Abstract
Purpose
Panobinostat is a potent oral pan-deacetylase inhibitor with promising clinical activity in hematologic malignancies. Panobinostat was shown to inhibit CYP2D6 activity in vitro; thus understanding the magnitude of the potential clinical inhibition of panobinostat on co-medications that are CYP2D6 substrates becomes important.
Methods
This study evaluated the effects of co-administration of panobinostat with a sensitive CYP2D6 substrate, dextromethorphan (DM), in patients with advanced cancer who have functional CYP2D6 genes. Patients received 60 mg DM alone on day 1, panobinostat at 20 mg alone on days 3 and 5, and both agents on day 8. Plasma concentrations of DM and its metabolite dextrorphan (DX) were determined by liquid chromatography–tandem mass spectrometry following serial blood collections on day 1 (DM alone) and day 8 (in combination with panobinostat).
Results
Panobinostat increased DM exposure by 64 % [geometric mean ratio (GMR), 1.64 (90 % confidence interval (CI), 1.17–2.31)] and DX exposure by 29 % (GMR, 1.29 [90 % CI, 1.10–1.51]). These results indicated that panobinostat weakly inhibited a sensitive CYP2D6 substrate in cancer patients by increasing DM exposure by less than twofold.
Conclusion
Safety monitoring of sensitive CYP2D6 substrates with narrow therapeutic index is recommended when co-administering with panobinostat in future clinical practice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Deacetylases (DACs) are enzymes that regulate numerous intracellular targets implicated in cancer cell survival, differentiation, and cell cycle progression through the removal of acetyl groups from target proteins [1]. Panobinostat, a novel cinnamic hydroxamic acid derivative, is a pan-DAC inhibitor (DACi) with low nanomolar activity against class I, II, and IV DACs [2, 3]. In vitro and in vivo studies demonstrated that panobinostat inhibited proliferation and induced apoptosis in several cell lines and murine xenografts bearing human cancers [2]. Promising clinical activity in hematologic malignancies led to pivotal phase 2/3 studies, which are currently ongoing, in patients with relapsed/refractory multiple myeloma [4, 5].
In human liver microsomes, the oxidative metabolism of panobinostat is largely mediated by the cytochrome P450 3A4 (CYP3A4) and, to a lesser extent, by CYP2D6 and CYP2C19 in the human liver [6, 7]. In addition, panobinostat competitively inhibited CYP2D6 activity with a K i value of 0.17 μM in pooled liver microsomes using a specific probe substrate.
At commonly administered oral doses of 20 mg, the mean maximum observed drug concentration (C max) of panobinostat, or the maximum inhibitory clinical concentration [I], was approximately 0.1 μM [8, 9]. Based on the [I]/K i ratio of 0.6, panobinostat is also likely to inhibit CYP2D6 activity in patients. It is important to recognize that a clinically relevant inhibition is more pronounced with a CYP2D6 substrate, which is mainly metabolized by CYP2D6 (i.e., sensitive substrate) than substrates that are only partly metabolized by CYP2D6 [10, 11].
Dextromethorphan (DM) is a common remedy in over-the-counter antitussives and expectorant products. Clinically significant safety risk of DM at the doses used in clinical practice is not evident (i.e., up to 120 mg/day) [12]. Therefore, as a sensitive CYP2D6 substrate with a wide therapeutic index, DM is a preferred probe to evaluate the magnitude of clinical drug interactions following CYP2D6 inhibition [13]. However, due to its high first-pass metabolism, variable oral absorption and metabolism by polymorphic CYP2D6 enzyme, DM is prone to have variable pharmacokinetics (PK). Co-administering an inhibitor of CYP2D6 may further complicate its PK.
