Abstract
Purpose
Sunitinib is an inhibitor of tyrosine-kinase receptors, and no biomarker predictive of sunitinib response is available. The purpose of this preclinical study was to show whether sunitinib molecular targets could be used as biomarkers to assess tumor response to sunitinib in human cancer cell line xenografts of three different tumor types.
Methods
Using mice xenografted with liver, breast and renal carcinoma cell lines, we sequentially analyzed the effect of 7-day sunitinib treatment on tumor and vascular compartments.
Results
In all xenografts, microvessel damage occurred from Day 1. Tumor damage also occurred in liver, breast, but not in renal xenografts. Using specific human and mouse probes for genes encoding sunitinib targets, we showed a significant relation between apoptotic tumor cell numbers and human PDGFRΒ and RET mRNA expression in liver cancer and to human VEGFR2 expression in breast cancer xenografts. In contrast, in renal cancer xenografts, vascular effect evaluated by measuring endothelial cell apoptosis was related to mouse Vegfr1, Vegfr2 and Vegfa-164 expression.
Conclusion
This study identifies sunitinib vascular and tumor effects according to different tumor types and shows that sunitinib molecular targets used as biomarkers enable assessment of therapeutic response.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recent therapies targeting tumor angiogenesis have shown clinical benefits in metastatic liver, breast and kidney carcinoma [1–3]. Nevertheless, biomarkers are still needed in order to better assess the therapeutic response or resistance in individual patients and to establish new treatment regimens. Sunitinib (Sutent, SU11248, Pfizer) is an orally administered inhibitor of tyrosine-kinase receptors. In advanced renal cell carcinoma, sunitinib-associated hypertension was shown to be associated with improved clinical outcomes, supporting its viability as an efficacy marker [4]. Nevertheless, primary resistance to sunitinib has occurred in about 30 % of patients [1, 5, 6]. In hepatocarcinoma and metastatic breast carcinoma, primary resistance to sunitinib is even more frequent [7–9], thereby limiting its use. Novel biomarkers are therefore needed to predict therapeutic response and also resistance to sunitinib.
Several studies have aimed to identify biomarkers associated with sunitinib response [10–12]. For example, VEGFA mRNA basal expression was found to be associated with response to sunitinib treatment in metastatic renal cell carcinoma [13]. In addition, a large set of data on VHL gene status [10] and HIF expression [14] is available, but results are contradictory and so far do not efficiently predict response to sunitinib. Preclinical studies, using mice xenografted with human cancer cell lines [15–17], have focused on the anti-angiogenic activity of sunitinib, showing a decrease in microvessel density [16–20]. However, little is known about its effects on tumor cells. Here, we explored the tissue effects of sunitinib on the microvessel network and on tumor cells at different time points using mice xenografted with liver, breast and renal carcinoma cell lines (SkHep1, MCF-7 and 786-O, respectively). We first demonstrated that sunitinib targets either the vascular stroma alone in 786-O cells or both the vascular stroma and tumor cells in SkHep1 and MCF-7 xenografts. The main sunitinib targets are VEGFR1, VEGFR2, PDGFRα and PDGFRβ, KIT, FLT3 and RET [18, 21]. Using quantitative RT-PCR, these xenograft models enabled us to perform separate analyses of the expression of these targets as well as VEGFA-165 in (1) human tumor cells, using specific human probes and (2) CD105-sorted mouse tumor endothelial cells, using mouse probes for the same targets as well Vegf-164. These analyses allowed us to assess whether the effects on tumor and endothelial cells were related to gene expression of sunitinib targets in each tumor-type. Altogether, our results show that sunitinib targets can be used as biomarkers, as their gene expression level correlates with the tumor-type-specific response to sunitinib treatment.
Materials and methods
Cell lines
Three human cell lines, 786-O (kidney carcinoma, with VHL mutation), MCF-7 (breast carcinoma) and SkHep1 (hepatocarcinoma), were obtained from the ATCC (Rockville, MD). Cell cultures are described in Supplemental Methods.
In vitro cytotoxicity and proliferation inhibition
Cells were exposed to increasing concentrations of sunitinib ranging from 0.5 to 16 μmol/L. After 2-day incubation, cytotoxicity was determined by the colorimetric conversion of tetrazolium MTT as described in Supplemental Methods.
Mice xenografted with human cell lines
Mice purchased from Janvier, France, were maintained in pathogen-free animal housing (University-Institute-Haematology, Paris). For each cell line studied, 5 × 106 cells were grafted subcutaneously in 6-week-old female NMRI nude mice, under xylazine (10 mg/kg) and ketamine (100 mg/kg) anesthesia. This study was approved by the University-Institute board ethics committee for animal studies. Tumor growth was measured daily in two perpendicular diameters with a caliper. Volumes were calculated as V = L × l 2 ÷ 2, L being the largest diameter (length), l the smallest (width) [22]. For each tumor, growth curves were established. When tumors reached a volume of 100 mm3, the mice were given sunitinib diluted in 0.9 % NaCl once daily.
Four concentrations of sunitinib, ranging from 0.84 to 40.00 mg/kg/day, were studied in the three xenograft models. These first results led us to treat mice at 2 mg/kg/day for the following experiments, where mice were euthanized at Day 0 (without treatment) and at Days 1, 3, 5 and 7 after treatment. Five mice were studied for each time point. Freshly dissected tumors were cut into four parts: one part was treated for endothelial cell sorting, one snap-frozen, one formaldehyde-fixed and paraffin-embedded and one glutaraldehyde-fixed for electron microscopy [23].
