or blood urea nitrogen. AUC area under the curve
Combination of kidney injury molecule-1 and monocyte chemotactic protein-1 enhances detection of cisplatin-induced acute kidney injury
Since a combination of two biomarkers may be better than a single biomarker, we tested whether a combination of KIM-1 and MCP-1 can detect cisplatin-induced AKI better than either biomarker alone. We defined the combination biomarker as follows:
In this equation, the KIM-1 and MCP-1 concentrations (denoted by i) are normalized to their ROC cutoff values. The AUC-ROC for this combination, 0.871, was higher than that of either KIM-1 or MCP-1 alone (p < 0.001; Fig. 4d).
Discussion
In this study, we examined whether KIM-1, NGAL, and MCP-1 can detect cisplatin-induced AKI in lung cancer patients. Our results suggested that KIM-1 and MCP-1, but not NGAL as well as NAG and β2-microglobulin, can discriminate between AKI (+) and AKI (−) urine samples. The potential usefulness of KIM-1 is consistent with a previous report that urinary KIM-1 is a sensitive and accurate biomarker of cisplatin-induced AKI in both preclinical and clinical settings [15]. Recently, Tekce et al. [16] also reported that urinary KIM-1 concentrations on the first day after treatment may predict cisplatin-induced AKI with high sensitivity and specificity in the clinical setting. Although there have not been any studies using MCP-1 as a biomarker of cisplatin-induced AKI, our previous findings that cisplatin-induced nephrotoxicity increases urinary MCP-1 in rats [12] also support this conclusion. Further, although the concentrations of these markers did not correlate with the Scr levels, our results indicate the possibility that these new urinary biomarkers are more sensitive and specific than Scr for cisplatin-induced AKI.
However, our NGAL findings contradict recent reports that it may be an early biomarker of AKI in cancer patients treated with cisplatin-based chemotherapy [17–19]. There are two possible reasons for this discrepancy. First, the NGAL levels may have differed among studies because urine samples are collected and analyzed at different time points. For example, Lin et al. [18] analyzed urine samples between 4 h and 4 days after cisplatin infusion and found that urinary NGAL levels significantly increased between 12 h and 3 days later in AKI (+) samples compared with the baseline levels. In contrast, we measured NGAL concentrations at later time points when they would be lower in AKI (+) samples and, thus, more similar to those in AKI (−) samples. In our study, analyzing urine samples at earlier time points was not possible because the patients were prehydrated, so their urine would have been too diluted during the first 2 or 3 days after cisplatin administration. Second, the NGAL levels may differ among the studies due to differences in the diagnostic criteria for AKI. Many investigators diagnose AKI using the Risk, Injury, Failure, Loss of kidney function and End-stage kidney disease or Acute Kidney Injury Network classifications, which are based on changes in the Scr levels and urine output. In our study, we used expedient criteria slightly modified from the KDIGO (Kidney Disease: Improving Guideline Outcomes) criteria; if the level of Scr was increased more than 1.5-fold compared to the baseline and/or that of BUN was over than 20 mg/dL after the administration of cisplatin, the sample was classified as AKI (+). There are two reasons for why we used the modified KDIGO criteria in this study. First, because the urine outputs of our patients were too high due to the prehydration, we could not accurately diagnose AKI based on the urine output. Second, because the Scr levels are influenced by the muscle mass, it is inadequate to monitor the kidney function based on only the Scr levels, particularly in elderly patients. Hence, we defined the criteria based on the levels of both Scr and BUN. However, further research is needed to determine more specific diagnostic criteria for cisplatin-induced AKI.
Finally, our finding that the AUC-ROC of the combination of KIM-1 and MCP-1 is higher than that of either biomarker alone (Fig. 4d) is consistent with previous reports that a combination of two biomarkers may have better diagnostic performance than a single biomarker [20, 21]. However, unlike these previous studies, which used a logistic regression model to combine two biomarkers, we simply summed the concentrations of two biomarkers normalized to their ROC cutoff values, because this would be easier to calculate in the clinical setting. Although a dipstick assay for KIM-1 has recently become available [15], there is no similar assay for measuring MCP-1 levels quickly and accurately; such an assay could enable early detection and improved treatment of cisplatin-induced AKI.
In conclusion, we here showed that urinary KIM-1 and MCP-1, either alone or in combination, may represent accurate biomarkers of cisplatin-induced AKI in lung cancer patients. These findings may also facilitate the development of new methods to monitor kidney function. However, because of the small number of patients in the present study, larger studies are required in the future to confirm our findings.
