Abstract
Purpose
This study evaluated the pharmacokinetic and safety profiles of arsenic trioxide given twice per week in adult cancer patients with advanced malignancies and varying degrees of renal function.
Methods
Patients received intravenous arsenic trioxide 0.15 mg/kg twice weekly for 4 weeks, followed by a 2-week rest period. The pharmacokinetic profiles of the pharmacologically active arsenical species, arsenious acid (AsIII), and its metabolites, monomethylarsonic acid (MMAV) and dimethylarsinic acid (DMAV), were evaluated during the first cycle for 72 h following doses on days 1 and 22. Safety assessments were made at each treatment visit.
Results
Twenty patients received an average of 11 doses. Compared with normal renal function, mild to severe renal impairment decreased urinary excretion of AsIII and increased exposure to MMAV and DMAV 1.4- to 8-fold after multiple dose administration. Only severe renal impairment substantially increased exposure to AsIII (AUC0–t increased by 18% after a single dose and 40% after multiple doses). The safety profile of arsenic trioxide after limited treatment on a twice-per-week schedule was comparable across all renal function groups.
Conclusion
Renal impairment did increase the systemic exposure to arsenic and its methylated metabolites following standard daily dosing of arsenic trioxide. The data from the limited number of patients with severe renal dysfunction did not suggest that severe renal impairment affected the safety profile of arsenic trioxide in cancer patients who received limited treatment with arsenic trioxide.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Arsenic trioxide has been used for centuries to treat infectious, inflammatory and malignant disorders, although it has been largely replaced by antibiotics and chemotherapy in Western medicine [1]. Reports from China about the efficacy and safety of arsenic trioxide in patients with previously incurable acute promyelocytic leukemia (APL) renewed interest in the drug in the USA, and pivotal US trials demonstrated significant clinical activity of arsenic trioxide in patients with refractory APL [2, 3]. Arsenic trioxide was subsequently approved in the USA in 2000 for induction of remission and for consolidation in patients with APL who relapsed on retinoid or anthracycline chemotherapy.
In clinical studies, arsenic trioxide administered by intravenous (IV) infusion at a dose of 0.15 mg/kg per day achieved hematological complete remission in 80–92% (and molecular remission in 73–76%) of patients with relapsed or refractory APL [2–4]. Similar activity has been demonstrated in patients with newly diagnosed, previously untreated APL [5, 6] Clinical activity of arsenic trioxide has also been demonstrated in other hematological cancers, including refractory multiple myeloma [7] and myelodysplastic syndrome [8]. The potential for the clinical application of arsenic trioxide in other indications, including solid tumors, is being explored in clinical trials.
Overall, arsenic trioxide is generally well tolerated; most toxicities are manageable and reversible and do not require discontinuation of treatment. The greatest safety concerns associated with arsenic trioxide include the potential for cardiac and hepatic toxicities. Arsenic trioxide can cause prolonged QT/QTc [9] intervals, which can be life-threatening, and electrocardiograph (ECG) monitoring during arsenic trioxide therapy is recommended. Arsenic trioxide has also been associated with hepatotoxicity, reflected by increased serum transaminases [4, 6]. One study has shown that an increased risk of hepatotoxicity in patients treated with arsenic trioxide may correlate with genetic polymorphisms affecting methylation and metabolism of inorganic arsenic [10].
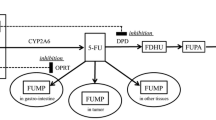
Inorganic arsenic exists in trivalent and pentavalent forms. The inorganic, lyophilized form of arsenic trioxide, when reconstituted, immediately forms the trivalent hydrolysis product, arsenious acid (AsIII), the pharmacologically active species of arsenic trioxide [11–14]. The metabolism of AsIII involves oxidation and oxidative methylation by methyltransferases (primarily in the liver and potentially also in the testes, lungs and kidneys) to pentavalent arsenic acid (AsV), monomethylarsonic acid (MMAV) and dimethylarsinic acid (DMAV) [11] (Fig. 1). These metabolites are primarily excreted in the urine.