Tumor endothelial cell sorting
Fresh tumors minced mechanically were strained through a 70-μm sieve to obtain a single-cell suspension. Tumor cells were washed three times with a PBS buffer containing 0.5 % bovine serum albumin. Cells were incubated 30 min at 4 °C with anti-mouse CD105 antibody (monoclonal rat, 1/50, clone MJ7/18, BD Pharmingen, France). Unbound antibody was washed off, and cells were incubated for 15 min at 4 °C with microbeads conjugated with a rat IgG2a antibody (Miltenyi Biotech, France). Sorting was performed as recommended by the manufacturer. Positive cells were counted and prepared for RNA extraction.
Quantitative RTPCR of sunitinib molecular targets and VEGFA isoforms
This study was performed using human probes on the three cancer cell lines, on untreated xenografted tumors (Day 0) and on sunitinib-treated xenografted tumors (Day 7). Mouse endothelial cells, sorted from xenografted tumor samples at Day 0 and Day 7, were studied using mouse probes.
RNA extraction, quantification and qualification and qPCR are explained in Supplemental Methods.
The following human genes were analyzed: VEGFA-165 [Hs00900058_m1], VEGFR1 [Hs01052936_m1], VEGFR2 [Hs00176676_m1], PDGFRΑ [Hs00183486_m1], PDGFRΒ [Hs00199831_m1], KIT [Hs00174029_m1], RET [Hs00240887_m1], FLT-3 [Hs00975659_m1] and TBP [Hs99999910_m1]. The following mouse genes were analyzed: Vegfa-164 [Mm00437308_m1], Vegfr1 [Mm00438980_m1], Vegfr2 [Mm01222431_m1], Pdgfrα [Mm01211694_m1], Pdgfrβ [Mm01262489_m1] and Gapdh [Mm99999915_g1] (Applied Biosystem, France).
Quantitative in situ studies: necrosis, cell proliferation, apoptosis
In all cases, analyses were performed by two pathologists (AJ, MV) unaware of the tumor-type and the day of treatment.
Necrosis was studied on virtual slides created on a Nanozoomer 2.0 HT scanner (Hamamatsu, Japon) from hematoxylin–eosin-stained paraffin sections. Areas of necrosis were delineated by the two pathologists on the virtual slides and quantified using DotSlide 2 software.
Microvessel density was assessed on tissue sections using CD31 monoclonal rat anti-mouse antibody (Dianova, Germany, clone SZ31) at 1:20 dilution as primary antibody. An indirect immunoperoxidase method was used, with controls having no primary antibody or an irrelevant primary antibody of same isotype. The number of stained blood vessels was counted on ten different fields at ×400 magnification, on a Provis-AX-70 microscope (Olympus–Tokyo). Results were expressed as mean ± standard error of the mean (SEM).
Human tumor cell proliferation and human tumor cell apoptosis were assessed on tissue sections using, respectively, a monoclonal mouse anti-human Ki67 antibody (clone MIB-1, DakoCytomation, France) and a polyclonal rabbit anti-human cleaved-caspase-3 antibody (Asp175, Cell Signaling, France) as primary antibodies. An indirect immunoperoxidase method was used. Immunostained cell counts were performed on ten different fields at ×400 magnification. Results were expressed as mean ± SEM.
Electron microscopy (Hitachi-7650) was used to count apoptotic endothelial cells in tumor microvessels. Apoptosis was defined according to consensus criteria [24]. A minimum of 50 microvessel sections was analyzed for each sample, and results expressed as mean ± SEM.
Statistical analyses
For quantitative in situ studies, the differences between analyses before and after treatment were assessed using the Wilcoxon signed-rank test. A p value ≤0.05 was considered to be significant. For correlation studies, the Pearson correlation coefficient was calculated between the level of mRNA expression of the molecular targets of sunitinib in tumor or endothelial cells at Day 0 and the apoptotic cell counts at Day 7. SPSS Statistics 17.0 software was used to perform the analyses.
Results
Sunitinib induces differential tumor and endothelial cell apoptosis in xenograft models of renal, breast and hepatic carcinomas
In vitro, cytotoxicity tests showed that sunitinib inhibitory concentration (IC50) values for MCF-7 (Fig. 1a), SkHep1 (Fig. 1b), 786-0 (Fig. 1c) human cancer cell lines were 5 μmol/L (±0.3), 5.4 μmol/L (±0.15) and 6.1 μmol/L (±0.22), respectively. The three human cancer cell lines were selected from a panel of more than 20 cell lines, because of greater sensitivity to sunitinib (Figure S1—supplementary Figure). In comparisons with untreated controls, sunitinib inhibited proliferation of MCF-7 (Fig. 1d) and SkHep1 (Fig. 1e) but not 786-0 cell lines (Fig. 1f), at 0.5 μmol/L concentration, a dose known to be pharmacologically active in vitro and in vivo [18, 25].
