Abstract
Purpose
Nasopharyngeal carcinoma (NPC) is a rare but aggressive malignancy in children and adolescents. An international, randomized phase 2 trial was conducted to compare induction chemotherapy with docetaxel plus cisplatin and 5-fluorouracil (TPF) with cisplatin and 5-fluorouracil (PF) in NPC patients under the age of 21.
Methods
Patients with stage IIB–IV NPC were randomly assigned, in a 2:1 ratio, to receive TPF or PF 3-weekly for three cycles, followed by chemoradiotherapy. The primary endpoint was the complete response rate achieved with TPF or PF. Docetaxel pharmacokinetics was also evaluated.
Results
Seventy-five patients (median 16 years old) were randomized, with 50 assigned to the TPF group and 25 to the PF group. Overall response was assessed after induction treatment: one patient in the TPF group and none in the PF group had a complete response. Partial response was achieved in 76.0 and 80.0 % in the TPF and PF groups, respectively. The overall safety profile was consistent with findings in adults. The estimated 3-year overall survival rate was 78.0 % for the PF group and 85.7 % for the TPF group (median follow-up 3.3 years). Mean docetaxel area under the curve was 3.41 µg h/mL, compared with 3.51 µg h/mL seen in adult patients.
Conclusion
This study demonstrated the feasibility of prospective randomized protocols, even for such rare tumors as pediatric NPC. Overall, there were no differences between the two treatment arms in terms of efficacy and toxicity. The pharmacokinetics of docetaxel in pediatric patients at 75 mg/m2 was similar to those observed in adults.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer of the nasopharynx (NPC) is one of the few malignant tumors in childhood that emerge from the epithelium. It is rare in children, and its incidence varies by geographical area, reflecting interactions between genetic and environmental factors, particularly exposure to the Epstein–Barr virus (EBV). According to the International Agency for Research on Cancer (IARC), the worldwide crude rate for NPC for 0- to 14-year-olds is 0.1/100,000. The most affected continent is Asia with 891 cases/year, followed by Africa with 469, America with 167, and Europe with 45 cases in 2002 [1]. In the North American population-based Surveillance, Epidemiology, and End Results (SEER) database, 129 children/adolescents (0- to 19-year-olds) were registered from 1988 to 2006, giving an incidence of 0.5/million person/years [2]; six thousand adult cases were registered during the same period.
NPC is potentially curable and generally considered more radio- and chemosensitive than other head and neck squamous cell carcinomas. Treatment for pediatric patients is generally extrapolated from guidelines for adults, while prospective trials in children and adolescents have been made unfeasible by the disease’s rarity and accrual difficulties [3–10]. Most young patients’ tumors are histological World Health Organization (WHO) type III cases, which are more likely to be advanced at onset, but these patients generally have a significantly better chance of survival than adults [3–10]. While concomitant chemoradiation, with or without adjuvant chemotherapy, is the standard of care for adult patients, optimal treatment modality for pediatric and young adolescent NPC has not yet been established. Induction chemotherapy before radiotherapy is well accepted in pediatric NPC. Cisplatin in combination with 5-fluorouracil (PF) is the most commonly used induction regimen [11], as seen in the recently completed American COG ARAR0331 study [3], the Italian Rare Tumors in Pediatric Age (TREP) project [4], and the NPC-2003 study of the German Society of Pediatric Oncology and Hematology (GPOH) [8]. Adding docetaxel (Taxotere®) (TPF regimen) to the PF regimen has had a significant effect in adults with locally advanced non-NPC head and neck squamous cell carcinoma [12]. Two pivotal phase 3 studies confirmed that TPF-based induction was associated with statistically significant better overall and progression-free survival rates than PF-based induction [13, 14], suggesting that docetaxel may improve response in induction.
This study (NCT00565448) was a multicenter, international, randomized phase 2 trial on the effect of TPF to evaluate early complete response (CR), compared with the standard PF combination, in patients under 21 years of age.
Patients and methods
Patients with NPC up to 21 years of age were randomized to receive TPF or PF. The main inclusion criteria were untreated histologically confirmed NPC, WHO type II/III, and stage IIB to IV with or without metastatic disease.
The protocol was approved from all independent ethics committees and/or institutional review boards. Written informed consent was obtained for each patient.