The primary purpose of this study was to evaluate the inhibitory effect of panobinostat on the PK of DM in patients with advanced cancer. As genetic variants of the CYP2D6 gene have been known to play a major role in CYP2D6 enzyme activities, a baseline CYP2D6 genotype will be determined after patients are enrolled into the study. Patients with a similar CYP2D6 metabolic status will be grouped to compare DM PK parameters with or without an inhibitor, specifically separating the poor metabolizers from the non–poor metabolizers of CYP2D6 (i.e., patients with functional CYP2D6 genes, including intermediate metabolizers [IM], extensive metabolizers [EM], and ultra-rapid metabolizers [UM]). The study is designed to group UM/EM/IM as it is known that substantial enzyme activity overlap among different phenotypes and differentiation is usually not discriminant in practice [14]. It is hypothesized that patients with null CYP2D6 genes or poor metabolizers (PM) have little or no CYP2D6 enzyme activity and that further enzyme inhibition from panobinostat would not result in further reduction in CYP2D6 activity; thus, no meaningful changes in the overall DM exposure would be observed. Conversely, CYP2D6 inhibition in patients with functional CYP2D6 genes (UM, EM, or IM of CYP2D6) converts them to a PM phenotype [15]. Hence, the current study aims to quantify the magnitude change in the exposure of DM and its metabolite DX in cancer patients who have functional CYP2D6 genes, as patients with null CYP2D6 genotype are not likely to show PK differences upon panobinostat inhibition.
Methods
Patients
This study adhered to the Declaration of Helsinki and Good Clinical Practice. Approval was obtained from an independent ethics committee and local institutional review boards from the following centers: Medical College of Georgia (Augusta, Georgia), Rush University Medical Center (Chicago, Illinois), Western Institutional Review Board (Olympia, Washington), University of Texas MD Anderson Cancer Center (Houston, Texas), and University Health Network (Toronto, Ontario, Canada). All patients provided written informed consent.
Adult patients with advanced or metastatic solid tumors who had progressed on or were no longer receiving standard cancer therapies were eligible for this study. Eligibility criteria included an Eastern Cooperative Oncology Group (ECOG) performance status ≤2; neutrophils ≥1.5 × 109/L and platelets ≥100 × 109/L; hemoglobin ≥9 g/dL; aspartate aminotransferase (AST) and alanine aminotransferase (ALT) ≤2.5 × upper limit of normal (ULN), or in patients with liver metastases, AST and ALT ≤5.0 × ULN; serum bilirubin ≤1.5 × ULN; serum albumin ≥3.0 g/dL; serum creatinine ≤1.5 × ULN or 24-h clearance ≥50 mL/min. Patients with gastrointestinal disorders that may significantly alter the absorption of oral panobinostat were not eligible. Due to the possible effects of DACi leading to QT prolongation, patients with impaired cardiac function or those at risk of torsades de pointes were not eligible. Prior treatment with DACi or monoamine oxidase inhibitors 14 days prior to first dose, and chemotherapy, any investigational drug, major surgery, or radiation therapy within 4 weeks prior to starting study were not permitted. Concomitant use of anticancer therapy, including radiation therapy, or CYP3A4/5 inhibitors was also not permitted.
CYP2D6 genotyping status and patient selection
DNA was extracted from baseline blood samples (1.5 mL), and genotyping analysis to characterize CYP2D6 metabolic status was performed at Epidauros Biotechnologie AG (Bernried, Germany). CYP2D6 variant alleles (AmpliChip CYP450 Test, Roche Molecular Diagnostics, Indianapolis, Indiana) included CYP2D6*2 (functional 1 or 2 alleles, EM); CYP2D6*3, *4, and *6 (nonfunctional alleles, PM); CYP2D6*5 (gene deletion/null allele, PM); CYP2D6*9, *10, *17, *29, and *41 (alleles with reduced activity, IM); and CYP2D6*MxN (multiple gene copies, UM). Patients were classified as EM, PM, IM, UM, or undetermined-status metabolizers of CYP2D6 according to published data [16, 17]. As the CYP2D6 enzyme activity can be further inhibited to observe meaningful change in DM exposure in non-PM patients, the primary endpoint of this study was to be based on PK results in those patients, although study subjects were enrolled regardless of CYP2D6 genotype status at baseline. PK results in PM patients were used as secondary endpoints when available. In the event that a CYP2D6 genotyping sample could not be obtained, a metabolic ratio (MR) of DM/DX in plasma was used to determine CYP2D6 metabolic status upon analysis of the DM and DX concentration data [18]. Patients with an MR <0.3 were considered non–poor metabolizers (UM/EM/IM) as previously described and considered in the primary analysis [12].
