Abstract
Purpose
Platinum-based chemotherapy is the recognized first-line treatment for metastatic nasopharyngeal carcinoma (NPC). However, no standard treatment regimens have been established. This phase II study was designed to evaluate the efficacy and safety of a paclitaxel, cisplatin and 5-FU combination in metastatic and/or recurrent NPC.
Methods
Patients with evaluable metastatic and/or recurrent NPC were entered into this study. Treatment consisted of paclitaxel at a dose of 135 mg/m2 on day 1, cisplatin 25 mg/m2/day from day 1 to day 3 and 5-FU-continuous infusion for 120 h at a variable dosage from 600 to 1,000 mg/m2/day according to prior radiation. This regimen was repeated every 3 weeks.
Results
A total of 95 patients were enrolled; 92 patients were evaluable for response. The overall response and disease control rates were 78.9 and 93.6 %, respectively. At a median follow-up of 24.8 months, the respective median overall survival (OS) and progression-free survival were 22.7 months (95 % CI 18.6–26.9 months) and 8.6 months (95 % CI 7.7–9.5 months). Toxicities were moderate and manageable. Grade 3/4 toxicities included leucopenia (14.7 %), neutropenia (17.9 %), anemia (3.2 %), thrombocytopenia (6.4 %), nausea (4.2 %), vomiting (9.5 %), stomatitis (9.5 %), diarrhea (3.2 %), aminotransferase (2.2 %) and sensory neuropathy (3.2 %).
Conclusion
Triplet combination chemotherapy with paclitaxel, cisplatin and 5-FU is an effective and safe option in the front-line treatment for recurrent and/or metastatic NPC. The encouraging results with high response rate and long OS suggest that this regimen might be especially considered where tumor shrinkage is required.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nasopharyngeal carcinoma (NPC) is a dominant form of cancer in well-defined populations, such as natives of southern China, Southeast Asia, the Middle East and North Africa, the Arctic [1, 2]. Almost eighty cases per 100,000 populations are reported in southern China. Although advances in treatment including intensity-modulated radiotherapy and chemoradiotherapy have improved local control, reports have shown that about 17–54 % of patients with NPC fail treatment due to distant metastases [3, 4].
Patients with recurrent and/or metastatic NPC have a dismal outcome. Platinum-based combination chemotherapy is considered a cornerstone in the management of metastatic and/or recurrent NPC. The combination of cisplatin and 5-FU by continuous infusion for 5 days has been the standard front-line regimen for decades, yielding ORR between 66 and 76 %, and median survival up to 11–14 months [5–7]. In recent studies, paclitaxel has been tested in the treatment for metastatic and/or recurrent NPC [8–11]. Au E et al. [8] reported a remarkable response rate of 21.7 % and a median PFS of 7.5 months for single-agent paclitaxel, and Tan EH et al. [11] reported a response rate of 75 % and a median PFS of 7 months for paclitaxel-platinum combination as first-line chemotherapy. Besides, platinum in combination with other agents including gemcitabine, docetaxel or capecitabine gives similar results: OR 25–73 % and median survival 9.5–19.6 months [12–17]. However, these chemotherapy regimens have not been randomly compared and established as new standards for patients with recurrent and/or metastatic NPC. The limitations of these doublet regimens are relatively low response rates and modest progression-free and median survivals in most series [5–17].
Triplet combination with paclitaxel, cisplatin and 5-FU (PCF) already has demonstrated feasibility, safety and promising activity in some types of tumors, including advanced gastric cancer and head and neck cancer [18, 19]. Ricardo Hitt et al. have compared the efficacy and toxicity of PCF versus CF in locally advanced head and neck cancer (HNC). The result shows that induction chemotherapy with PCF was better tolerated and resulted in a higher CR rate than CF [20].
Based on the above knowledge, we conducted this phase II study to investigate the efficacy and safety profile of the PCF triplet regimen as front-line treatment in patients with recurrent and/or metastatic NPC.
Patients and methods
Eligibility of patients
Participants of this study were recruited from Sun Yat-Sen University Cancer Center between 2005 and 2011. Patients aged 18–75 years with pathologically confirmed recurrent and/or metastatic NPC, and measurable disease was eligible for the study. Prior palliative chemotherapy was not permitted. However, neoadjuvant (adjuvant) or concurrent chemotherapy was allowed, providing that the treatment was completed at least 6 months before the start of the current study. Patients were required to have ECOG performance status of 0–2, with a life expectancy ≥3 months, adequate bone marrow, liver and kidney functions. This study was approved by the Institutional Ethics Committee of the Sun Yat-Sen University Cancer Center, and all patients were given written informed consents to participate in this study.