Treatment schema
The study consisted of an induction and consolidation phase over a total period of 18 weeks. The induction period consisted of three cycles of chemotherapy administered 3-weekly. Patients were randomized in a 2:1 ratio to either the TPF or PF treatment group with the aim to enroll as many patients as possible to the experimental arm while maintaining enough patients in the control arm for safety and efficacy comparison.
The TPF regimen consisted of docetaxel at a dose of 75 mg/m2, given as a 1-h infusion on Day 1, with cisplatin at a dose of 75 mg/m2, given as a 6-h infusion on Day 1, and 5-fluorouracil at a dose of 750 mg/m2/day, given as continuous infusion on Days 1–4. The PF regimen consisted of cisplatin at a dose of 80 mg/m2, given as a 6-h infusion on Day 1, and 5-fluorouracil at a dose of 1000 mg/m2/day, given as continuous infusion on Days 1–4.
In the TPF arm, two doses of oral or intravenous dexamethasone (3 mg/m2) were given every 6 h beginning at 12 h before docetaxel administration. It was recommended that all patients receive prophylactic antibiotic therapy such as ciprofloxacin at 500 mg orally twice daily (Days 5–15). No primary prophylactic granulocyte colony-stimulating factor was administered, but it could be used after the second and/or subsequent cycles in case of toxicity.
The consolidation period consisted of locoregional radiotherapy over 6 weeks with three 21-day cycles of cisplatin at 100 mg/m2 administered concurrently, beginning on weeks 10, 13, and 16.
Either three-dimensional planning or intensity-modulated radiation therapy (IMRT) was recommended, but the use of IMRT was strongly encouraged.
After completing treatment, patients were followed for up to 3 years, assessing survival status every 3 months in the first year and every 6 months for the second and third year.
Objectives
The primary objective of the study was to estimate the CR rate of TPF compared with PF after induction treatment. The secondary objectives were safety of TPF in comparison with PF, pharmacokinetics (PK) of docetaxel when added to PF, and overall survival (OS) rates between TPF and PF.
Methods
Patients were staged according to the 5th edition of the American Joint Committee on Cancer staging system. Modified Response Evaluation Criteria in Solid Tumors (RECIST) guidelines were used to assess response rate for primary tumor, nodal disease, target metastatic lesions, and overall response. The modifications included a volumetric measurement of primary NPC and a bi-dimensional assessment of nodal disease at magnetic resonance imaging. Measurable and non-measurable metastatic lesions were assessed according to standard RECIST [15]. CR was defined as the complete disappearance of all target and non-target lesions.
Patient characteristics were tabulated based on the intent-to-treat population including all randomized patients. OS was analyzed using the intent-to-treat population, based on the treatment assigned. The safety population included all patients given at least one cycle. Adverse events (AEs) were presented by worst National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) grade. Serious AEs (SAEs) were recorded starting from informed consent signature until 30 days after the last treatment.
A CR rate of 20 % after 3 cycles for the control arm (PF) and a 31 % CR rate in the experimental arm (TPF) were assumed. For this rare disease in a pediatric population, a pick-a-winner study design was used to randomize 75 patients in a 2:1 ratio to identify a treatment arm with the best CR rate with 85 % probability. This was the first randomized trial in such a disease population although it was not powered with the sample size of a phase 3 trial.
Assuming a CR rate of 20 % after 3 cycles for the control arm (PF) and predicting a 31 % CR rate in the experimental arm (TPF), randomizing 75 patients in a 2:1 ratio was calculated to identify a treatment arm with the best CR rate with 85 % probability.
OS was defined from the date of randomization to date of death for any reason. Patients lost to follow-up or with missing data were censored at their last contact. The OS distribution was estimated using the Kaplan–Meier method [16], computing the 95 % confidence intervals (CIs) with the Brookmeyer–Crowley method [17] and linear transformation [18].
A data monitoring committee of four independent experts (pediatrician, oncologist, radiotherapist, and statistician) and an independent review committee of three board-certified radiologists were established.