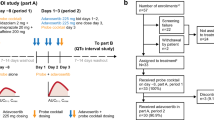
Study design and drug administration
This study consisted of 2 phases: the PK assessment phase (10 days) and the extension phase. The PK assessment phase was designed to investigate CYP2D6 inhibition via panobinostat on the PK of DM and its metabolite DX in patients with advanced cancer who were also CYP2D6 non–poor metabolizers. A single 60-mg dose of DM was administered alone on day 1 followed by PK sampling. On days 3, 5, and 8, a single oral panobinostat dose of 20 mg was administered. On day 8, a second DM dose of 60 mg was co-administered with panobinostat, followed by PK sampling. On day 10, patients entered the extension phase, designed to further evaluate the safety, tolerability, and potential activity of single-agent panobinostat (20 mg 3 times a week) in 28-day cycles until disease progression, patients withdrawing consent or starting another anticancer therapy, or intolerability.
Pharmacokinetic sampling and analysis
Serial whole blood samples were obtained on days 1 and 8 at predose and 0.5, 1, 1.5, 2, 2.5, 3, 4, 6, 8, 10 (or 12), 24, and 48 h post DM doses. Blood samples were centrifuged at 800×g for 15 min at 4 °C to yield plasma within 60 min after blood collection and stored below −60 °C until analysis. Plasma concentrations of DM and total DX (free + glucuronidated) were assayed using liquid chromatography–tandem mass spectrometry (LC–MS/MS) method as developed by Taylor Technology (Princeton, NJ). The lower limit of quantification (LLOQ) was 20 pg/mL for DM with a dynamic range from 0.05 to 20 ng/mL and 400 pg/mL for DX with a dynamic range from 1 to 400 ng/mL. The analytes, DM and DX, and their internal standards (IS) dextromethorphan-d3 and dextrorphan-d3, were extracted from 0.100 mL of human plasma by a liquid–liquid extraction procedure. Potassium carbonate buffer (pH 10) and hexane/ethyl acetate (90:10; v/v) were added to a 96-well plate. A Tomtec Quadra 96-well pipettor system was used to mix and transfer the samples. The transferred organic layer was evaporated to dryness and then reconstituted with acetonitrile and water (90:10; v/v). The extracts were chromatographed under HILIC conditions on a Betasil Silica HPLC column using an isocratic system with ammonium acetate in acetonitrile/water (90:10; v/v). The compounds were detected and quantified by tandem mass spectrometry in positive-ion mode on an MDS Sciex API 4000 equipped with a Turbo Ionspray® interface. The assay was linear within the DM dynamic ranges with the bias (%) and coefficient of variance (CV%) values of the QC sample results ranged from 0 to 0.7 % and 2.7 to 11.3 %, respectively, based on the within-study validation during the sample analysis. Similarly, the bias (%) and CV (%) values of the QC sample results ranged from −0.7 to −1.3 % and 2.7–9.3 %, respectively for DX.
PK parameters of DM, DX, and panobinostat were derived from the individual concentration versus time profile using noncompartmental analysis WinNonlin Pro software (version 5.01; Pharsight Corporation, Cary, North Carolina). C max and time to reach C max (T max) were obtained by visual inspection of the plasma concentration–time curve. The apparent t 1/2 was estimated using the best-fit variables of a single exponential to the log-linear portion of the plasma concentration–time curve using nonweighted linear regression. The area under the concentration–time curve (AUC) from zero to infinity (AUC0−∞) was calculated using the linear-up/log-down method up to the last measured concentrations, and extrapolation using the last concentration and the value of apparent t 1/2. MR was calculated as the plasma AUC from time 0 to 48 h (AUC0–48) ratio of DM/DX after a single oral dose of DM on day 1.
Safety assessment
Patients were monitored throughout the trial with regular laboratory monitoring consisting of hematology and biochemistry 2 days/week and thyroid function, coagulation profile, urinalysis, and pregnancy tests once per cycle. Adverse events (AEs) were assessed according to National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) version 3.0 throughout the study and for at least 28 days following the last dose of panobinostat. Independent review of QTc intervals was conducted on an electrocardiogram (ECG) once at baseline; on a minimum of 6 sequential ECGs predosing followed by 3 sequential ECGs postdosing on days 1 and 3; 3 sequential ECGs predosing followed by 3 sequential ECGs postdosing on day 8; 3 sequential ECGs on days 2, 4, and 10; and 1 ECG on day 9 of the PK assessment. In the extension phase, 3 sequential ECGs predosing were evaluated once a week.
