Abstract
Purpose
Temozolomide pharmacokinetics were evaluated in children receiving concurrent O6-benzylguanine (O6BG), which enhanced the hematological toxicity of temozolomide.
Methods
Temozolomide was administered orally, daily for 5 days starting at 28 mg/m2 per day with escalations to 40, 55, 75 and 100 mg/m2 per day with O6BG intravenously daily for 5 days at doses of 60, 90 or 120 mg/m2 per day. Plasma samples were drawn over 48 h after the day 5 dose. Temozolomide was quantified with a validated HPLC/tandem mass spectroscopic assay.
Results
Temozolomide was rapidly absorbed (mean T max, 2.1 h). The mean apparent clearance (CL/F) (96 mL/min/m2) was similar to the CL/F for temozolomide alone and was not age- or gender-dependent. There was minimal inter-patient variability.
Conclusions
The enhanced hematologic toxicity resulting from combining O6BG with temozolomide does not appear to be the result of a pharmacokinetic interaction between the agents.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Temozolomide is a prodrug that undergoes spontaneous decomposition in solution at physiological pH to the reactive intermediate 5-(3-methyl-1-triazeno)imidazole-4-carboxamide (MTIC). MTIC is an alkylating agent that methylates the N7 and O6 positions of guanine, as well as the N3 position of adenine. These methyl adducts result in a continuous cycle of DNA base mismatch and repair with eventual strand breaks and cellular apoptosis [3, 4, 10, 14]. Methylation of the O6 position of guanine is thought to be the most cytotoxic adduct [2, 15].
Resistance to temozolomide is mediated through the DNA repair protein, O6-alkylguanine-DNA alkyltransferase (AGT), which removes the methyl adduct from the O6 position of guanine. O6-benzylguanine (O6BG) is a modulating agent that inactivates and depletes AGT by serving as substrate and transferring its benzyl group to the active site of AGT. In preclinical studies, the cytotoxicity of alkylating agents was enhanced when administered in combination with O6BG, and there was a direct correlation between intracellular AGT levels and the degree of enhanced activity [1, 5, 6, 18, 19].
We conducted a pediatric phase I trial of oral temozolomide in combination with intravenous O6BG administered once daily for 5 days and repeated every 28 days in children with refractory solid tumors to determine the maximum tolerated temozolomide dose when given with an O6BG dose that was known to deplete AGT [17]. The O6BG dose was initially escalated over 3 dose levels (60, 90, 120 mg/m2 per day) in combination with the starting dose of temozolomide (28 mg/m2 per day). The temozolomide dose was escalated to 40, 55, 75 and 100 mg/m2 per day in combination with 120 mg/m2 per day of O6BG. The maximum tolerated dose (MTD) of temozolomide was 75 mg/m2 per day.
Prior pharmacokinetic studies of temozolomide alone in children [7, 8, 11, 12] included 97 patients who received oral doses ranging 60–240 mg/m2 once daily for 5 days, repeated every 21–28 days. Temozolomide was rapidly absorbed with a mean ± SD time to maximum concentration (T max) of 1.3 ± 0.4 h. The mean terminal half-life (t 1/2) was 1.7 ± 0.2 h, and the apparent clearance (Cl/F) was 91 ± 15 mL/min/m2. A linear relationship was observed between dose and maximum temozolomide concentration (C max) and area under the concentration–time curve (AUC).
We studied the pharmacokinetics of temozolomide over a dosage range of 28–100 mg/m2 in a subset of patients enrolled on our phase I pediatric trial of temozolomide in combination with O6BG using a sensitive high performance liquid chromatography/tandem mass spectrometry (HPLC/MS/MS) assay.
Materials and methods
Phase I trial design
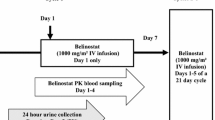
The trial design, patient eligibility criteria, treatment regimen, monitoring parameters and definitions of MTD and dose limiting toxicity (DLT) for the pediatric phase I trial of temozolomide and O6BG have been previously reported [17]. Patients ≤21 years of age with relapsed or refractory solid tumors including brain tumors received O6BG (Pharmaceutical Management Branch of the Cancer Therapy Evaluation Program, NCI, Bethesda, MD, USA), administered intravenously (IV) over 1 h followed by oral temozolomide (Schering-Plough, Kenilworth, NJ, USA) administered in capsule form 30 min after the end of the O6BG infusion once daily for 5 days, repeated every 28 days. A two-part dose-escalation scheme was utilized to define the MTD of temozolomide administered with a biologically active dose of O6BG. The dose levels are presented in Table 1.
Sample handling
Pharmacokinetic samples were collected on day 5 of cycle 1. Blood was drawn into heparinized tubes immediately prior to the dose of temozolomide then 15 and 30 min and 1, 2, 3.5, 5.5, 7.5, 9.5, 24 (×2 samples) and 48 (×2 samples) h after the dose. Five mL blood samples were placed immediately into ice, and plasma was separated by centrifugation in a pre-chilled centrifuge. Two mL of plasma was transferred to a pre-chilled tube containing 0.1 mL of 8.5% phosphoric acid to maintain stability. Acidified samples were vortexed and stored at −70°C until analysis. Sample handling and processing were based on published methods with particular attention paid to timely cooling, acidification and freezing of patient samples, all of which have been shown to impact temozolomide stability [9, 13].