Treatment plan
Patients were treated with paclitaxel 135 mg/m2 by 3-hour infusion on day 1, cisplatin 25 mg/m2/day from day 1 to 3, 5-FU by continuous IV infusion for 120 h (from day 1 to 5). The doses of 5-FU varied according to prior radiation. 5-FU 600 mg/m2/day was given for those patients who received radiation within a year; 800 mg/m2/day for those patients who were radiation-free for more than a year, while 5-FU 1,000 mg/m2/day for radiation naive patients. This regimen was repeated every 3 weeks up to a maximum of 6 cycles or until disease progression, the development of intolerable toxic effects or patients’ voluntary withdrawal. Response assessments were carried out clinically and radiologically following every two cycles of treatment.
The dose was modified based on the following criteria
-
1.
In cases of febrile neutropenia, grade 4 neutropenia, grade 3 or 4 thrombocytopenia and anemia, the dose of paclitaxel/cisplatin/5-FU for the following cycle was reduced to 80 %. Subsequent toxicities required a further 20 % dose reduction in these agents. If hematological toxicity persisted despite these dose reductions, treatment was discontinued.
-
2.
In cases of grade 3 hepatotoxicity, the dose of paclitaxel for the following cycle was reduced to 80 %. If the hepatotoxicity was grade 4, the study was discontinued.
-
3.
In cases for the stomatitis and diarrhea of grade 3 or higher, 5-FU was reduced to 80 % for the next cycle.
-
4.
In cases where the creatinine clearance rate ranged between 30 and 50 ml/min due to the nephrotoxicity, the dose of cisplatin was reduced by 50 %. If the creatinine clearance rate was lower than 30 ml/min, the study was discontinued.
-
5.
Dose reductions were also required for other related grade 3 or 4 toxicities.
Baseline and treatment evaluations
The following tests were evaluated before entry into the study: medical history, physical examination, performance status (ECOG), weight, blood routine and blood chemistry, chest radiograph, bone scan, abdominal sonography, and CT scan or MRI of the relevant region(s). The blood routine was repeated before each dose of chemotherapy and at the nadir of the leukocytes and platelets. Blood chemistry was repeated before each cycle of chemotherapy. An imaging study of the relevant region(s) was performed after every two courses of treatment and then every 3 months after the completion of the chemotherapy for follow-up.
Statistical analysis
The sample size was calculated based on a null hypothesis of 65 % versus an alternative hypothesis of 80 %. This expected range was used because the historically reported response rates to platinum-based doublets varied between 22 and 76 %, a range which reflects the variations in patient selection, trial designs and criteria of response assessment across different phase II studies. A two-stage design by Simon was used, where if more than 21 of the 31 patients recruited during the first stage of enrollment responded to PCF; 62 additional patients would be accrued until an expected total of 93 patients had been reached (type I error, 0.05; type II error, 0.1). Objective response was measured using the RECIST criteria, and toxicity was graded according to the CTCAE version 3. PFS was measured until the date when objective disease progression was observed, while OS was measured until the date of death from any cause. Patients were censored if they remained alive without progression at the time of data analysis. All enrolled patients were included in the analysis of efficacy and AEs. The primary endpoint was overall response rate as assessed by the investigators. The 95 % CI for response rate was calculated. PFS and OS were estimated by Kaplan–Meier analysis.
Results
Patient characteristics
Ninety-five patients were recruited from 2005 to 2011. All patients had WHO type II/III NPC with a male predominance. Seventy-one patients (74.7 %) had prior RT for primary NPC; 51.6 % of patients received prior platinum-based neoadjuvant, concurrent or adjuvant chemotherapy. Sixty-nine patients had metachronous metastases. Lung, liver and bone were primary involved sites. There were also 17 loco-regional recurrences besides distant metastases. The interval from the end of the primary treatment to the diagnosis of metastases/recurrence ranged from 3.0 months to 7 years. Eighteen patients were discovered with metachronous metastases/recurrence within a year of radiotherapy. Other details of their characteristics are summarized in Table 1. Results are reported at a median follow-up of 24.8 months.