Efficacy assessment
Antitumor response was assessed during the extension phase. Computed tomography scans (or magnetic resonance imaging) were scheduled at baseline and at the end of every other cycle while patients were treated with panobinostat. Response was assessed by endpoints defined by standard Response Evaluation Criteria in Solid Tumors (RECIST). Objective responses required confirmation after at least 4 weeks [19]. Disease progression was based on objective evidence documented by radiological or physical examination.
Statistical analyses
It was estimated that 12 patients under a fixed-sequence design would provide at least 80 % power to demonstrate lack of drug interaction on DM PK parameters, assuming a true ratio of 1.05 and an intra-subject CV% of 15.2 %. An estimated CV% of 12.7 % was reported in healthy volunteers [20]. At least a 20 % increase in CV% is assumed for the patient population, which resulted in a CV% of 15.2 %.
Statistical analyses on PK included non-PM patients who received DM and panobinostat doses as per protocol and had evaluable PK data on day 1 (in the absence of panobinostat) and day 8 (in the presence of panobinostat). A linear mixed-effects model was fit to the log-transformed PK parameters (C max, AUC0–48, AUC0–∞) of DM and DX separately. The model included treatment (DM + panobinostat or DM alone) as a fixed effect and patient as a random effect. In the model, the combination treatment (DM + panobinostat) was the test and DM alone was the reference. Proper contrasts were used to estimate the treatment differences (i.e., DM + panobinostat vs. DM alone). The point estimate of the treatment difference and the corresponding 90 % confidence intervals (CIs) were calculated and antilogged to obtain the point estimate and CI on the linear scale as represented by the ratio of geometric means of the test as compared with the reference. T max differences between treatment groups were captured using nonparametric methods. Descriptive statistics of PK parameters included geometric mean and geometric CV% for all parameters except for T max, where median (range) was used.
Results
Patient demographics and characteristics
The study population was composed of 17 adult patients with advanced or metastatic cancer, including mesothelioma (n = 4), colon, ovary, prostate, and stomach cancer (n = 1 each), and lung cancer (n = 9). Demographic characteristics are summarized in Table 1. The median age was 67 years (range 49–74), and the majority were male (n = 10), Caucasian (n = 11) and extensive metabolizers (n = 11). Most patients presented with an ECOG performance status ≤1 (n = 14). None were poor-metabolizers or ultra-metabolizers. Of the 17 enrolled patients, 1 patient who took a single DM dose on day 1 withdrew consent from the study, and therefore, no genotype or PK information was reported. Fifteen of the remaining 16 patients were characterized as non-PM by the genotyping method (13) or metabolic ratio (2), and 1 patient could not be assessed due to invalidated genotyping results. All 16 patients entered into the extension phase, and all subsequently discontinued from the study. Disease progression was the reason for treatment discontinuation in 65 % of patients, whereas 24 % patients discontinued due to AEs.
Pharmacokinetics of dextromethorphan and dextrorphan
Fourteen patients had evaluable PK profiles of DM and DX for both days 1 and 8 and followed the dose administrations as per protocol. These 14 patients were considered in the PK statistical analyses. Figure 1 depicts mean plasma DM concentration–time data for DM and DX in the absence (day 1) or presence (day 8) of panobinostat. DM plasma concentrations were generally quantifiable up to 48 h postdose in both the presence and absence of panobinostat. Summary PK parameters of DM and DX are presented in Table 2. In the presence of panobinostat, the overall C max and exposure (AUC0–∞) of DM increased by 83 and 64 %, based on the geometric mean ratio (GMR) of 1.83 (90 % CI, 1.44–2.34) and 1.64 (90 % CI, 1.17–2.31), respectively. The overall DX exposure increased by 29 % in the presence of panobinostat [GMR, 1.29 (90 % CI, 1.10–1.51)], but C max did not increase [GMR, 1.05 (90 % CI, 0.85–1.29)]. The terminal t 1/2 of DM or DX was similar in the presence or absence of panobinostat.