Pharmacokinetic parameters
The PKs of docetaxel have been previously studied in a phase 1 trial in 29 pediatric patients who received docetaxel as a 1-h infusion at doses ranging from 55 to 235 mg/m2 [19]. This study has shown that Bayesian estimation, using three concentration–time data and the adult population PK model as prior information [20], performs well with respect to both bias (mean relative-error prediction +11.4 %) and precision (root-mean-squared relative-error prediction 21.7 %) for estimating docetaxel clearance when compared to classical compartmental analysis. Therefore, optimal sampling strategy was applied in this phase 2 study, and a series of 1 mL blood samples were collected just before the end of docetaxel infusion, at 45 min, and 5 h post-infusion. Plasma samples were analyzed using liquid chromatography/tandem mass spectrometry with a lower limit of quantification of 1 ng/mL [21]. The three-compartment structural adult model with first-order elimination was used as prior information. Estimates of adult population PK were clearance of 36.8 L/h, volume of distribution of central compartment of 7.83 L, a steady-state volume of distribution of 122 L, and a terminal half-life of 10.0 h. The combined administration of docetaxel, cisplatin, and 5-fluorouracil in adults had no influence on the PKs of the individual drugs [22]. The analysis was focused on docetaxel plasma clearance and area under the curve (AUC) parameters.
Results
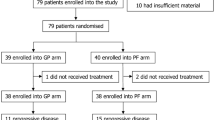
Between November 2007 and November 2008, a total of 75 patients, accrued in 26 centers in 14 countries (Table 1), were randomized, 50 to receive TPF and 25 PF.
The treatment groups were comparable regarding demographics and baseline disease characteristics (Table 2). Median patient age was 16 years: 64 % of patients in both groups were Caucasian/white, while the proportion of Asians was lower in the TPF arm. Most patients had undifferentiated NPC and stage IV disease (Appendix 1). Five (6.6 %) patients had distant metastasis at diagnosis.
The vast majority of patients (94 % in the TPF and 92 % in the PF arm) completed the induction treatment as per protocol. Of the five patients who did not complete the induction treatment, three in the TPF group were due to transaminase elevation, disease progression, or patient’s request, and two in the PF group were due to one case of neutropenic infection with thrombocytopenia and one case of septic shock. One patient in the PF group had a major deviation (received 5-floxuridine instead of 5-fluorouracil in Cycle 1), and 37 patients had minor deviations involving small changes in dosage (13 patients in the TPF and two in the PF group). Most patients received docetaxel and cisplatin as specified in the protocol, with a median relative dose intensity of 98.6 % for both drugs. The median relative dose intensity of 5-fluorouracil was 98.6 % in the TPF group and 98.5 % in the PF group (Table 3).
Efficacy
As shown in Table 4, six patients were not evaluable; one had inappropriate and five had no radiological assessments after induction chemotherapy.
One patient in the TPF group and no patient in the PF group had a CR in all target and non-target lesions. Concerning the primary tumor, CR rate was 12 % in the TPF and 8 % in the PF group. For regional nodal disease, CR rate was 4 % for TPF and 0 % for PF group. Overall, there were no differences between the two treatment groups. Thirty-eight patients (76 %) given TPF and 20 (80 %) given PF experienced a partial response.
The median follow-up time from the date of randomization was 3.3 years (range 0.1–48.1 months). The estimated 3-year OS rate was 78.0 % (95 % CI 60.8–95.1) for the PF group and 85.7 % (95 % CI 75.9–95.5) for the TPF group (p value 0.48) (Fig. 1). By the end of the 3-year follow-up, 13 of the 75 patients had died (five in the PF and eight in the TPF group): Four deaths in the PF and seven in the TPF group were due to disease progression; one in the PF group was due to febrile bone marrow aplasia and septic shock during treatment and one in the TPF group to severe hematemesis during the follow-up period.
Safety
An overview of safety from visit 1 to the start of the consolidation period is available in Appendix 2. The overall incidence and severity of AEs were similar in both induction treatment groups, with vomiting (84 %), nausea (62 %), alopecia (56 %), diarrhea (36 %), and neutropenia (30 %) being the most frequent in the TPF group and vomiting (80 %), nausea (32 %), neutropenia (28 %), and pyrexia (16 %) in the PF group. AEs occurring at least 10 % more often in the TPF group than in PF group were anemia (16 vs 4 %), abdominal pain (38 vs 12 %), constipation (12 vs 0 %), nausea (62 vs 32 %), diarrhea (36 vs 12 %), stomatitis (18 vs 4 %), hyponatremia (10 vs 0 %), and alopecia (56 vs 4 %).