In vitro and in vivo dose effects of sunitinib on three human cancer cell lines and in three xenograft models. For the in vitro data (a–f), experiments were done in triplicate. Inhibitory concentration 50 (IC50) toward sunitinib was 5 μmol/L (±0.3), 5.4 μmol/L (±0.15) and 6.1 μmol/L (±0.22) for MCF-7, SkHep1 and 786-0 cell lines, respectively. For MCF-7 and SkHep1 cell lines, inhibition of proliferation was observed at the pharmacologically active dose of 0.5 μmol/L, while it was not for 786-0 cell line. For the in vivo experiments (g–i), mice (n = 5 per dose) were treated daily with sunitinib at 0.84, 2, 8 and 40 mg/kg, respectively. Tumors were measured at Day 0 and Day 7, and tumor volumes were calculated. A dose-dependent tumor growth inhibition was observed for the three xenograft models (SkHep-1, MCF-7 and 786-O), with a significant tumor growth inhibition at 2 mg/kg/day. *p < 0.05
In vivo, in the three types of xenografts treated with increasing doses of sunitinib (0.84; 2; 8; 40 mg/kg/day), we showed that the dose of 2 mg/kg/day was the first efficient dose in inhibiting tumor growth (p < 0.05) (Fig. 1g–i). In addition, we hypothesized that a dose of 40 mg/kg/day was too high for modeling in mice sunitinib tumor cell effects observed in patients. In patients, after 28 days of continuous treatment with a 50-mg daily dose of sunitinib, the peak of concentration (C max) in serum is around 70 ng/mL [25]. In mice, a comparable C max is obtained after a dose of 5 mg/kg. After a dose of 40 mg/kg, the C max is much higher than 100 ng/mL [18]. In addition, in the mice we treated with a 40-mg/kg daily dose of sunitinib, after 7 days of treatment, we observed severe cardiac and hepatic toxicities (i.e, round steatosis vacuoles with picnotic hepatocytes surrounding centrolobular veins, congestive capillaries with reduced thickness of cardiomyocytes; see Supplementary Figure S2). By contrast, at the minimal efficient dose of 2 mg/kg/day, in the three xenograft models, our results showed that sunitinib induced mild liver or cardiac toxicity.
We then performed kinetic analyses of microvessel density, necrosis, proliferation and apoptosis in the three models treated with 2 mg/kg/day sunitinib. Microvessel distribution was tumor-type dependent at baseline, typically more peripheral in MCF-7 xenografts (Supplementary Figure S3), and microvessel density significantly decreased from Day 1 in all three xenografts (Fig. 2a). Tumor necrosis occurred at Day 3 in SkHep1 and MCF-7 xenografts, whereas it was detected only at Day 5 in 786-O (Fig. 2b; Supplementary Figure S4). In addition, at this 2 mg/kg/day dose, sunitinib efficiently inhibited tumor cell proliferation at Day 3 in SkHep1 and MCF-7 xenografts, whereas this was never observed in 786-O tumors (Fig. 2c; Supplementary Figure S4). We then wondered whether tumor cell apoptosis was affected in the same manner. Sunitinib significantly induced tumor cell apoptosis in SkHep1 and MCF-7 xenografts but not in 786-O tumors (Fig. 2d; Supplementary Figure S4).
In vivo cell and tissue effects of sunitinib on three human cancer cell line xenografts. At the dose of 2 mg/kg/day, quantitative data were assessed on five different fields for each tumor and for five different mice in each group. For necrosis extent (b), a significant difference was found at Day 3 in both SkHep1 and MCF-7 xenografts and at Day 5 in 786-O xenografts. For tumor cell proliferation (c), Ki67 positive cells counts significantly decreased at Day 3 in SkHep1 and MCF-7 xenografts, but not in 786-O xenograft. For tumor cell apoptosis (d), cleaved-caspase 3 positive cells counts significantly increased at Day 5 in MCF-7 and SkHep1 xenografts, but not in 786-O xenograft. *p < 0.05. ns = not significant
Having observed that sunitinib was able to induce necrosis and decrease microvessel density, we analyzed how the vascular compartment was targeted in these three xenograft models of renal, breast and hepatic carcinomas. Using electron microscopy, apoptotic endothelial cells were quantified on semi-thin sections. This analysis made it possible to determine that sunitinib displayed the same efficacy in inducing endothelial apoptosis in these three models (Fig. 3, panel a–c).
Sunitinib-induced endothelial cell apoptosis in tumor cell line xenografts. Using electron microscopy, on semi-thin sections, endothelial cells with large nuclei and regularly distributed chromatin (white arrow) were found within microvessel section at Day 0 (white broken lines = microvessel basal membrane). At Day 7 in SkHep1 xenograft (a), microvessel sections showed major damage with apoptotic endothelial cells (white arrow heads) and microthrombi (T) at Day 7. In MCF-7 xenografts (b), on semi-thin sections, microvessels had a more irregular shape (white broken lines = microvessel basal membrane) and apoptotic endothelial cells with reduction in cellular volume (pyknosis) were observed (white arrow head). At higher magnification, chromatin condensation and distribution in the nucleus periphery (double black arrow) are clearly seen on apoptotic endothelial cells. In 786-O xenografts, microvessel sections showed endothelial damage with pycnotic nuclei (white arrow heads), together with an accumulation of apoptotic nuclei and fibrin deposits in the vessel lumen. The number of apoptotic endothelial cells significantly increased between Day 0 and Day 7 in all xenografted models. RBC = red blood cell. *p < 0.05
Altogether, these results suggest that sunitinib targets both the tumor and vascular compartments in SkHep1 and MCF-7 xenografts, but only the vascular compartment in 786-O tumors. Interestingly, this translates as early necrosis at Day 3 in SkHep1 and MCF-7 xenografts, whereas it was only detected later, at Day 5, in 786-O.