a The arithmetic mean (standard deviation vertical lines) of plasma dextromethorphan (DM) concentration–time profiles (pg/mL) in semilogarithmic scale following oral administration of DM in the absence (closed circle) and presence (open circle) of panobinostat, quantifiable up to 48 h. Non–poor metabolizers include ultra-extensive, extensive, and intermediate metabolizers. b The arithmetic mean (standard deviation vertical lines) of plasma dextrorphan concentration–time profiles (pg/mL) in semilogarithmic scale following oral administration of DM in the absence (closed circle) and presence (open circle) of panobinostat, quantifiable up to 48 h. Non–poor metabolizers include ultra-extensive, extensive, and intermediate metabolizers
There was a high inter-individual variability in the DM PK with or without panobinostat (Table 2), but with a slightly reduced variability following panobinostat. Variability in DM C max or AUC fold-change upon panobinostat inhibition was also observed (Fig. 2). The highest fold-change of 7.2 was seen in a 67-year-old female Japanese patient with stage IV non-small-cell lung carcinoma (NSCLC). This patient was characterized as EM based on genotyping, which showed a combination of *10 and 2 copies of the *2 allele. The patient had received 4 oral doses of panobinostat (20 mg/dose) when CTCAE grade 3 thrombocytopenia was observed together with atrial fibrillation and cerebral hemorrhage. A computed tomography scan demonstrated the presence of new central nervous system metastases, and treatment was discontinued due to these events. Atrial fibrillation resolved 4 days after the last dose of panobinostat. However, 7 days after the last dose, the patient died of cerebral hemorrhage. Removing this patient from the analysis reduced the GMR of DM AUC0–∞ to 1.45 (90 % CI, 1.14–1.84), representing a 45 % increase with panobinostat combination rather than an average of 64 % increase reported with all available patients.
Individual symbols represent the DM C max ratio on the left and DM AUC0–48h ratio on the right. The ratio is calculated by comparing the DM C max or AUC0–48h following DM + panobinostat on day 8 and DM alone on day 1. Open triangles denote CYP2D6 intermediate metabolizers and open circles denote CYP2D6 extensive metabolizers. The vertical bars represent the 90 % confidence interval, and the horizontal bars represent the geometric mean ratio
Safety and efficacy
All 17 patients were included in the safety analysis for the PK assessment phase and 16 patients (94.1 %) in the extension phase. During the PK assessment phase, the majority of patients (88.2 %) experienced at least 1 treatment-emergent AE, with most events being CTCAE grade 1 and 2 in severity. The most frequent AEs were nausea (35.3 %), diarrhea (23.4 %), constipation (17.6 %), and fatigue (17.6 %). There was no evidence that the incidence or severity of the AEs was different with the combination of DM and panobinostat compared with panobinostat alone. All 16 patients in the extension phase experienced at least 1 treatment-emergent AE. The most frequently reported AEs were dyspnea (43.8 %), fatigue (37.5 %), and thrombocytopenia (25.0 %). The incidence of CTCAE grade 3 or 4 AEs increased in the extension phase, with 9 patients (56.3 %) experiencing grade 3 or 4 AEs. However, these AEs were considered to be study-drug related in only 3 patients: 1 patient with grade 3 thrombocytopenia, 1 patient with grade 4 thrombocytopenia and grade 3 hypothyroidism, and a third patient with grade 3 QT prolongation. None of these events led to treatment discontinuation. Two patients reported a new QTcF reading >450 ms in the extension phase (compared with 3 in the PK assessment phase). No patient had a new QTcF reading >480 ms. Three deaths were reported in the extension phase, 1 due to disease progression and 2 due to AEs and disease progression. None of the deaths were considered to be related to study drug.
Efficacy was assessed during the extension phase of the study and was based on the investigator’s assessment of overall response. Of the 16 patients included in the efficacy analysis set, 1 patient (6 %) had a partial response (PR) and 3 patients (19 %) had stable disease (SD). The PR was observed in a patient with a pleural mesothelioma who had an objective response lasting 125 days and progression-free survival of 288 days. SD was noted in 3 patients with NSCLC. Progressive disease was the best response in 6 patients (38 %), whereas response status was unknown (no or insufficient postbaseline tumor assessment) in an additional 6 patients (38 %).