Response and survival
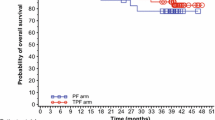
Ninety-two patients were assessable for response. Two patients were not assessable because of treatment refusal for personal reasons after one cycle and one patient withdrew the consent because of grade 2 renal damage after the first cycle. For all the 95 patients, 1 patient (1.1 %) had CR, 74 patients (77.8 %) had PR, 14 patients (14.7 %) had SD and 3 patients had PD (3.2 %). The confirmed response rate was 78.9 % (95 % CI 70.7–87.1 %), and the disease control rate (CR + PR + SD) was 93.6 % (95 % CI 88.6–98.5 %). The median follow-up period was 24.8 months. The median PFS for all patients was 8.6 months (95 % CI 7.7–9.5 months; Fig. 1a). The median OS was 22.7 months (95 % CI 18.6–26.9 months; Fig. 1b).
For the 17 patients with loco-regional recurrence, the overall response rate was 82.3 % with 14 PRs, 1 SD, 1PD and 1NA. The median PFS and OS for these patients were 9.1 (95 % CI 5.3–12.9 months) and 27.2 months (95 % CI 22.6–31.7 months), respectively.
Follow-up treatment
Sixty-three patients (66.3 %) had disease progression after PCF chemotherapy. Fifty-seven patients (90.5 %) switched to another anti-tumor treatment. Of the 57 patients, 52 patients (82.5 %) received second-line salvage chemotherapy. The regimen included Gem-NVB, Gem-Xeloda, Gem-DDP, BLM-DDP-FUDR and so on. The other five patients with liver metastasis underwent CT-guided percutaneous radiofrequency ablation. Of the remaining 32 patients, 22 patients were progression-free at the time of analysis and 10 patients were lost to follow-up.
Tolerability of PCF in this population
A total of 493 cycles of PCF were delivered to 95 patients who were assessable for toxicities. The median number of cycles given was 6. Seventy (73.7 %) patients completed the planned maximum of 6 cycles of PCF. The dose of cisplatin was reduced in 10 patients because of renal function damage and hematological toxicity. Twelve patients required a dose reduction of paclitaxel, and 23 patients required a dose reduction in 5-FU. The causes of dose reduction were hematological toxicity, diarrhea, hepatotoxicity, sensory neuropathy and stomatitis.
Toxicities were expressed as the number of patients who experienced a certain grade of an adverse event at least once during the study. As outlined in Table 2, the most common grade 3–4 hematological toxicity was neutropenia, followed by leucopenia. The most common non-hematological toxicity of grade 3/4 was diarrhea and stomatitis related to 5-FU. Paclitaxel-related sensory neuropathy, fatigue and nausea were mostly grade 1–2 in severity. Only one patient developed neutropenic fever, while most febrile events were mild and unrelated to neutropenia or serious infections. One patient with grade 2 renal damage after one cycle of treatment withdrew his consent. One patient tested positive for hepatitis B surface antigen experienced hepatitis B virus reactivation during the study, resulting in permanent withdrawal from study. No treatment-related death occurred during this study.
Prognostic factor analysis
Factors that were analyzed in univariate analysis are listed in Tables 3 and 4. Statistically significant negative prognostic factors included age >45 years, multiple metastatic sites and elevated baseline serum LDH levels. Cox multivariate analysis also identified as the independent negative prognostic factors for overall survival. However, further analyzed association between baseline serum LDH and clinical outcome showed no differences in response rates and PFS were identified between different baseline serum LDH levels in the exploratory analysis. (Table 5).
Discussion
NPC is prevalent in southern China, especially in Guangdong Province [21]. More than half of the patients eventually fail treatment due to distant metastases or recurrence [4]. Platinum-based regimens are the mainstay front-line chemotherapy in the treatment for advanced NPC. However, no standard treatment regimens have been established. Table 6 summarizes data from several phase II trials of platinum-based chemotherapy in the front-line setting among patients with advanced/recurrent NPC. As outlined in Table 6, phase II trials of platinum-based doublets with 5-fluorouracil, taxanes or gemcitabine have reported overall response rates ranging from 59 to 75 % and complete response rates of 3–20 % in the front-line setting [5–17]. This characteristic intuitively supports the approach of using aggressive, multidrug regimens to achieve disease remission or control. Studies from the East [5, 22] and the West [23, 24] have reported on multidrug regimens resulting in high response rates.