Tumor-type-specific regulation of genes encoding sunitinib targets in vitro and in vivo
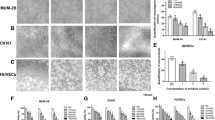
Having shown that sunitinib is able to target either the tumor or the vascular stroma in these models, we next wondered whether its differential effect on tumor and endothelial apoptosis might be due to differential expression of genes encoding its targets, i.e, VEGFR1, VEGFR2, PDGFRα, PDGFRβ, KIT, RET and FLT-3. We also studied VEGFA-165 isoform expression. The expression levels of these genes were therefore investigated both in vitro and in vivo in the three tumor cell types. In vitro, we showed a different gene expression pattern in the three cell types. Only VEGFR2, RET, PDGFRΒ and VEGFA-165 mRNAs were expressed in SkHep1 cells (Fig. 4a), whereas MCF-7 cells expressed VEGFR1, VEGFR2, KIT, RET, FLT-3 and VEGFA-165 mRNAs (Fig. 4b), and 786-O cells expressed VEGFR2, PDGFRΑ, PDGFRΒ, RET and VEGFA-165 mRNA (Fig. 4c).
qRTPCR for sunitinib targets on cell lines (a–c) and tumor xenografts using human (d–f) or mouse (g–i) probes. For the cell lines (a–c), experiments were done in triplicate. The housekeeping gene tbp was used to normalize gene expression results. The results were expressed in (−ΔCT). For the xenografts (d–i), five mice of each type were used and experiments were done in triplicate. The same concentration of RNA was used for all the samples, and the quality of RNA was checked using Agilent electrophoresis (Agilent, France). The housekeeping genes TBP (d–f) or Gapdh (g–i) were used. At Day 0, we showed a loss of mRNA expression for most human targets of sunitinib compared to human tumor cell lines. At Day 7, decreased mRNA expression of PDGFRΒ (RQ = 0.07, p < 0.05), RET (RQ = 0.12, p < 0.05), and VEGFA-165 (RQ = 0.18, p < 0.05) was found in SkHep1 xenograft (D), and decreased mRNA expression of human VEGFR2 (RQ = 0.33, p < 0.05) in MCF-7 xenograft (e). In mouse CD105-positive cells from treated tumors (Day 7), we showed decreased mRNA expression of Vegfa-164 in the MCF-7 (h) and 786-O xenografts (i), but not in the SkHep1 xenograft (G). In MCF-7 xenograft, mRNA expression of Vegfr1 (RQ = 0.2, p < 0.05), Vegfr2 (RQ = 0.28, p < 0.05), Pdgfrα (RQ = 0.23, p < 0.05) and Pdgfrβ (RQ = 0.07, p < 0.05) decreased at Day 7 (h). In 786-O, Vegfr1 (RQ = 0.39, p < 0.05) and Vegfr2 (RQ = 0.22, p < 0.05) expression decreased at Day 7 (i)
We then performed quantitative RT-PCR analyses using either human-specific or mouse-specific probes on tumor samples, thereby making it possible to decipher which genes encoding sunitinib targets are expressed in human cancer cells versus those expressed in the mouse tumor stroma. These analyses revealed striking differences when compared to the in vitro results. In untreated tumors, human VEGFR1 mRNA expression was up-regulated in SkHep1 xenografts (Fig. 4d, dark bar), whereas it was down-regulated in MCF-7 xenografts (Fig. 4e, dark bar) and unchanged in 786-O cells (Fig. 4f, dark bar). Human RET expression was also modulated in vivo when compared to in vitro, as its expression was lost in MCF-7 xenografts (Fig. 4e, dark bar). We then wondered whether the expression of these genes was affected by sunitinib treatment. We demonstrated that mRNA expression of PDGFRΒ, RET and VEFGA-165 was affected by sunitinib in SkHep1 xenograft, whereas only VEGFR2 was affected in MCF-7 xenograft (Fig. 4d–e, gray bar). Interestingly, and in line with our observations that neither proliferation nor apoptosis was affected by sunitinib in 786-O xenografts, we demonstrated here that none of the genes encoding sunitinib targets were differentially regulated in vivo in 786-O xenografts.
We next explored whether the expression of these genes was affected in the vascular stroma (sorted CD105-positive mouse endothelial cells of these tumors), using mouse-specific probes. We first showed that Vegfr1, Vegfr2, Pdgfrα, Pdgfrβ and Vegfa-164 were expressed in the three untreated xenografts (Fig. 4g–i—black bar). In sunitinib-treated tumors, none of these genes were affected in SkHep1 xenografts (Fig. 4g, gray bar), whereas Vegfr1, Vegfr2, Pdgfrα, Pdgfrβ and Vegfa-164 were all affected in MCF-7 xenografts (Fig. 4h, gray bar) and Vegfr1, Vegfr2, Vegfa-164 in 786-O xenografts (Fig. 4i, gray bar). Altogether, these data therefore showed that sunitinib has tumor-type-specific effects and specifically affects its target genes expression both in the tumor and vascular compartments in MCF-7 xenografts and either in the tumor compartment in SkHep-1 or only in the vascular compartment in 786-O xenografts.