Discussion
This study investigated the PK and clinical impact of panobinostat inhibition on a sensitive CYP2D6 substrate, DM, in advanced cancer patients who had similar CYP2D6 metabolic status (i.e., functional CYP2D6 genes). Identification of patients with similar CYP2D6 metabolic status by genotype was a key consideration in this study because panobinostat inhibitory effect may differ depending on the inherent baseline CYP2D6 enzyme activity. In the study, the majority were Caucasian and no patient was identified as a poor metabolizer and this seemed to be consistent with PM frequencies reported in Caucasians (5–10 %). It is hypothesized that patients who are CYP2D6 poor metabolizers (i.e., null CYP2D6 gene with little or no CYP2D6 activity) are not likely to show further CYP2D6 inhibition; the absence of this phenotype allows the study to show the impact of panobinostat inhibition on the disposition of a CYP2D6 substrate in non–poor metabolizers, who represent the majority of the patients.
The observed mean panobinostat C max value at steady state was 25.8 ng/mL, or 0.074 μM as total concentration (free and bound), in the current study. The mean panobinostat C max value was in the range of prior studies using the 20-mg dosing [8, 9]. Based on the empirical predication using [i]/K i ratio of 0.43 observed in the current study and preclinical inhibitory IC50 value, a clinical inhibition of CYP2D6 by panobinostat is still likely in patients [21]. However, only a modest inhibition was observed in patients with an increase in DM exposure by an average 1.64-fold (90 % CI, 1.17–2.31) following repeated panobinostat doses. Although these results were not in the default “no-effect boundaries” of 0.8–1.25, the observed drug–drug interaction can be classified as weak. There is a general consensus on the risk category of drug interaction based on the observed magnitude of the resulting AUC change (e.g., US Food and Drug Administration guidance): a more than fivefold increase in substrate AUC upon inhibition is deemed to have a strong interaction; an increase between two- and fivefold, a moderate interaction; and a less than twofold increase, a weak interaction [22]. In the current study, panobinostat was shown to have a weak clinical CYP2D6 inhibition (less than twofold). The observed results are not unexpected as the magnitude of exposure increase of a victim drug (DM) by an inhibitor (panobinostat) is also determined by the affinity at the enzyme-binding sites which are estimated by parameters such as maximum rate of drug elimination (V max), drug concentration at one-half of V max (K m), inhibition constant (K i ), substrate concentration ([S]) and [I]. In addition, the fold-change by an inhibitor is also driven by the fraction of the metabolic pathway inhibited. The empirical prediction used above does not account for all these factors.
In comparison with studies in healthy volunteers, the inter-individual variability in DM/DX exposures observed in this study was substantial [18, 23]. It is possible that the heterogeneity of the cancer population as well as variable absorption of a high first-pass oral agent with low bioavailability may have contributed to the large variability observed in DM/DX exposures.
A weak increase of approximately 30 % in the exposure of the DX metabolite (free + glucuronidated) paralleled the increase in DM exposure consequent to panobinostat inhibition. This observation was unexpected and suggested additional pathway inhibition following panobinostat co-administration. In addition to CYP2D6 mediated metabolism, DX was shown to be further metabolized by CYP3A4 to form 3-hydroxymorphinan (HYM) and DM was simultaneously metabolized by CYP3A4 to 3-methoxymorphlnan (MEM) in parallel to DX formation [24]. It is possible that panobinostat can be a partial CYP3A4 inhibitor in addition to CYP2D6. A future drug–drug interaction study with a sensitive CYP3A4 substrate is warranted to confirm the inhibitory potential of panobinostat.
Overall, the combination treatment was well tolerated. There was no evidence that the administration of DM with panobinostat altered the safety profile of panobinostat. In patients who continued with single-agent panobinostat treatment following the completion of the drug–drug interaction phase, the most common AEs were grade 1 or 2 fatigue and gastrointestinal-related events (e.g., nausea, diarrhea, and vomiting). These AEs have been reported with similar frequencies and severities in other clinical studies of panobinostat [4, 25, 26]. No serious myelosuppressive effects or QTcF increases >480 ms were noted. Although antitumor activity was not the primary endpoint of this study, 1 patient with pleural mesothelioma achieved a partial response lasting 4 months.