In our current study, we firstly reported the efficacy and safety of the triplet regimen with paclitaxel, cisplatin and 5-FU in metastatic and/or recurrent NPC. First of all, the regimen achieved encouraging response rate (78.9 %) when compared with other platinum-based doublets. Although a higher response rate may not consistently translate into an improved progression-free survival or overall survival, this triplet regimen could be considered where tumor shrinkage is required. For instance, a higher response has been commonly observed with better palliation of tumor-related symptoms for those patients with high tumor burden, thereby improving the quality of life which is one of the purposes of palliative chemotherapy. However, unfortunately, patient quality of life (QoL) during treatment was not formally assessed in this study. Meanwhile, the fact that the triplet combination regimen achieved a high response rate also indicates that it may be reasonable to use this regimen as induction therapy which is widely used for locally advanced NPC [25]. Phase III clinical trials from western countries have shown that induction chemotherapy with the addition of taxanes to the PF (cisplatin and 5-FU) regimen could result in a higher CR rate and significantly improve overall survival for patients with head and neck squamous cell carcinoma compared with PF alone [20]. Therefore, the role of PCF in induction chemotherapy for locally advanced NPC is promising and worthy of further study. Moreover, the PCF regimen also shows a high response rate for loco-regional lesions in our current study. For the 17 patients with loco-regional recurrence, the overall response rate was as high as 82.3 % with 14 PRs, 1 SD, 1PD and 1NA. As we know, recurrence is still one of the common failures after radiotherapy for NPC. However, external re-irradiation of NPC with curative intent is difficult for some patients due to previous high doses of irradiation and the short recurrence-free interval. The high incidence of fatal complications following re-irradiation is also of concern [26]. Therefore, the use of PCF regimen may be suitable for recurrent disease where further radiation therapy is contraindicated. It is also reasonable to consider using this regimen as induction to shrink tumor bulk, thereby reducing the required re-irradiation dose and treatment volume.
Successive phase II studies have defined the activity of platinum-based doublets in patients with recurrent and/or metastatic NPC, which are now commonly used in clinical practice in Asia. The expected median PFS and OS reported in these studies ranges from 3.5 to 10.6 months and 11 to 19.6 months, as is shown in Table 6 [5–17]. The combination of 5-FU and cisplatin has been reported to achieve a median PFS of 8 months and a median OS of 11 months [6]. The combination of cisplatin and gemcitabine had a median PFS of 10.6 months and a median OS of 15 months [14], while the combination of carboplatin and paclitaxel was reported to achieve a median PFS of 7 months and a median OS of 12 months [11]. No randomized trial has been reported comparing the different chemotherapy regimens in the treatment for metastatic and/or recurrent NPC. The median PFS reported with PCF regimen falls well within these expected ranges. However, the median OS of 22.7 months reported in our current study is impressive and appears to lie above the expected range when compared with our historic data of median OS with two-drug regimens. However, these results are still insufficient to determine whether PCF is superior to other platinum-based regimen. The observed differences by cross-trial comparisons may be explained by the heterogeneity in trial designs, patient selection, the use of effective salvage regimens and improved ancillary support at progression. For instance, in this study, all patients were diagnosed in endemic area and were relatively young. Secondly, the majority of patients (90.5 %) with disease progression after first-line of PCF treatment received second-line salvage treatment. It has been reported that patients receiving salvage chemotherapy for metastatic NPC had significantly longer median OS than patients receiving only one line of chemotherapy [27]. Further randomized trial to compare TPF versus PF regimen is warranted.
Previous studies have shown that multidrug regimens are associated with a higher response rate, but also associated with increased toxicities [22–24]. However, our study showed that the PCF regimen resulted in a high response rate with a relatively mild toxicity profile when compared with other triplet regimens. The incidence of neutropenic sepsis was very low. Only one patient experienced neutropenic febrile in our study. Notably, only around 10 % of our patients experienced other grade 3–4 non-hematological toxicity. These indicate that the use of paclitaxel combined with 5-FU and cisplatin at this dose level is safe. Stomatitis is the most common grade 3–4 non-hematological toxicity, and it is also well tolerated by appropriate dose modifications.
Furthermore, in prognostic factor analysis, our study demonstrated an elevated level of LDH was correlated with a worse overall survival in a uniformly treated patient cohort with metastatic NPC for the first time. But there was not any correlation between baseline LDH levels and response rate or PFS. In fact, elevated serum baseline LDH levels have historically been interpreted as a surrogate for large tumor burden [28]. This might be of clinical importance.