The most common SAEs consisted of hematologic disorders (14 % TPF, 16 % PF). The incidence of gastrointestinal AEs was similar in the two groups, but gastrointestinal SAEs were more frequent in the TPF group (14 % TPF, 0 % PF).
Other significant AEs were hypersensitivity reactions (four patients in the TPF group) and convulsions in three patients (one in TPF and two in PF patients—none had a history of seizures, but two revealed grade 4 hyponatremia at the time of their seizures).
Two patients in each group had their treatment discontinued due to AEs. In the TPF group, these were a grade 4 and a grade 2 transaminase elevation (the latter patient discontinued 5-fluorouracil only), and in the PF group, one patient had grade 3 neutropenic infection with grade 4 thrombocytopenia and one had grade 4 septic shock. Nineteen patients (13 TPF, six PF) had their treatment interrupted or delayed during the induction period due to AEs, mainly involving neutropenia or hypersensitivity. The number of patients that delayed starting consolidation phase was 52 % in PF group and 56 % in TPF group.
Pharmacokinetics
The docetaxel PK analysis was performed in 28 of the 50 patients (19 male, nine female) with a median age of 16 years (range 10–21). The median body surface area was 1.49 m2 (range 0.81–1.89). The mean (±SD) docetaxel clearance was 40.0 ± 18.8 (range 11.8 to −85.4) L/h corresponding to a mean AUC of 3.41 ± 1.98 (range 1.64–8.82) µg h/mL. Mean AUC was similar compared with that observed in adult patients (n = 52) with several tumor types [23, 24], treated at docetaxel monotherapy dose of 75 mg/m2 with a mean value of 3.51 ± 1.76 (range 1.74–12.7) µg h/mL (mean adult clearance 42.4 ± 13.3 L/h, 24.3 ± 7.09 L/h/m2) (Fig. 2).
Discussion
Although radiotherapy has been the mainstay of treatment in both adult and pediatric NPC patients, concomitant chemoradiotherapy with three adjuvant PF cycles was accepted as standard treatment of adult NPC patients after the publication of the landmark Intergroup Study 0099 [10]. While most of the experiences were with cisplatin/5-fluorouracil-based chemotherapy, either as induction or concurrent treatment, the role of taxanes in the management of adult NPC is not definite. Initially, cisplatin and docetaxel were used as induction treatment in adult NPC patients with outstanding compliance and efficacy [25]. Neoadjuvant TPF, followed by concurrent chemoradiation, was also reported to be well tolerated and produced encouraging outcomes in patients with locally advanced NPC [26]. However, both strategies had not yet been compared against standard treatment of a PF regimen in randomized, controlled trials.
Here, we report the results of the first randomized phase 2 trial in pediatric and adolescent NPC patients, comparing TPF and PF regimens. It represents the largest published series of NPC pediatric patients treated prospectively in the context of a trial.
This study was developed with the hope that adding docetaxel to a PF regimen would yield better response rates than a PF regimen. However, analysis of the primary efficacy endpoint showed no significant difference between the two groups. The CR rates reported in this study were lower than those assumed when the sample size was calculated (31 % for TPF and 20 % for PF), based on the North American Pediatric Oncology Group (POG) study [6] that used four courses of a regimen consisting of methotrexate, cisplatin, 5-fluorouracil, and leucovorin. It is noteworthy to mention that this rate was calculated in only 16 cases. Conversely, the CR rate was 14 % after three courses of the same regimen in the GPOH study on 55 patients [7]. The GPOH series resembled ours in regard to the higher proportion of stage IV disease (53 %) as compared to the POG series (13 %). These differences may partially explain the difference in the observed response rate compared to the one initially assumed, but the accuracy and the conservative approach to assessing response in the present study may have played a part as well. In our study, the response was assessed blindly by an independent panel of experts who reviewed the radiological scans of patients to assign appropriate objective response—whereas none of the previously published pediatric studies used blinded investigators. This hypothesis may be confirmed by looking at overall response rate (76 and 80 % in our TPF and PF groups vs 93.7 % in the POG and 90 % in the Italian series) [4, 6].