Historically, assays were available to quantify total arsenic; however, the recent development of sensitive and specific assays has allowed for differentiation and measurement of individual arsenical species. Because AsIII has been reported to be more toxic than AsV [15–17], the toxicity of arsenic trioxide may be related to the metabolism and excretion patterns of arsenic metabolites in individual patients. The purpose of this study was to determine the effect of renal impairment on the pharmacokinetic (PK) profile of AsIII and its metabolites and on the resulting safety profile of arsenic trioxide treatment in patients with advanced malignancies.
Methods
Study design
This was a noncomparative, open-label pharmacokinetic and safety study of arsenic trioxide in patients with various levels of renal function. Patients with advanced malignancies, for which standard curative or palliative therapies did not exist or were no longer effective, were stratified by renal function based on a 24 h urine collection for creatinine clearance (CrCl) into five groups: (1) normal renal function (CrCl > 80 mL/min); (2) mild renal impairment (CrCl 50–80 mL/min); (3) moderate renal impairment (CrCl 30–49 mL/min); (4) severe renal impairment (CrCl < 30 mL/min); and (5) end-stage renal disease (patients requiring dialysis). Enrollment of up to six patients per renal function group was planned, according to regulatory guidance on the conduct of PK studies in patients with renal impairment [18, 19)]. In addition, with six patients in each cohort, there was at least a 74% chance of observing a true dose-limiting toxicity (DLT) rate ≥20%. Specifically, two or more patients experiencing a DLT in a given cohort would indicate unacceptable toxicity of the dose in a particular renal function group. A DLT in this study was defined as: a grade 4 neutropenia lasting 7 days or more; febrile neutropenia defined as a fever (temperature of 38.5°C or more) of unknown origin without clinically or microbiologically documented infection when absolute neutrophil count (ANC) was less than 1.0 × 109/L; thrombocytopenia with a platelet nadir of less than 25 × 109/L; a study treatment-related grade 3 or grade 4 nonhematologic toxicity (excluding nausea and vomiting of all grades) or any drug-related death.
Eligibility criteria also included age ≥18 years; life expectancy of ≥3 months; Karnofsky performance status of ≥70; serum bilirubin within normal limits; alanine aminotransferase and aspartate aminotransferase no more than 1.5 times the upper limit of normal; a serum potassium value of >4.0 mEq/L and a serum magnesium value of >1.8 mg/dL; and a demonstrated commitment to use medically acceptable birth control, if applicable, for the duration of the study.
Exclusion criteria included: a QTc interval of >460 ms; pre-existing neurotoxicity or neuropathy (grade ≥2); ANC of <1,000/mm3, platelets <75,000/mm3 or hemoglobin of <10 g/dL; concurrent treatment or treatment within 30 days before study entry with chemotherapy, radiation therapy, hormonal therapy or immunotherapy; any previous treatment with arsenic compounds; significant underlying cardiac dysfunction, including conduction defects, unstable angina, myocardial infarction <6 months before study entry, congestive heart failure from any cause or class ≥ II New York Heart Association functional classification; uncontrolled diabetes mellitus; any active serious infections; and a history of grand mal seizures.
Patients were treated with arsenic trioxide 0.15 mg/kg infused intravenously over 2 h twice weekly for the first 4 weeks of two 6-week cycles. The study was defined as two complete cycles. The standard dose of arsenic trioxide for the treatment of APL is 0.15 mg/kg per day. Based on simulation studies that predicted steady-state plasma AsIII trough concentrations nearly doubling after once-daily administration to patients with normal renal function, a twice weekly administration schedule, which was not associated with significant drug accumulation, was chosen (data on file). Informed consent was obtained before any study-specific assessments were performed. Patients who received at least one dose of arsenic trioxide were considered evaluable for PK and safety analyses.
Blood sampling and drug analysis
Plasma and urine samples for PK evaluation were collected during cycle 1 on days 1–4 (following a single dose) and days 22–25 (following dose 7). Patients were confined in a general clinical research center at these times to allow accurate collection of PK samples. Trough samples were also collected at interim visits (weeks 2 and 3) during cycle 1. Heparinized blood samples were collected before dosing and 2, 2.5, 3, 4, 6, 8, 12, 16, 20, 24, 48 and 72 h after initiation of drug infusion. Pooled urine samples were also collected over 72 h during the following time intervals: 0–4, 4–8, 8–12, 12–16, 16–24, 24–48 and 48–72 h after initiation of infusion. Samples were kept frozen at −20°C in the dark until analysis.