In conclusion, the results of our study demonstrate that triplet combination chemotherapy with paclitaxel, cisplatin and 5-FU is an effective and safe option in the front-line treatment for recurrent and/or metastatic NPC. The encouraging results with high response rate and long overall survival suggest that this regimen might be especially considered where tumor shrinkage is required. Further randomized controlled study is warranted to determine the optimal treatment regimen for metastatic NPC. The role of PCF in induction chemotherapy for locally advanced or loco-regional recurrent NPC is also deserving of further study.
References
Yu MC, Yuan JM (2002) Epidemiology of nasopharyngeal carcinoma. Semin Cancer Biol 12(6):421–429
Chang ET, Adami HO (2006) The enigmatic epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol Biomarkers Prev 15(10):1765–1777
Chiesa F, De Paoli F (2001) Distant metastases from nasopharyngeal cancer. ORL J Otorhinolaryngol Relat Spec 63(4):214–216
Liu MT, Hsieh CY, Chang TH, Lin JP, Huang CC, Wang AY (2003) Prognostic factors affecting the outcome of nasopharyngeal carcinoma. Jpn J Clin Oncol 33(10):501–508
Chi KH, Chan WK, Cooper DL, Yen SH, Lin CZ, Chen KY (1994) A phase II study of outpatient chemotherapy with cisplatin, 5-fluorouracil, and leucovorin in nasopharyngeal carcinoma. Cancer 73(2):247–252
Au E, Ang PT (1994) A phase II trial of 5-fluorouracil and cisplatinum in recurrent or metastatic nasopharyngeal carcinoma. Ann Oncol 5(1):87–89
Wang TL, Tan YO (1991) Cisplatin and 5-fluorouracil continuous infusion for metastatic nasopharyngeal carcinoma. Ann Acad Med Singap 20(5):601–603
Au E, Tan EH, Ang PT (1998) Activity of paclitaxel by three-hour infusion in Asian patients with metastatic undifferentiated nasopharyngeal cancer. Ann Oncol 9(3):327–329
Yeo W, Leung TW, Chan AT, Chiu SK, Yu P, Mok TS, Johnson PJ (1998) A phase II study of combination paclitaxel and carboplatin in advanced nasopharyngeal carcinoma. Eur J Cancer 34(13):2027–2031
Ciuleanu TE, Fountzilas G, Ciuleanu E, Plataniotis M, Todor N, Ghilezan N (2004) Paclitaxel and carboplatin in relapsed or metastatic nasopharyngeal carcinoma: a multicenter phase II study. J BUON 9(2):161–165
Tan EH, Khoo KS, Wee J, Fong KW, Lee KS, Lee KM, Chua ET, Tan T, Hoo-Tan HS, Yang TL, Au E, Tao M, Ong YK, Chua EJ (1999) Phase II trial of a paclitaxel and carboplatin combination in Asian patients with metastatic nasopharyngeal carcinoma. Ann Oncol 10(2):235–237
Chua DT, Sham JS, Au GK (2005) A phase II study of docetaxel and cisplatin as first-line chemotherapy in patients with metastatic nasopharyngeal carcinoma. Oral Oncol 41(6):589–595
McCarthy JS, Tannock IF, Degendorfer P, Panzarella T, Furlan M, Siu LL (2002) A Phase II trial of docetaxel and cisplatin in patients with recurrent or metastatic nasopharyngeal carcinoma. Oral Oncol 38(7):686–690
Ngan RK, Yiu HH, Lau WH, Yau S, Cheung FY, Chan TM, Kwok CH, Chiu CY, Au SK, Foo W, Law CK, Tse KC (2002) Combination gemcitabine and cisplatin chemotherapy for metastatic or recurrent nasopharyngeal carcinoma: report of a phase II study. Ann Oncol 13(8):1252–1258
Ma BB, Tannock IF, Pond GR, Edmonds MR, Siu LL (2002) Chemotherapy with gemcitabine-containing regimens for locally recurrent or metastatic nasopharyngeal carcinoma. Cancer 95:2516–2523
Ma BB, Hui EP, Wong SC, Tung SY, Yuen KK, King A, Chan SL, Leung SF, Kam MK, Yu BK, Zee B, Chan AT (2009) Multicenter phase II study of gemcitabine and oxaliplatin in advanced nasopharyngeal carcinoma–correlation with excision repair cross-complementing-1 polymorphisms. Ann Oncol 20(11):1854–1859