Tumor and endothelial cell apoptosis is correlated with the level of molecular expression of sunitinib targets
We then sought to determine whether tumor or endothelial apoptosis induced by sunitinib might be correlated with differences in the expression levels of genes encoding its targets. We performed correlation analyses between the number of apoptotic cells in either tumor or endothelial cells and the expression of these genes. A significant correlation was observed between the number of apoptotic tumor cells and tumor mRNA expression levels of human PDGFRΒ and RET in SkHep1 xenograft (R 2 = 0.91 and 0.96, respectively, p < 0.01) (Fig. 5a) and VEGFR2 in MCF-7 xenograft (R 2 = 0.81, p < 0.05) (Fig. 5b). The same analysis was performed on genes expressed by mouse endothelial cells in 786-O xenografts. We showed reduced mRNA expression of Vegfr1 (R 2 = 0.91, p < 0.05) and Vegfr2 (R 2 = 0.93, p < 0.05) and Vegfa-164 (R 2 = 0.91, p < 0.05), but of none of the human genes encoding sunitinib targets (Fig. 5c).
Correlations between vegfa isoform and molecular targets of sunitinib mRNA expression levels at Day 0 and apoptotic cell counts at Day 7 in MCF-7, SkHep1 and 786-O tumor xenografts. mRNA expression levels at Day 0 are expressed in –dCT, the housekeeping genes TBP (a, b) and Gapdh (c) being used to normalize gene expression results. The Pearson coefficient of correlation (R 2) was calculated between the percentage of apoptotic cell at Day 7 and the mRNA expression levels of molecular targets of sunitinib at Day 0. The SPSS statistics 17.0 was used to perform the analyses. A significant correlation was shown between percentages of apoptotic tumor cells at Day 7 and mRNA expression levels at Day 0 of human PDGFRΒ and RET in SkHep1 xenograft (R 2 = 0.96 and 0.91, respectively) (a) and of human VEGFR2 in MCF-7 xenograft (R 2 = 0.81) (b). For 786-O xenograft (c), in CD105 mouse endothelial cells at Day 0, we found a significant correlation between mRNA expression levels of Vegfr1 (R 2 = 0.91), Vegfr2 (R 2 = 0.93), Vegfa164 (R 2 = 0.91) and the number of tumor endothelial apoptotic cells at Day 7. *p value <0.05
Discussion
In metastatic clear cell carcinoma patients treated with sunitinib, Rini et al. [26] reported that the plasma levels of VEGF-A and PlGF increased significantly after 28 days. Deprimo et al. [1] also reported that VEGF and PlGF levels increased more than threefold in 44 and 40 % of cases, respectively, after 28 days of treatment. In parallel, sVEGFR-2 levels decreased by at least 20 % in all patients, and sVEGFR-3 levels decreased by ≥30 in 87 % cases [1]. These effects were dependent on drug exposure, since levels tended to return to near baseline after 2 weeks without sunitinib. These analyses support the hypothesis that these angiogenesis-associated proteins might be used as circulating biomarkers, and research is ongoing to better understand the underlying mechanisms and assess their utility. In addition, hypertension [4] and circulating endothelial cells [27, 28] have also been reported to correlate with outcome in advanced renal cell carcinoma patients treated with sunitinib. Nevertheless, it is still a matter of debate as to whether hypertension, circulating endothelial cells or angiogenesis-related circulating proteins are bona fide reliable markers in metastatic clear cell carcinoma for use in clinical settings. In addition, the use of the proposed biomarkers in clear cell carcinoma as universal biomarkers for any other type of cancer is as yet unproven.
In our study, we first analyzed tissue response to sunitinib in xenograft models of renal, breast and liver carcinoma. It can be noted that despite the efficacy of sunitinib in the treatment of ccRCC, its targets and mechanisms of action remain unclear, and direct anti-tumoral effects of sunitinib have not been clearly established in vivo. One study showed apoptotic effects of sunitinib on ccRCC cells in vivo but the reported doses were far higher than pharmacologically relevant doses [29]. In contrast, Huang et al. [19] proposed that sunitinib acts primarily on endothelial cells rather than on tumor cells, as sunitinib is not cytotoxic for ACHN, A-498 or 786-O human renal cancer cell lines, while it is cytotoxic for HUVEC and HLMVEC endothelial cells, at relevant pharmacological doses. It is therefore yet not fully understood whether sunitinib acts primarily through an anti-angiogenic mechanism or whether it also acts by directly targeting ccRCC tumor cells. In the present study, we have first shown that 2 mg/kg/day sunitinib has significant anti-tumoral activity in all three models tested. In these models, sunitinib targets the vascular stroma, inducing endothelial apoptosis, as expected for anti-angiogenics. Our analysis further showed that sunitinib also directly targets tumor cells in SkHep-1 and MCF-7 xenografts, but not in 786-O xenografts. Necrosis is a well-known effect of VEGFR inhibition [18, 30–32], and it is partially related to vessel damage. Accordingly, tumor necrosis was observed as soon as Day 3 in SkHep1 and MCF-7 xenografts, whereas it was detected only at Day 5 in 786-O.