Most of the patients in our study received their chemotherapy in accordance with the protocol with a dose intensity higher than 95 % in both arms. The incidence and severity of AEs were similar in the two induction treatment groups. As expected, most AEs involved the gastrointestinal and blood/lymphatic system. Delays or interruptions were mainly due to neutropenia and hypersensitivity reactions. PK analysis showed that the mean AUC in pediatric patients treated with 75 mg/m2 docetaxel was similar to findings in adults [23]. A narrower range of AUC might suggest that the variability of PK in children may be less than in adults, possibly due to better or more homogeneous organ function. As in adults, combining cisplatin and 5-fluorouracil had no influence on the PK of docetaxel [22].
There are no other published experiences with TPF chemotherapy in pediatric patients. Docetaxel was evaluated in monotherapy in phase 1 and 2 studies [19, 27, 28]. The maximum tolerated dose was 125 mg/m2, and neutropenia was the dose-limiting toxicity [19]. When docetaxel was given with granulocyte colony-stimulating factor, the maximum tolerated dose was higher (185 mg/m2), and generalized erythematous desquamating skin rash and myalgias were the dose-limiting toxicity [27]. The combination of cisplatin and docetaxel was previously evaluated in a limited series of ten pediatric NPC patients in a preradiation setting [29]. The 2-year OS rate was 90 %, and the event-free survival rate was 70 %.
Survival analysis in the present study showed no statistically significant difference among the two treatments: The 3-year OS rate was 78.0 and 85.7 % in the PF and TPF groups, respectively, and in the same range as in other studies including metastatic patients. The best OS rate in non-metastatic patients was reported in the last GHOP study (97.1 %), using maintenance therapy with interferon to boost the immune system’s capacity to capture cells harboring the EBV genome [8]. The role of interferon in NPC treatment remains controversial, even if interest in immunotherapy is growing and, in particular, the possibility of adding autologous cellular immunotherapy with EBV-specific cytotoxic T cells in EBV-positive patients [3].
Although the use of neoadjuvant chemotherapy in NPC among pediatric oncologists is quite standard, its use is not evidence based. The absence of improvement from adding docetaxel to PF could be justified by the lack of benefit of induction chemotherapy or by the fact that two drugs may reach a plateau of efficacy.
Strong collaboration between pharmaceutical companies, regulatory agencies and pediatric oncology cooperative groups should be encouraged in order to determine the best way to provide innovative drugs for rare pediatric tumors. This study is an example of such worldwide collaborative efforts, and while it did not show a substantial clinical benefit by adding docetaxel in this population, the conduction and demonstration of feasibility of such a study were successful.
References
Globocan 2002. IARC. http://www-dep.iarc.fr/globocan/database.htm
Sultan I, Casanova M, Ferrari A, Rihani R, Rodriguez-Galindo C (2010) Differential features of nasopharyngeal carcinoma in children and adults: a SEER study. Pediatr Blood Cancer 55:279–284
Rodriguez-Galindo C, Krailo M, Frazier L, Chintagumpala M, Amatruda J, Katzenstein H, Malogolowkin M, Spector L, Pashankar F, Meyers R, Tomlinson G, COG Rare Tumors Disease Committee (2013) Children’s Oncology Group’s 2013 blueprint for research: rare tumors. Pediatr Blood Cancer 60:1016–1021
Casanova M, Bisogno G, Gandola L, Cecchetto G, Di Cataldo A, Basso E, Indolfi P, D’Angelo P, Favini F, Collini P, Potepan P, Ferrari A, Rare Tumors in Pediatric Age Group (2012) A prospective protocol for nasopharyngeal carcinoma in children and adolescents. Cancer 118:2718–2725