Li YH, Wang FH, Jiang WQ, Xiang XJ, Deng YM, Hu GQ, Xu DM, Chen Y, Lin Q, He YJ (2008) Phase II study of capecitabine and cisplatin combination as first-line chemotherapy in Chinese patients with metastatic nasopharyngeal carcinoma. Cancer Chemother Pharmacol 62(3):539–544
Worden FP, Moon J, Samlowski W, Clark JI, Dakhil SR, Williamson S et al (2006) A phase II evaluation of a 3-hour infusion of paclitaxel, cisplatin, and 5-fluorouracil in patients with advanced or recurrent squamous cell carcinoma of the head and neck: Southwest Oncology Group study 0007. Cancer 107(2):319–327
Kang GH, Kim GS, Lee HR, Yuh YJ, Kim SR (2008) A phase II trial of paclitaxel, 5-fluorouracil (5-FU) and cisplatin in patients with metastatic or recurrent gastric cancer. Cancer Res Treat 40(3):106–110
Hitt R, Lopez-Pousa A, Martinez-Trufero J, Escrig V, Carles J, Rizo A, Isla D, Vega ME, Marti JL, Lobo F, Pastor P, Valenti V, Belon J, Sanchez MA, Chaib C, Pallares C, Anton A, Cervantes A, Paz-Ares L, Cortes-Funes H (2005) Phase III study comparing cisplatin plus fluorouracil to paclitaxel, cisplatin, and fluorouracil induction chemotherapy followed by chemoradiotherapy in locally advanced head and neck cancer. J Clin Oncol 23(34):8636–8645
Chan AT, Teo PM, Johnson PJ (2002) Nasopharyngeal carcinoma. Ann Oncol 13(7):1007–1015
Hong RL, Sheen TS, Ko JY, Hsu MM, Wang CC, Ting LL (1999) Induction with mitomycin C, doxorubicin, cisplatin and maintenance with weekly 5-fluorouracil, leucovorin for treatment of metastatic nasopharyngeal carcinoma: a phase II study. Br J Cancer 80(12):1962–1967
Hasbini A, Mahjoubi R, Fandi A, Chouaki N, Taamma A, Lianes P, Cortès-Funes H, Alonso S, Armand JP, Cvitkovic E, Raymond E (1999) Phase II trial combining mitomycin with 5-fluorouracil, epirubicin, and cisplatin in recurrent and metastatic undifferentiated carcinoma of nasopharyngeal type. Ann Oncol 10(4):421–425
Taamma A, Fandi A, Azli N, Wibault P, Chouaki N, Hasbini A, Couteau C, Armand JP, Cvitkovic E (1999) Phase II trial of chemotherapy with 5-fluorouracil, bleomycin, epirubicin, and cisplatin for patients with locally advanced, metastatic, or recurrent undifferentiated carcinoma of the nasopharyngeal type. Cancer 86(7):1101–1108
Langendijk JA, Leemans CR, Buter J, Berkhof J, Slotman BJ (2004) The additional value of chemotherapy to radiotherapy in locally advanced nasopharyngeal carcinoma: a meta-analysis of the published literature. J Clin Oncol 22(22):4604–4612
Suarez C, Rodrigo JP, Rinaldo A, Langendijk JA, Shaha AR, Ferlito A (2010) Current treatment options for recurrent nasopharyngeal cancer. Eur Arch Otorhinolaryngol 267(12):1811–1824
Tay MH, Ong YK, Foo KF (2001) The role of salvage chemotherapy in metastatic nasopharyngeal carcinoma (NPC). Proc Am Soc Clin Oncol 20 (abstract 2547)
Liaw CC, Wang CH, Huang JS, Kiu MC, Chen JS, Chang HK (1997) Serum lactate dehydrogenase level in patients with nasopharyngeal carcinoma. Acta Oncol 36(2):159–164
Acknowledgments
We thank Sharlene Gill for providing sincerely writing assistance for our study.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Cui Chen and Feng-hua Wang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Chen, C., Wang, Fh., An, X. et al. Triplet combination with paclitaxel, cisplatin and 5-FU is effective in metastatic and/or recurrent nasopharyngeal carcinoma. Cancer Chemother Pharmacol 71, 371–378 (2013). https://doi.org/10.1007/s00280-012-2020-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-012-2020-x