The current study showed that panobinostat, a novel oral DACi, weakly inhibited a sensitive CYP2D6 substrate in cancer patients. Extrapolation of the results from this study to clinical practice suggests that no special monitoring beyond routine clinical practice is necessary when administering panobinostat with sensitive CYP2D6 substrates (e.g., atomoxetine, desipramine, dextromethorphan, metoprolol, nebivolol, perphenazine, tolterodine, or venlafaxine) [27]. However, close monitoring of sensitive CYP2D6 substrates with known narrow therapeutic index (e.g., thioridazine) (FDA Web site) is recommended, and dose titration should be considered when such agents are co-administered with panobinostat. These recommendations are incorporated in the currently ongoing and future studies of panobinostat in various malignancies.
References
Bolden JE, Peart MJ, Johnstone RW (2006) Anticancer activities of histone deacetylase inhibitors. Nat Rev Drug Discov 5:769–784
Atadja P (2009) Development of the pan-DAC inhibitor panobinostat (LBH589): successes and challenges. Cancer Lett 280:233–241
Witt O, Deubzer HE, Milde T, Oehme I (2009) HDAC family: what are the cancer relevant targets? Cancer Lett 277:8–21
Alsina M, Schlossman RL, Weber DM, Coutre SE, Lonial S, Gasparetto C, Warsi G, Ondovik MS, Mukhopadhyay S, Paley CS, Richardson PGG (2012) PANORAMA 2: a phase II study of panobinostat in combination with bortezomib and dexamethasone in patients with relapsed and bortezomib-refractory multiple myeloma. ASCO Meet Abstr 30:8012
San-Miguel JF, Moreau P, Yoon S, Dimopoulos MA, VT de M Hungria, Wiktor-Jedrzejczak W, Elghandour A, Corradini P, Gunther A, Beksac M, Yong K, Lee JH, Lonial S, Hou J, Einsele H, Wroclawska-Swacha M, Weber H, Bourquelot P, Richardson PG (2012) Phase III study of panobinostat with bortezomib and dexamethasone in patients with relapsed multiple myeloma (PANORAMA 1). ASCO Ann Meet Abstr e18572
Clive S, Woo MM, Nydam T, Kelly L, Squier M, Kagan M (2012) Characterizing the disposition, metabolism, and excretion of an orally active pan-deacetylase inhibitor, panobinostat, via trace radiolabeled 14C material in advanced cancer patients. Cancer Chemother Pharmacol 70:513–522
Hamberg P, Woo MM, Chen LC, Verweij J, Porro MG, Zhao L, Li W, van der Biessen D, Sharma S, Hengelage T, de Jonge M (2011) Effect of ketoconazole-mediated CYP3A4 inhibition on clinical pharmacokinetics of panobinostat (LBH589), an orally active histone deacetylase inhibitor. Cancer Chemother Pharmacol
Shapiro GI, Frank R, Dandamudi UB, Hengelage T, Zhao L, Gazi L, Porro MG, Woo MM, Lewis LD (2012) The effect of food on the bioavailability of panobinostat, an orally active pan-histone deacetylase inhibitor, in patients with advanced cancer. Cancer Chemother Pharmacol 69:555–562
Woo MM, Culver K, Li W, Liu A, Scott J, Parker K, Jalaluddin M, Laird G, Cooper MR, Schran HF (2008) Panobinostat (LBH589) pharmacokinetics (PK): implication for clinical safety and efficacy. Ann Oncol 19:viii161 [abstract 487P]
Gonzalez FJ, Lee YH (1996) Constitutive expression of hepatic cytochrome P450 genes. FASEB J 10:1112–1117
Ingelman-Sundberg M, Sim SC, Gomez A, Rodriguez-Antona C (2007) Influence of cytochrome P450 polymorphisms on drug therapies: pharmacogenetic, pharmacoepigenetic and clinical aspects. Pharmacol Ther 116:496–526
Kohler D, Hartter S, Fuchs K, Sieghart W, Hiemke C (1997) CYP2D6 genotype and phenotyping by determination of dextromethorphan and metabolites in serum of healthy controls and of patients under psychotropic medication. Pharmacogenetics 7:453–461
Bjornsson TD, Callaghan JT, Einolf HJ, Fischer V, Gan L, Grimm S, Kao J, King SP, Miwa G, Ni L, Kumar G, McLeod J, Obach RS, Roberts S, Roe A, Shah A, Snikeris F, Sullivan JT, Tweedie D, Vega JM, Walsh J, Wrighton SA, Pharmaceutical Research and Manufacturers of America (PhRMA) Drug Metabolism/Clinical Pharmacology Technical Working Group, FDA Center for Drug Evaluation and Research (CDER) (2003) The conduct of in vitro and in vivo drug–drug interaction studies: a Pharmaceutical Research and Manufacturers of America (PhRMA) perspective. Drug Metab Dispos 31:815–832