Plasma and urine samples were analyzed by Cantest, Ltd. (formerly Elemental Research Inc; North Vancouver, BC, Canada) for four arsenical species (arsenious acid [AsIII(OH)3], arsenic acid [AsVO(OH)3], monomethylarsonic acid [MMAV, AsVO(CH3)(OH)2] and dimethylarsinic acid [DMAV, AsVO(CH3)2OH]) using validated liquid chromatography methods with inductively coupled plasma mass spectrometric detection (LC-ICPMS), following separation of the compounds by ion chromatography. Calibration standards and quality control (QC) samples were prepared in either human plasma containing sodium heparin or human urine. Phenylarsonic acid was employed as the internal standard for the analysis of all specimen samples, calibration standards and QC samples. For all arsenical species in plasma, the linear range was from 5.0 to 500 ng/mL. For all arsenical species in urine, the linear range was from 50.0 to 1,500 ng/mL. Plasma and urine concentrations of arsenical species below the quantifiable limits of the assay were treated as zero in calculations of mean concentrations.
Pharmacokinetic analysis
Pharmacokinetic parameters for each arsenical species in plasma were estimated by standard noncompartmental methods using WinNonlin® software (Enterprise Version 4.1, Pharsight Corporation, Mountain View, CA, USA, 2003). To facilitate comparisons between arsenical compounds, pharmacokinetic parameters were calculated on an arsenic-equivalent basis, rather than based on the actual measured concentrations of the arsenical species.
Maximum plasma concentration (C max) was defined as the highest observed plasma concentration; time to peak plasma concentration (T max) was the corresponding time when C max was observed. The terminal rate constant for elimination from plasma (λz) was determined by linear regression of the terminal portion of the semi-logarithmic plasma concentration-versus-time curve. The terminal phase was identified for each patient’s profile by visual inspection of the data, with a minimum of three values included for each λz determination. The terminal elimination half-life (t 1/2) was calculated for each profile as ln [2] divided by λz. The areas under the plasma concentration-versus-time curve from time zero to the time of the last measurable concentration (AUC0–t ) and to 72 h (AUC0–72) for each profile were determined by log-linear trapezoidal summation. The area from time zero to infinity (AUC0–∞) was calculated as the sum of AUC0–t and the area extrapolated from the last measurable plasma concentration to infinity (C last/λz). The percentage of AUC0–∞ extrapolated was calculated as (AUC0–∞ − AUC0–t )/(AUC0–∞) × 100. Systemic clearance (CL) was calculated as dose divided by AUC0–∞, and the steady-state volume of distribution (V ss) was calculated as [(AUMC/AUC0–∞) − TI/2] × CL, where AUMC was the area under the first moment curve to infinity and TI was the length of the infusion. Renal clearance (CLR) was calculated as the amount of AsIII excreted over 72 h divided by AUC0–72, and nonrenal clearance (CLNR) was calculated as CL − CLR. The observed ratio of exposure (R obs) was calculated as the day 22 AUC0–72 divided by the day 1 AUC0–72. CL, CLR, CLNR, V ss and R obs were calculated for AsIII only.
Urinary excretion amounts were calculated for each arsenical species and collection interval by multiplying the urinary arsenic concentration by the urine volume for the respective interval; cumulative amounts were also tabulated. Urinary excretion rates for AsIII were calculated for each collection interval by dividing the excretion amount by the length of the collection interval. Cumulative percentages of the arsenic dose excreted as AsIII and as the sum of all arsenical species measured were calculated on an arsenic-equivalent basis.