Orbach D, Brisse H, Helfre S, Klijanienko J, Bours D, Mosseri V, Rodriguez J (2008) Radiation and chemotherapy combination for nasopharyngeal carcinoma in children: radiotherapy dose adaptation after chemotherapy response to minimize late effects. Pediatr Blood Cancer 50:849–853
Rodriguez-Galindo C, Wofford M, Castleberry RP, Swanson GP, London WB, Fontanesi J, Pappo AS, Douglass EC (2005) Preradiation chemotherapy with methotrexate, cisplatin, 5-fluorouracil, and leucovorin for pediatric nasopharyngeal carcinoma. Results of a Pediatric Oncology Group (now Children’s Oncology Group) Study 9486. Cancer 103:850–857
Mertens R, Granzen B, Lassay L, Bucsky P, Hundgen M, Stetter G, Heimann G, Weiss C, Hess CF, Gademann G (2005) Treatment of nasopharyngeal carcinoma in children and adolescents. Definitive results of a multicenter study (NPC-91-GPOH). Cancer 104:1083–1089
Buehrlen M, Zwaan CM, Granzen B, Lassay L, Deutz P, Vorwerk P, Staatz G, Gademann G, Christiansen H, Oldenburger F, Tamm M, Mertens R (2012) Multimodal treatment, including interferon beta, of nasopharyngeal carcinoma in children and young adults: preliminary results from the prospective, multicenter study NPC-2003-GPOH/DCOG. Cancer 118:4892–4900
Küpeli S, Varan A, Özyar E, Atahan IL, Yalçin B, Kutluk T, Akyüz C, Büyükpamukçu M (2006) Treatment results of 84 patients with nasopharyngeal carcinoma in childhood. Pediatr Blood Cancer 46:454–458
Özyar E, Selek U, Laskar S, Uzel O, Anacak Y, Ben-Arush M, Polychronopoulou S, Akman F, Wolden SL, Sarihan S, Miller RC, Ozsahin M, Abacioğlu U, Martin M, Caloglu M, Scandolaro L, Szutowicz E, Atahan IL (2006) Treatment results of 165 pediatric patients with non-metastatic nasopharyngeal carcinoma: a rare cancer network study. Radiother Oncol 81:39–46
Al-Sarraf Al-Sarraf M, Leblanc M, Giri PGS, Fu KK, Cooper J, Vuong T, Forastiere AA, Adams G, Sakr WA, Schuller DE, Ensley JF (1998) Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: phase III randomized intergroup study 0099. J Clin Oncol 16:1310–1317
Haddad R, Colevas AD, Tishler R, Busse P, Goguen L, Sullivan C, Norris CM, Lake-Willcutt B, Case MA, Costello R, Posner M (2003) Docetaxel, cisplatin, and 5-fluorouracil induction chemotherapy in patients with locally advanced squamous cell carcinoma of the head and neck: the Dana Farber Cancer Institute experience. Cancer 97:412–418
Vermorken JB, Remenar E, vanHerpen C, Gorlia T, Mesia R, Degardin M, Stewart JS, Jelic S, Betka J, Preiss JH, van den Weyngaert D, Awada A, Cupissol D, Kienzer HR, Rey A, Desaunois I, Bernier J, Lefebvre JL, EORTC 24971/TAX 323 Study Group (2007) Cisplatin, fluorouracil, and docetaxel in unresectable head and neck cancer. N Engl J Med 357:1695–1704
Posner MR, Hershock DM, Blajman CR, Mickiewicz E, Winquist E, Gorbounova V, Tjulandin S, Shin DM, Cullen K, Ervin TJ, Murphy BA, Raez LE, Cohen RB, Spaulding M, Tishler RB, Roth B, Viroglio Rdel C, Venkatesan V, Romanov I, Agarwala S, Harter KW, Dugan M, Cmelak A, Markoe AM, Read PW, Steinbrenner L, Colevas AD, Norris CM Jr, Haddad RI, TAX 324 Study Group (2007) Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. N Engl J Med 357:1705–1715
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG (2000) New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst 92:205–216
Kaplan EL, Meier P (1958) Nonparametric estimation from incomplete observations. J Am Stat Assoc 53:45–81
Brookmever R, Crowley JA (1982) Confidence interval for the median survival time. Biometrics 38:29–41
Kalbfleisch JD, Prentice RL (1980) The statistical analysis of failure time data. Wiley, New York
Blaney SM, Seibel NL, O’Brien M, Reaman GH, Berg SL, Adamson PC, Poplack DG, Krailo MD, Mosher R, Balis FM (1997) Phase 1 trial of docetaxel administered as a 1-hour infusion in children with refractory solid tumors: a collaborative Pediatric Branch, National Cancer Institute and Children’s Cancer Group trial. J Clin Oncol 15:1538–1543