Considering the pharmacological profile of sunitinib, we then postulated that quantifying the different expression levels of genes encoding its targets (VEGFR1, VEGFR2, PDGFRΑ, PDGFRΒ, KIT, RET and FLT-3) and the VEGFA-165 isoform might enable us to determine biomarkers. Indeed, although sunitinib may induce tumor cell apoptosis in MCF-7 xenograft by blocking VEGFR2, it is not clear how it could affect the level of expression of this target. It was recently reported that blocking VEGFR2 with sunitinib inhibits VEGFR2 translocation to the nucleus and its own transcription [33]. Therefore, we first analyzed the expression of these specific genes in all three xenografts using specific human and mouse primers and performed quantitative RT-PCR analyses. Our results showed (1) that these xenografts expressed various combinations of genes and (2) that these genes are expressed at different levels. The results therefore raise the hypothesis that sunitinib action may be tumor-specific. To our knowledge, this has never been demonstrated before in vivo at the molecular level. A correlation between the cytotoxic effect of sunitinib and basal mRNA expression of PDGFRB in three human cancer cell lines of human RCC has previously been reported in vitro [34]. In 67 patients with clear cell RCC, the protein expression of PDGFRB in cancer cells before treatment with sunitinib is associated with a better response [35].
In our study, we then analyzed whether the differential expression of these genes correlated with the effect of sunitinib, evaluated by measuring either the number of apoptotic tumor cells or the number of apoptotic endothelial cells. A significant correlation was observed between the number of apoptotic tumor cells at Day 7 and mRNA basal expression levels of human PDGFRΒ and RET in SkHep1 xenografts and VEGFR2 in MCF-7 xenografts. In 786-O xenografts, in which sunitinib targeted only the vascular stroma, we showed a reduced basal expression of Vegfr1, Vegfr2 (R 2 = 0.93, p < 0.05) and Vegfa-164 genes in isolated mouse tumor endothelial cells.
Although this study was restricted to a small number of gene expression analyses, performed in only three tumor types, it nevertheless identifies a tumor-type-specific effect of sunitinib, targeting either the vascular stroma only or both the vascular stroma and tumor cells. This study also shows that sunitinib molecular targets, the expression of which is altered in endothelial cells or in both endothelial and tumor cells, could be used as biomarkers to assess response to sunitinib treatment providing confirmatory clinical data. Indeed, in patients, a biological test assessing mRNA expression levels of sunitinib targets on the tumor cells could be easily implemented in the daily practice. In addition, sorting of CD105-positive endothelial cells from tumor samples is also feasible [36], enabling further assessment of mRNA expression levels of sunitinib targets on tumor endothelial cells. In conclusion, these findings open new avenues that might contribute to better evaluation of the therapeutic response and better treatments among cancer patients treated with anti-angiogenics.
References
Deprimo SE, Bello CL, Smeraglia J, Baum CM, Spinella D, Rini BI, Michaelson MD, Motzer RJ (2007) Circulating protein biomarkers of pharmacodynamic activity of sunitinib in patients with metastatic renal cell carcinoma: modulation of VEGF and VEGF-related proteins. J Transl Med 5:32. doi:10.1186/1479-5876-5-32
Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Haussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J (2008) Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 359:378–390. doi:10.1056/NEJMoa0708857
Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA, Shenkier T, Cella D, Davidson NE (2007) Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med 357:2666–2676. doi:10.1056/NEJMoa072113
Rini BI, Cohen DP, Lu DR, Chen I, Hariharan S, Gore ME, Figlin RA, Baum MS, Motzer RJ (2011) Hypertension as a biomarker of efficacy in patients with metastatic renal cell carcinoma treated with sunitinib. J Natl Cancer Inst 103:763–773. doi:10.1093/jnci/djr128
Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Oudard S, Negrier S, Szczylik C, Pili R, Bjarnason GA, Garcia-del-Muro X, Sosman JA, Solska E, Wilding G, Thompson JA, Kim ST, Chen I, Huang X, Figlin RA (2009) Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol 27:3584–3590. doi:10.1200/JCO.2008.20.1293
Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, Oudard S, Negrier S, Szczylik C, Kim ST, Chen I, Bycott PW, Baum CM, Figlin RA (2007) Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med 356:115–124. doi:10.1056/NEJMoa065044