Toxicity assessment
Toxicity was assessed by the National Cancer Institute Common Toxicity Criteria (NCI-CTC) Version 2 at each treatment visit. Laboratory (hematology, clinical chemistry) and ECG monitoring was performed once a week during weeks 1 through 4 and at the end of week 6 of each treatment cycle or as clinically indicated. Patients with disease progression, any DLT, or deterioration by 1 grade in renal function were withdrawn from the study. Follow-up safety assessments were performed within 30 days of the last arsenic trioxide dose. Adverse events were classified as serious or nonserious. A serious adverse event was defined as any experience (which may be a combination of toxicities, clinical presentation, diagnoses and therapies) at any dose that suggested a significant hazard, contraindication, side effect or precaution. A serious adverse event was further defined as one that was fatal, life-threatening (NCI-CTC grade 4), resulted in persistent disability or incapacity, required inpatient hospitalization or prolonged existing hospitalization or was a congenital anomaly.
Response assessment
Disease progression and disease response were evaluated by radiography, physical examination, ultrasonography, magnetic resonance imaging, positron-emission tomography or other methods at the end of each cycle.
Statistical analyses
Pharmacokinetic data analyses were summarized using descriptive statistics. To evaluate the relationship between each pharmacokinetic parameter and creatinine clearance, a Spearman rank correlation and its 95% asymptotic confidence limits were calculated using values of creatinine clearance and pharmacokinetic parameters. All data were processed and summarized by the use of Statistical Analysis Software (SAS®) version 9.1.3.
Results
Patients
Between January 2003 and October 2004, 20 patients (mean age, 63.1 years; range, 29–85 years) with advanced malignancies were enrolled. Patient demographics are summarized by renal function group in Table 1.
All 20 patients received at least one dose of the study drug. Enrollment included six, five, six and three patients in the normal, mild, moderate and severe renal disease cohorts, respectively. No patients were enrolled in the planned end-stage renal disease cohort. As much as 15 patients received at least one complete cycle, 6 patients received two complete cycles, and 1 patient received three complete cycles. The median [±standard deviation (SD)] cumulative dose was 89.0 mg (range, 35.6–349.4). Disease progression was the primary cause for early termination, including seven (35%) patients before and five (25%) patients after the start of cycle 2. Plasma samples from five patients were stored for a period of time that exceeded the documented stability window of 30 days for AsIII. In addition, plasma samples from one of these patients were in frozen storage for a period of time that exceeded the 71-day stability window for arsenic acid prior to assay. Data collected for these samples have been omitted from all analyses and summaries presented within the current report.
Pharmacokinetic evaluation
Single dose
Following a single intravenous dose of arsenic trioxide, the pharmacokinetic profile of AsIII was similar among the four renal function groups (Table 2). The plasma concentration-versus-time profile of AsIII was characterized by C max being reached immediately after drug administration (2 h), followed by a biphasic decline (Fig. 2a) with an initial rapid distribution followed by elimination with mean t 1/2 ranging from 9.5 to 12.7 h (Table 2). Renal impairment was associated with decreased renal clearance of AsIII, with renal clearance accounting for approximately 3% of total systemic clearance in patients with severe renal impairment compared with 18% in patients with normal renal function (Table 2). The percentage of the total arsenic dose excreted in urine decreased from 42% in the normal renal function group to 24% in the severe renal function group (Table 2). The percentage of the arsenic dose excreted as AsIII also decreased by 50–60% in patients with renal impairment compared with patients with normal renal function (Table 2).
MMAV and DMAV were detected in the plasma at quantifiable levels 8–12 h after the start of the initial infusion of arsenic trioxide and reached maximum values at 16–24 and 24–48 h, respectively. The mean AUC0–t of both MMAV and DMAV were inversely correlated with creatinine clearance (Spearman rank correlation −0.727 and −0.600, respectively) (Table 2). In the mild, moderate and severe renal dysfunction groups, the AUC0–t for MMAV increased by approximately threefold in the mild and moderate groups and sixfold in the severe group, and the AUC0–t for DMAV increased by approximately two to threefold across the renal dysfunction groups. AsV plasma levels were below quantifiable levels.
Multiple doses
Following 3 weeks (total 7 doses) of arsenic trioxide treatment, the plasma concentration-versus-time curve of AsIII (Fig. 2b) was qualitatively similar to that following a single dose (Fig. 2a). However, after multiple doses of arsenic trioxide, the mean AUC0–t of AsIII was 40% higher in the severe renal impairment group compared with the normal renal function group (Table 3), although data were available for only two patients from each of these subgroups. Similar to the single-dose setting, renal clearance was approximately 5% of total systemic clearance in patients with severe renal impairment compared with approximately 16% in patients with normal renal function.