Bruno R, Vivler N, Vergniol JC, De Phillips SL, Montay G, Sheiner LB (1996) A population pharmacokinetic model for docetaxel (Taxotere): model building and validation. J Pharmacokinet Biopharm 24:153–172
Pascual MH, Vergniol JC (2007) Method and validation of a LC/MS/MS assay for the quantitation of RP56976 in lithium-heparin human plasma upgrade of the assay to API4000 mass spectrometer. In: DOH0601 16th October, Sanofi (data on file)
Felici A, Loos WJ, Verweij J, Cirillo I, de Bruijn P, Nooter K, Mathijssen RH, de Jonge MJ (2006) A pharmacokinetic interaction study of docetaxel and cisplatin plus or minus 5-fluorouracil in the treatment of patients with recurrent or metastatic solid tumors. Cancer Chemother Pharmacol 58:673–680
Harver V, Mouridsen H, Semiglazov V, Jakobsen E, Voznyi E, Robinson BA, Groult V, Murawsky M, Cold S (2006) Phase III trial comparing three doses of docetaxel for second-line treatment of advanced breast cancer. J Clin Oncol 24:4963–4970
Bruno R, Hille D, Riva A, Vivier N, ten Bokkel Huinnink WW, van Oosterom AT, Kaye SB, Verweij J, Fossella FV, Valero V, Rigas JR, Seidman AD, Chevallier B, Fumoleau P, Burris HA, Ravdin PM, Sheiner LB (1998) Population pharmacokinetics/pharmacodynamics (PK/PD) of docetaxel in phase II studies in patients with cancer. J Clin Oncol 16:187–196
Zhong YH, Dai J, Wang XY, Xie CH, Chen G, Zeng L, Zhou YF (2013) Phase II trial of neoadjuvant docetaxel and cisplatin followed by intensity-modulated radiotherapy with concurrent cisplatin in locally advanced nasopharyngeal carcinoma. Cancer Chemother Pharmacol 71:1577–1583
Kong L, Hu C, Niu X, Zhang Y, Guo Y, Tham IW, Lu JJ (2013) Neoadjuvant chemotherapy followed by concurrent chemoradiation for locoregionally advanced nasopharyngeal carcinoma: interim results from 2 prospective phase 2 clinical trials. Cancer 119:4111–4118
Seibel NL, Blaney SM, O’Brien M, Krailo M, Hutchinson R, Mosher RB, Balis FM, Reaman GH (1999) Phase I trial of docetaxel with filgrastim support in pediatric patients with refractory solid tumors: a collaborative Pediatric Oncology Branch, National Cancer Institute and Children’s Cancer Group trial. Clin Cancer Res 5:733–737
Zwerdling T, Krailo M, Monteleone P, Byrd R, Sato J, Dunaway R, Seibel N, Chen Z, Strain J, Reaman G, Children’s Oncology Group (2006) Phase II Investigation of docetaxel in pediatric patients with recurrent solid tumors. Cancer 106:1821–1828
Varan A, Özyar E, Çorapçioğlu F, Köksal Y, Aydin B, Yazici N, Akyüz C, Büyükpamukçu M (2009) Pediatric and young adult nasopharyngeal carcinoma patients treated with preradiation cisplatin and docetaxel chemotherapy. Int J Radiat Oncol Biol Phys 73:1116–1120
Acknowledgments
This study was sponsored by Sanofi. We thank the individual research teams at all participating study sites (Appendix 3), patients and their families. We thank Dr. Lia Gore for her writing and editing input and Tania Bojanowski for editorial assistance. Editorial support was provided by MediTech Media, funded by Sanofi.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Christine Veyrat-Follet and Liji Shen are Sanofi employees and shareholders. Grzegorzewski Krzysztof is a former employee of Sanofi. Hassan Errihani declares a consultant/advisory role with Novartis, Pfizer, Lilly, Bristol-Myers Squibb, and Merck Sharp and Dohme and has received research funding from Roche. The remaining authors have no conflicts to declare.
Additional information
Krzysztof J. Grzegorzewski was previously working for Sanofi.
Rights and permissions
About this article
Cite this article
Casanova, M., Özyar, E., Patte, C. et al. International randomized phase 2 study on the addition of docetaxel to the combination of cisplatin and 5-fluorouracil in the induction treatment for nasopharyngeal carcinoma in children and adolescents. Cancer Chemother Pharmacol 77, 289–298 (2016). https://doi.org/10.1007/s00280-015-2933-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-015-2933-2