Barrios CH, Liu MC, Lee SC, Vanlemmens L, Ferrero JM, Tabei T, Pivot X, Iwata H, Aogi K, Lugo-Quintana R, Harbeck N, Brickman MJ, Zhang K, Kern KA, Martin M (2010) Phase III randomized trial of sunitinib versus capecitabine in patients with previously treated HER2-negative advanced breast cancer. Breast Cancer Res Treat 121:121–131. doi:10.1007/s10549-010-0788-0
Faivre S, Raymond E, Boucher E, Douillard J, Lim HY, Kim JS, Zappa M, Lanzalone S, Lin X, Deprimo S, Harmon C, Ruiz-Garcia A, Lechuga MJ, Cheng AL (2009) Safety and efficacy of sunitinib in patients with advanced hepatocellular carcinoma: an open-label, multicentre, phase II study. Lancet Oncol 10:794–800. doi:10.1016/S1470-2045(09)70171-8
Koeberle D, Montemurro M, Samaras P, Majno P, Simcock M, Limacher A, Lerch S, Kovacs K, Inauen R, Hess V, Saletti P, Borner M, Roth A, Bodoky G (2010) Continuous Sunitinib treatment in patients with advanced hepatocellular carcinoma: a Swiss Group for Clinical Cancer Research (SAKK) and Swiss Association for the Study of the Liver (SASL) multicenter phase II trial (SAKK 77/06). Oncologist 15:285–292. doi:10.1634/theoncologist.2009-0316
Jonasch E, Futreal A, Davis I, Bailey S, Kim WY, Brugarolas J, Giaccia AJ, Kurban G, Pause A, Frydman J, Zurita A, Rini BI, Sharma P, Atkins M, Walker C, Rathmell WK (2012) State-of-the-science: an update on renal cell carcinoma. Mol Cancer Res. doi:10.1158/1541-7786.MCR-12-0117
Harmon CS, DePrimo SE, Raymond E, Cheng AL, Boucher E, Douillard JY, Lim HY, Kim JS, Lechuga MJ, Lanzalone S, Lin X, Faivre S (2011) Mechanism-related circulating proteins as biomarkers for clinical outcome in patients with unresectable hepatocellular carcinoma receiving sunitinib. J Transl Med 9:120. doi:10.1186/1479-5876-9-120
Zhu AX, Duda DG, Ancukiewicz M, di Tomaso E, Clark JW, Miksad R, Fuchs CS, Ryan DP, Jain RK (2011) Exploratory analysis of early toxicity of sunitinib in advanced hepatocellular carcinoma patients: kinetics and potential biomarker value. Clin Cancer Res 17:918–927. doi:10.1158/1078-0432.CCR-10-0515
Paule B, Bastien L, Deslandes E, Cussenot O, Podgorniak MP, Allory Y, Naimi B, Porcher R, de La Taille A, Menashi S, Calvo F, Mourah S (2010) Soluble isoforms of vascular endothelial growth factor are predictors of response to sunitinib in metastatic renal cell carcinomas. PLoS One 5:e10715. doi:10.1371/journal.pone.0010715
Gordan JD, Lal P, Dondeti VR, Letrero R, Parekh KN, Oquendo CE, Greenberg RA, Flaherty KT, Rathmell WK, Keith B, Simon MC, Nathanson KL (2008) HIF-alpha effects on c-Myc distinguish two subtypes of sporadic VHL-deficient clear cell renal carcinoma. Cancer Cell 14:435–446. doi:10.1016/j.ccr.2008.10.016
Cumashi A, Tinari N, Rossi C, Lattanzio R, Natoli C, Piantelli M, Iacobelli S (2008) Sunitinib malate (SU-11248) alone or in combination with low-dose docetaxel inhibits the growth of DU-145 prostate cancer xenografts. Cancer Lett 270:229–233. doi:10.1016/j.canlet.2008.05.007
de Bouard S, Herlin P, Christensen JG, Lemoisson E, Gauduchon P, Raymond E, Guillamo JS (2007) Antiangiogenic and anti-invasive effects of sunitinib on experimental human glioblastoma. Neuro Oncol 9:412–423. doi:10.1215/15228517-2007-024
Tanaka Y, Shibata MA, Morimoto J, Otsuki Y (2011) Sunitinib suppresses tumor growth and metastases in a highly metastatic mouse mammary cancer model. Anticancer Res 31:1225–1234
Mendel DB, Laird AD, Xin X, Louie SG, Christensen JG, Li G, Schreck RE, Abrams TJ, Ngai TJ, Lee LB, Murray LJ, Carver J, Chan E, Moss KG, Haznedar JO, Sukbuntherng J, Blake RA, Sun L, Tang C, Miller T, Shirazian S, McMahon G, Cherrington JM (2003) In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: determination of a pharmacokinetic/pharmacodynamic relationship. Clin Cancer Res 9:327–337
Huang D, Ding Y, Li Y, Luo WM, Zhang ZF, Snider J, Vandenbeldt K, Qian CN, Teh BT (2010) Sunitinib acts primarily on tumor endothelium rather than tumor cells to inhibit the growth of renal cell carcinoma. Cancer Res 70:1053–1062. doi:10.1158/0008-5472.CAN-09-3722
Zhou Q, Guo P, Gallo JM (2008) Impact of angiogenesis inhibition by sunitinib on tumor distribution of temozolomide. Clin Cancer Res 14:1540–1549. doi:10.1158/1078-0432.CCR-07-4544
Maluf FC, Fernandes Gdos S, Kann AG, Aguilar-Ponce JL, de la Garza J, Buzaid AC (2008) Exploring the role of novel agents in the treatment of renal cell carcinoma. Cancer Treat Rev 34:750–760. doi:10.1016/j.ctrv.2008.07.002
Varna M, Lehmann-Che J, Turpin E, Marangoni E, El-Bouchtaoui M, Jeanne M, Grigoriu C, Ratajczak P, Leboeuf C, Plassa LF, Ferreira I, Poupon MF, Janin A, de The H, Bertheau P (2009) p53 dependent cell-cycle arrest triggered by chemotherapy in xenografted breast tumors. Int J Cancer 124:991–997. doi:10.1002/ijc.24049