Abbreviations
- AKI:
-
Acute kidney injury
- KIM-1:
-
Kidney injury molecule-1
- MCP-1:
-
Monocyte chemotactic protein-1
- NGAL:
-
Neutrophil gelatinase-associated lipocalin
- AUC-ROC:
-
Area under the receiver operating characteristic curve
- Scr:
-
Serum creatinine
- BUN:
-
Blood urea nitrogen
References
Lebwohl D, Canetta R (1998) Clinical development of platinum complexes in cancer therapy: an historical perspective and an update. Eur J Cancer 34(10):1522–1534
Hayes DM et al (1977) High dose cis-platinum diammine dichloride: amelioration of renal toxicity by mannitol diuresis. Cancer 39(4):1372–1381
Bonventre JV et al (2010) Next-generation biomarkers for detecting kidney toxicity. Nat Biotechnol 28(5):436–440
Yonezawa A et al (2005) Association between tubular toxicity of cisplatin and expression of organic cation transporter rOCT2 (Slc22a2) in the rat. Biochem Pharmacol 70(12):1823–1831
Sieber M et al (2009) Comparative analysis of novel noninvasive renal biomarkers and metabonomic changes in a rat model of gentamicin nephrotoxicity. Toxicol Sci 109(2):336–349
Ichimura T et al (2004) Kidney injury molecule-1: a tissue and urinary biomarker for nephrotoxicant-induced renal injury. Am J Physiol Renal Physiol 286(3):F552–F563
Ichimura T et al (1998) Kidney injury molecule-1 (KIM-1), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is up-regulated in renal cells after injury. J Biol Chem 273(7):4135–4142
Mishra J et al (2003) Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol 14(10):2534–2543
Mishra J et al (2005) Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet 365(9466):1231–1238
Bonventre JV (2009) Kidney injury molecule-1 (KIM-1): a urinary biomarker and much more. Nephrol Dial Transplant 24(11):3265–3268
Mishra J et al (2004) Neutrophil gelatinase-associated lipocalin: a novel early urinary biomarker for cisplatin nephrotoxicity. Am J Nephrol 24(3):307–315
Nishihara K et al (2013) Urinary chemokine (C–C motif) ligand 2 (monocyte chemotactic protein-1) as a tubular injury marker for early detection of cisplatin-induced nephrotoxicity. Biochem Pharmacol 85(4):570–582
Rice JC et al (2002) Monocyte chemoattractant protein-1 expression correlates with monocyte infiltration in the post-ischemic kidney. Ren Fail 24(6):703–723
Vaidya VS et al (2010) Kidney injury molecule-1 outperforms traditional biomarkers of kidney injury in preclinical biomarker qualification studies. Nat Biotechnol 28(5):478–485
Vaidya VS et al (2009) A rapid urine test for early detection of kidney injury. Kidney Int 76(1):108–114
Tekce BK et al (2015) Does the kidney injury molecule-1 predict cisplatin-induced kidney injury in early stage? Ann Clin Biochem 52(Pt 1):88–94
Gaspari F et al (2010) Predicting cisplatin-induced acute kidney injury by urinary neutrophil gelatinase-associated lipocalin excretion: a pilot prospective case-control study. Nephron Clin Pract 115(2):c154–c160
Lin HY et al (2013) Urinary neutrophil gelatinase-associated lipocalin levels predict cisplatin-induced acute kidney injury better than albuminuria or urinary cystatin C levels. Kaohsiung J Med Sci 29(6):304–311
Peres LA et al (2014) Evaluation of the cisplatin nephrotoxicity using the urinary neutrophil gelatinase-associated lipocalin (NGAL) in patients with head and neck cancer. J Bras Nefrol 36(3):280–288
Katagiri D et al (2012) Combination of two urinary biomarkers predicts acute kidney injury after adult cardiac surgery. Ann Thorac Surg 93(2):577–583
Liang XL et al (2010) Combination of urinary kidney injury molecule-1 and interleukin-18 as early biomarker for the diagnosis and progressive assessment of acute kidney injury following cardiopulmonary bypass surgery: a prospective nested case-control study. Biomarkers 15(4):332–339
Acknowledgments
This work was supported in part by a Grant-in-Aid for Scientific Research (KAKENHI) from the Ministry of Education, Science, Culture, Sports, and Technology of Japan (MEXT); a Grant-in-Aid for Research on Biological Markers for New Drug Development and Health and Labour Sciences Research Grants from the Ministry of Health, Labour, and Welfare of Japan (08062855); and a funding program for Next Generation World-Leading Researchers (LS073 to SM) from the Council for Science and Technology Policy of the Japan Society for the Promotion of Science.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shinke, H., Masuda, S., Togashi, Y. et al. Urinary kidney injury molecule-1 and monocyte chemotactic protein-1 are noninvasive biomarkers of cisplatin-induced nephrotoxicity in lung cancer patients. Cancer Chemother Pharmacol 76, 989–996 (2015). https://doi.org/10.1007/s00280-015-2880-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-015-2880-y