Systemic exposure (as measured by AUC0–t and C max) to all measureable arsenic species (AsIII, MMAV, and DMAV) was increased in all renal function groups following multiple doses, compared with single-dose administration, although it was greatest in patients with severe renal impairment (Tables 2, 3). MMAV and DMAV exposure was increased 1.4- to 8-fold in the mild, moderate and severe renal dysfunction groups as compared with values in subjects with normal renal function. AsV plasma levels were generally below quantifiable levels. AsIII accumulation was not observed with increased renal dysfunction (R obs; Table 3).
Safety evaluation
Adverse events were reported by all patients and were equally distributed across all renal function groups. Overall, the most frequent adverse events were nausea and fatigue, each occurring in 45% of all patients; diarrhea (35%); anorexia and headache (30% each); vomiting, cough and constipation (25% each); and abdominal pain, stomatitis, peripheral edema, dyspnea and rash (20% each).
The most frequent treatment-related adverse events included nausea in six (30%) patients, fatigue in three (15%) patients and vomiting in three (15%) patients (Table 4). Treatment-related adverse events were similar in frequency among normal renal function and mild and moderate renal impairment function groups [normal, 4 (67%); mild, 4 (80%); and moderate, 3 (50%)]. All three (100%) patients in the severe renal function group experienced at least one treatment-related event.
Six patients experienced one or more serious adverse events, including two that were considered to be DLTs (Table 5). One patient with normal renal function experienced sudden cardiac death after having received three doses of arsenic trioxide. No autopsy was performed, but the investigator considered the event to be possibly related in view of the known effects of arsenic trioxide on QT prolongation. The baseline ECG showed sinus tachycardia, and a follow-up ECG 2 days before the patient’s death showed left axis deviation and sinus tachycardia. The QT/QTc interval of 308/417 ms was essentially unchanged from baseline. Another patient with mild renal dysfunction had a dose-limiting adverse event of grade 3 congestive cardiac failure after four doses of arsenic trioxide. This event was preceded by grade 1 ventricular extrasystoles (palpitations) and a nonserious prolonged QT interval considered to be related to the study drug. The patient had a history of cardiomyopathy and congestive cardiac failure during initial chemotherapy with doxorubicin; these conditions resolved after discontinuation of doxorubicin and modification of the patient’s medications.
Only one (33%) patient with severe renal impairment experienced any adverse event above grade 2 (grade 3 fatigue and grade 3 hyperkalemia), and none of the patients with severe renal function suffered any serious adverse events, as defined in the protocol.
In addition to the two patients with DLTs discussed above, two (10%) patients withdrew from the study before starting cycle 2 because of clinical deterioration without meeting the criteria for disease progression. In addition, one patient in the mild renal impairment group with a grade 2 urinary tract infection and one patient in the moderate renal impairment group with grade 2 renal failure discontinued study treatment because of adverse events. None of the three patients with severe renal impairment experienced side effects requiring study drug discontinuation.
Discussion
Arsenic trioxide is used in the induction, consolidation and maintenance of remission in patients with relapsed/refractory APL at a standard dose of 0.15 mg/kg per day for a maximum of 60 consecutive days. Because arsenic is excreted in the urine [13] renal impairment has the potential to alter the pharmacokinetics and safety of arsenic trioxide in cancer patients. Our study was designed to examine the pharmacokinetics of the AsIII, AsV, MMAV and DMAV arsenic species in cancer patients with varying degrees of renal function. To our knowledge, this is the first report of its kind. We report that mild to severe renal impairment results in increased systemic exposure to the methylated arsenic metabolites, but only severe renal impairment results in increased exposure to AsIII. Although renal impairment reduces the urinary excretion of both the total arsenic dose and AsIII, this effect is somewhat mitigated following multiple doses of arsenic trioxide. With impaired renal function, both total arsenic excretion and the percentage of arsenic dose excreted as AsIII were reduced, and systemic exposure to AsIII increased by up to 40% in the severely impaired renal function group compared with the normal renal function group. Despite increased exposure to both methylated arsenic metabolites and inorganic arsenic in patients with renal impairment, our study indicates little correlation between renal function and the occurrence or severity of adverse events. A DLT occurred in 1 of 6 patients in the normal renal function group, 1 of 5 patients in the mild impairment group, 0 of 6 patients in the moderate impairment group, and 0 of 3 patients in the severe renal impairment group. As such, the data in this study do not indicate a clear relationship between the rate of DLTs and the degree of renal impairment or increase in systemic exposure to arsenic or its metabolites as a result of renal impairment.