Zhao WL, Mourah S, Mounier N, Leboeuf C, Daneshpouy ME, Legres L, Meignin V, Oksenhendler E, Maignin CL, Calvo F, Briere J, Gisselbrecht C, Janin A (2004) Vascular endothelial growth factor-A is expressed both on lymphoma cells and endothelial cells in angioimmunoblastic T-cell lymphoma and related to lymphoma progression. Lab Invest 84:1512–1519. doi:10.1038/labinvest.3700145
Kroemer G, Galluzzi L, Vandenabeele P, Abrams J, Alnemri ES, Baehrecke EH, Blagosklonny MV, El-Deiry WS, Golstein P, Green DR, Hengartner M, Knight RA, Kumar S, Lipton SA, Malorni W, Nunez G, Peter ME, Tschopp J, Yuan J, Piacentini M, Zhivotovsky B, Melino G (2009) Classification of cell death: recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ 16:3–11. doi:10.1038/cdd.2008.150
Faivre S, Delbaldo C, Vera K, Robert C, Lozahic S, Lassau N, Bello C, Deprimo S, Brega N, Massimini G, Armand JP, Scigalla P, Raymond E (2006) Safety, pharmacokinetic, and antitumor activity of SU11248, a novel oral multitarget tyrosine kinase inhibitor, in patients with cancer. J Clin Oncol 24:25–35. doi:10.1200/JCO.2005.02.2194
Rini BI, Michaelson MD, Rosenberg JE, Bukowski RM, Sosman JA, Stadler WM, Hutson TE, Margolin K, Harmon CS, DePrimo SE, Kim ST, Chen I, George DJ (2008) Antitumor activity and biomarker analysis of sunitinib in patients with bevacizumab-refractory metastatic renal cell carcinoma. J Clin Oncol 26:3743–3748. doi:10.1200/JCO.2007.15.5416
Gruenwald V, Beutel G, Schuch-Jantsch S, Reuter C, Ivanyi P, Ganser A, Haubitz M (2010) Circulating endothelial cells are an early predictor in renal cell carcinoma for tumor response to sunitinib. BMC Cancer 10:695. doi:10.1186/1471-2407-10-695
Farace F, Gross-Goupil M, Tournay E, Taylor M, Vimond N, Jacques N, Billiot F, Mauguen A, Hill C, Escudier B (2011) Levels of circulating CD45(dim)CD34(+)VEGFR2(+) progenitor cells correlate with outcome in metastatic renal cell carcinoma patients treated with tyrosine kinase inhibitors. Br J Cancer 104:1144–1150. doi:10.1038/bjc.2011.72
Xin H, Zhang C, Herrmann A, Du Y, Figlin R, Yu H (2009) Sunitinib inhibition of Stat3 induces renal cell carcinoma tumor cell apoptosis and reduces immunosuppressive cells. Cancer Res 69:2506–2513. doi:10.1158/0008-5472.CAN-08-4323
Duignan IJ, Corcoran E, Pennello A, Plym MJ, Amatulli M, Claros N, Iacolina M, Youssoufian H, Witte L, Samakoglu S, Schwartz J, Surguladze D, Tonra JR (2011) Pleiotropic stromal effects of vascular endothelial growth factor receptor 2 antibody therapy in renal cell carcinoma models. Neoplasia 13:49–59
Ellis LM, Hicklin DJ (2008) VEGF-targeted therapy: mechanisms of anti-tumour activity. Nat Rev Cancer 8:579–591. doi:10.1038/nrc2403
Kerbel RS (2008) Tumor angiogenesis. N Engl J Med 358:2039–2049. doi:10.1056/NEJMra0706596
Domingues I, Rino J, Demmers JA, de Lanerolle P, Santos SC (2011) VEGFR2 translocates to the nucleus to regulate its own transcription. PLoS One 6:e25668. doi:10.1371/journal.pone.0025668
Miyake M, Anai S, Fujimoto K, Ohnishi S, Kuwada M, Nakai Y, Inoue T, Tomioka A, Tanaka N, Hirao Y (2012) 5-fluorouracil enhances the antitumor effect of sorafenib and sunitinib in a xenograft model of human renal cell carcinoma. Oncol Lett 3:1195–1202. doi:10.3892/ol.2012.662
Garcia-Donas J, Leandro-Garcia LJ, Gonzalez Del Alba A, Morente M, Alemany I, Esteban E, Arranz JA, Climent MA, Gallardo E, Castellano DE, Bellmunt J, Mellado B, Puente J, Moreno F, Font A, Hernando S, Robledo M, Rodriguez-Antona C (2013) Prospective study assessing hypoxia-related proteins as markers for the outcome of treatment with sunitinib in advanced clear-cell renal cell carcinoma. Ann Oncol. doi:10.1093/annonc/mdt219
Xiong YQ, Sun HC, Zhang W, Zhu XD, Zhuang PY, Zhang JB, Wang L, Wu WZ, Qin LX, Tang ZY (2009) Human hepatocellular carcinoma tumor-derived endothelial cells manifest increased angiogenesis capability and drug resistance compared with normal endothelial cells. Clin Cancer Res 15:4838–4846. doi:10.1158/1078-0432.CCR-08-2780
Acknowledgments
We thank C.Dosquet for critical review of the manuscript, S.Lefrançois and S.Arien for electron microscopy techniques. Ms A.Swaine reviewed the English language. This study was supported by grants from University-Paris-Diderot, Inserm, Agence Nationale pour la Recherche (ANR), and Canceropole—Institut National du Cancer (InCa).
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guilhem Bousquet and Mariana Varna have contributed equally to this work.
Stéphane Germain and Anne Janin are equally senor authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bousquet, G., Varna, M., Ferreira, I. et al. Differential regulation of sunitinib targets predicts its tumor-type-specific effect on endothelial and/or tumor cell apoptosis. Cancer Chemother Pharmacol 72, 1183–1193 (2013). https://doi.org/10.1007/s00280-013-2300-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-013-2300-0