Zhou SF (2009) Polymorphism of human cytochrome P450 2D6 and its clinical significance: Part I. Clin Pharmacokinet 48:689–723
Nielsen MD, Brosen K, Gram LF (1990) A dose-effect study of the in vivo inhibitory effect of quinidine on sparteine oxidation in man. Br J Clin Pharmacol 29:299–304
Sachse C, Brockmoller J, Bauer S, Roots I (1997) Cytochrome P450 2D6 variants in a Caucasian population: allele frequencies and phenotypic consequences. Am J Hum Genet 60:284–295
Bradford LD (2002) CYP2D6 allele frequency in European Caucasians, Asians, Africans and their descendants. Pharmacogenomics 3:229–243
Pope LE, Khalil MH, Berg JE, Stiles M, Yakatan GJ, Sellers EM (2004) Pharmacokinetics of dextromethorphan after single or multiple dosing in combination with quinidine in extensive and poor metabolizers. J Clin Pharmacol 44:1132–1142
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92:205–216
Gorski JC, Jones DR, Wrighton SA, Hall SD (1994) Characterization of dextromethorphan N-demethylation by human liver microsomes. Contribution of the cytochrome P450 3A (CYP3A) subfamily. Biochem Pharmacol 48:173–182
Obach RS, Walsky RL, Venkatakrishnan K (2007) Mechanism-based inactivation of human cytochrome p450 enzymes and the prediction of drug–drug interactions. Drug Metab Dispos 35:246–255
U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER) (2012) Guidance for industry: Drug interaction studies-study design, data analysis, implications for dosing, and labeling recommendations. In: http://www.fda.gov Web site. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM292362.pdf. Accessed 02/29 2012
Capon DA, Bochner F, Kerry N, Mikus G, Danz C, Somogyi AA (1996) The influence of CYP2D6 polymorphism and quinidine on the disposition and antitussive effect of dextromethorphan in humans. Clin Pharmacol Ther 60:295–307
Yu A, Haining RL (2001) Comparative contribution to dextromethorphan metabolism by cytochrome P450 isoforms in vitro: can dextromethorphan be used as a dual probe for both CTP2D6 and CYP3A activities? Drug Metab Dispos 29:1514–1520
Ellis L, Pan Y, Smyth GK, George DJ, McCormack C, Williams-Truax R, Mita M, Beck J, Burris H, Ryan G, Atadja P, Butterfoss D, Dugan M, Culver K, Johnstone RW, Prince HM (2008) Histone deacetylase inhibitor panobinostat induces clinical responses with associated alterations in gene expression profiles in cutaneous T-cell lymphoma. Clin Cancer Res 14:4500–4510
Younes A, Sureda A, Ben-Yehuda D, Zinzani PL, Ong TC, Prince HM, Harrison SJ, Kirschbaum M, Johnston P, Gallagher J, Le Corre C, Shen A, Engert A (2012) Panobinostat in patients with relapsed/refractory Hodgkin’s lymphoma after autologous stem-cell transplantation: results of a phase II study. J Clin Oncol 30:2197–2203
U.S. Food and Drug Adminstration (2013) Drug Interactions & Labeling http://www.fda.gov/Drugs/DevelopmentApprovalProcess/DevelopmentResources/DrugInteractionsLabeling/ucm080499.htm
Acknowledgments
We are grateful to the participating patients and their families. We thank Wenkui Li, PhD, Stefan de Buck, PhD, Ronit Elshtein, and the study teams at our respective institutions for contributions to the conduct and analysis of the trial. Financial support for medical editorial assistance was provided by Novartis Pharmaceuticals Corporation. We thank Kerry Brinkman, PhD, Articulate Science, for medical editorial assistance with this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Feld, R., Woo, M.M., Leighl, N. et al. A clinical investigation of inhibitory effect of panobinostat on CYP2D6 substrate in patients with advanced cancer. Cancer Chemother Pharmacol 72, 747–755 (2013). https://doi.org/10.1007/s00280-013-2237-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-013-2237-3