A study by Fujisawa et al. [20] evaluating the pharmacokinetics of arsenic trioxide given daily to 12 Japanese patients with APL (renal function status not reported), showed that accumulation of the methylated metabolites accounts for most of the increase in total arsenic concentrations following repeated exposure to arsenic trioxide. In this study, inorganic arsenic reached steady-state levels over a 4-week dosing period, whereas MMAV and DMAV continued to rise. Mean total arsenic excretion was 20% following a single dose of arsenic trioxide, but increased to 60% during weeks 1–4. However, renal clearance accounted for only 6–10% of the total clearance of inorganic arsenic, suggesting that hepatic elimination accounts for the majority of systemic clearance of the drug.
Our data are generally consistent with these previously reported observations. Although we observed higher rates of total arsenic excretion (among patients with normal renal function), the trend was similar with about 42% of daily dose excreted after a single dose and 70% of daily dose excreted after multiple doses. Also similarly, we report that inorganic arsenic (AsIII) accounts for 12–14% of the excreted arsenic dose. Although renal excretion plays a minor role in the elimination of inorganic arsenic in patients with normal renal function, in patients with severe renal impairment, even when the administered dose is reduced (i.e., administered twice weekly), systemic exposure to AsIII is increased. Our data suggest that these changes may not significantly affect the safety profile of arsenic trioxide in patients with renal impairment when administered using a twice-weekly schedule. The consequences of the observed increases in systemic exposure to both methylated arsenic metabolites and inorganic arsenic as a result of renal impairment on patient safety with prolonged dosing schedules (i.e., daily for up to 60 days) are unknown. Future clinical studies are needed to examine these relationships in more detail. This could include a study of APL patients treated with arsenic trioxide with curative intent and who have renal dysfunction. This would allow a better estimation of the risks and benefits in this patient population.
In conclusion, definitive recommendations on dosing for each renal dysfunction level cannot be made. The limited toxicity data suggest that dose reductions are not required when arsenic trioxide is administered on a biweekly schedule. However, the PK data indicate caution as well as consideration of dose reduction in the treatment of patients with arsenic trioxide who are potentially at risk for increased toxicity, including patients with renal impairment. A pragmatic recommendation is therefore to weigh the risk and benefit of treatment. With APL, the goal is cure. In this case, one approach could be to dose patients with renal dysfunction using the dose as per the package insert (i.e., 0.15 mg/kg per day) and monitor the patient’s cardiac rhythm via continuous telemetry monitoring. Dose alterations would be instituted if any safety concerns are noted (e.g., QTc interval increases greater than 60 ms above baseline, increases to an absolute value greater than 500 ms, and/or any evidence of ventricular triplets or “R on T” phenomena. Therapy should be held until resolution. If a clinical decision is made to continue therapy for cure, then dose reduction should be considered. Concomitant medications that are known to affect the QT interval should be avoided during treatment with arsenic trioxide. Overall, caution is advised in the use of arsenic trioxide in the treatment of patients with renal impairment, and the risk versus benefit must be carefully assessed.
References
Kwong YL, Todd D (1997) Delicious poison: arsenic trioxide for the treatment of leukemia. Blood 89(9):3487–3488
Soignet SL, Maslak P, Wang ZG et al (1998) Complete remission after treatment of acute promyelocytic leukemia with arsenic trioxide. N Engl J Med 339(19):1341–1348
Soignet SL, Frankel SR, Douer D et al (2001) United States multicenter study of arsenic trioxide in relapsed acute promyelocytic leukemia. J Clin Oncol 19(18):3852–3860
Shen Y, Shen ZX, Yan H et al (2001) Studies on the clinical efficacy and pharmacokinetics of low-dose arsenic trioxide in the treatment of relapsed acute promyelocytic leukemia: a comparison with conventional dosage. Leukemia 15(5):735–741
Mathews V, Balasubramanian P, Shaji RV, George B, Chandy M, Srivastava A (2002) Arsenic trioxide in the treatment of newly diagnosed acute promyelocytic leukemia: a single center experience. Am J Hematol 70(4):292–299
Mathews V, George B, Lakshmi KM et al (2006) Single-agent arsenic trioxide in the treatment of newly diagnosed acute promyelocytic leukemia: durable remissions with minimal toxicity. Blood 107(7):2627–2632
Munshi NC, Tricot G, Desikan R et al (2002) Clinical activity of arsenic trioxide for the treatment of multiple myeloma. Leukemia 16(9):1835–1837
Vey N, Bosly A, Guerci A et al (2006) Arsenic trioxide in patients with myelodysplastic syndromes: a phase II multicenter study. J Clin Oncol 24(16):2465–2471
Barbey JT, Pezzullo JC, Soignet SL (2003) Effect of arsenic trioxide on QT interval in patients with advanced malignancies. J Clin Oncol 21(19):3609–3615
Mathews V, Desire S, George B et al (2006) Hepatotoxicity profile of single-agent arsenic trioxide in the treatment of newly diagnosed acute promyelocytic leukemia, its impact on clinical outcome and the effect of genetic polymorphisms on the incidence of hepatotoxicity. Leukemia 20(5):881–883
Vahter M (1999) Methylation of inorganic arsenic in different mammalian species and population groups. Sci Prog 82(Pt 1):69–88
Vahter M (2002) Mechanisms of arsenic biotransformation. Toxicology 181–182:211–217
Aposhian HV, Aposhian MM (2006) Arsenic toxicology: five questions. Chem Res Toxicol 19(1):1–15
Chen GQ, Zhou L, Styblo M et al (2003) Methylated metabolites of arsenic trioxide are more potent than arsenic trioxide as apoptotic but not differentiation inducers in leukemia and lymphoma cells. Cancer Res 63(8):1853–1859
Styblo M, Del Razo LM, Vega L et al (2000) Comparative toxicity of trivalent and pentavalent inorganic and methylated arsenicals in rat and human cells. Arch Toxicol 74(6):289–299
Styblo M, Drobna Z, Jaspers I, Lin S, Thomas DJ (2002) The role of biomethylation in toxicity and carcinogenicity of arsenic: a research update. Environ Health Perspect 110(Suppl 5):767–771
Petrick JS, Jagadish B, Mash EA, Aposhian HV (2001) Monomethylarsonous acid (MMA(III)) and arsenite: LD(50) in hamsters and in vitro inhibition of pyruvate dehydrogenase. Chem Res Toxicol 14(6):651–656
US Food and Drug Administration (1998) Guidance for industry:pharmacokinetics in patients with impaired renal function-study design, data analysis, and impact on dosing and labeling. US Department of Health and Human Services, US Food and Drug Administration, Rockville
European Medicines Agency (2004) Note for guidance on the evaluation of the pharmacokinetics of medicinal products in patients with impaired renal function. European Medicines Agency, London
Fujisawa S, Ohno R, Shigeno K et al (2007) Pharmacokinetics of arsenic species in Japanese patients with relapsed or refractory acute promyelocytic leukemia treated with arsenic trioxide. Cancer Chemother Pharmacol 59(4):485–493
Acknowledgments
The authors gratefully acknowledge the editorial support provided by Bridget O’Keeffe, PhD, and Helix Medical Communications LLC. This study was funded by Cephalon, Inc., Frazer, Pennsylvania with additional support from NIH Grant No. M01 RR-000080 (SR) and NIH Grant GCRC M01RR00750 (CS).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sweeney, C.J., Takimoto, C., Wood, L. et al. A pharmacokinetic and safety study of intravenous arsenic trioxide in adult cancer patients with renal impairment. Cancer Chemother Pharmacol 66, 345–356 (2010). https://doi.org/10.1007/s00280-009-1169-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-009-1169-4