Temozolomide assay
After thawing, temozolomide was extracted from plasma samples using a small volume liquid/liquid extraction with ethyl acetate. In a 1.5 mL polypropylene eppendorf tube, 1,000 mcL of ethyl acetate was added to 100 mcL of plasma. Samples were vortexed for 30 s, placed on a shaker for 5 min then centrifuged at 10,000 rpm for 5 min. The top organic layer was removed and placed in a borosilicate glass tube (12 × 75 mm). A second extraction was performed with the remaining aqueous layer in the same manner described above to maximize recovery. The organic layer was evaporated to dryness under a stream of nitrogen at a room temperature in a water bath. The dried sample was reconstituted in 100 mcL of ethyl acetate, transferred to an eppendorf tube and centrifuged at 10,000 rpm for 5 min. The supernatant was transferred to an injection vial for same day analysis by HPLC/MS/MS. The mean recovery of temozolomide from the extraction procedure was 83%. Patient sample set extraction and analysis were performed in duplicate or triplicate.
Temozolomide was quantified with an Applied Biosystems MDS Sciex API 5000 triple quadrupole mass spectrometer (Foster City, CA, USA). The HPLC system included Shimadzu LC-20AD pumps and Shimadzu SIL 20AC autosampler and column heater (Columbia, MD, USA). Mass spectrometer conditions for this assay were optimized in the positive electrospray ionization, multiple reaction monitoring mode measuring the transition of temozolomide parent to daughter ion, 193.3–138.2 amu. The collision gas was set at 6 psi, curtain gas 10 psi and ion source gas 1 at 30 psi. Ion spray voltage was 5,000 V, declustering potential 51 V, entrance potential 13 V and collision energy 13 V. Ion source temperature was 500°C, autosampler temperature set to 4°C and the column chamber to 40°C. Chromatographic separation was optimal with a Shimadzu Premier C18, 5 micron, 50 × 4.6 mm internal diameter column (Columbia, MD, USA) and injection volume of 20 mcL. The mobile phase consisted of 0.5% acetic acid and methanol (80:20) at a flow rate of 0.15 mL/min. Temozolomide retention time was 5.37 ± 0.04 min and total run time was 7 min with a 3 min delay between samples. Analyst® software 1.4.2 was used for data analysis.
Validation of the HPLC/MS/MS assay for temozolomide quantification was conducted according to the FDA Bioanalytical Method Validation guidelines [16]. Temozolomide (Pharmaceutical Management Branch of the Cancer Therapy Evaluation Program, DCTD, Bethesda, MD, USA) calibration curves, which were prepared from a 1 mM ethyl acetate stock solution stored at −20°C, covered a concentration range from 0.2 to 194 ng/mL (0.001–1 mcM) and were used for the assay validation. Plasma temozolomide standard curves and quality control samples were prepared by adding 100 mcL of ethyl acetate (blank) or 100 mcL of temozolomide at 10, 19 and 194 ng/mL (0.05, 0.1 and 1 mcM) in ethyl acetate to 100 mcL of pooled plasma prior to undergoing extraction. Temozolomide was not shown to co-elute with other compounds. The lower limit of quantification for aqueous standards was 1 ng/mL (0.005 mcM). Extracted samples maintained at 4°C for >24 h were stable with mean difference of 6.9% between values obtained prior to and following storage. Intra-day precision ranged 4.1–16% and accuracy ranged 11.8–21.7% with the lower limit of quantification of temozolomide in plasma of 2 ng/mL (0.01 mcM). The inter-day coefficient of variation was <20%. Patient samples were assayed in duplicate or triplicate, and the concentration was the average value from these measurements.
Pharmacokinetic analysis
The temozolomide plasma concentration–time data were analyzed using non-compartmental methods. The peak temozolomide concentration (C max) and time to peak concentration (T max) were determined from a concentration–time plot of each patient’s data. Area under the concentration curve to the last measured time point (AUC0–last) was calculated with the linear trapezoidal method and extrapolated to infinity (AUC0–∞) by adding the final measured plasma concentration divided by the terminal rate constant derived from the slope of the natural log-transformed concentrations and times on the terminal elimination phase of the decay curve. Terminal half-life (t 1/2) was calculated by dividing 0.693 by the terminal rate constant. Apparent clearance (Cl/F) was calculated by dividing the temozolomide dose by the AUC0–∞.
Results
Patient Characteristics
Of the 32 patients who were evaluable for toxicity on the phase I trial, [17] 21 patients had complete or near complete temozolomide pharmacokinetic sample sets and were selected for analysis (Tables 1, 2). Characteristics of this cohort of patients are described in Table 2.
Pharmacokinetic analysis
The mean plasma concentration–time profile of temozolomide for the four patients studied at the 55 mg/m2 dose level is shown in Fig. 1. The drug was rapidly absorbed (T max ≤2 h in 15/21 patients), but the T max, which reflects the absorption rate, appeared to be dose- ependent. The median T max at the three lower dose levels (28, 40, and 55 mg/m2) was 1 h, but at the two highest dose levels (75 and 100 mg/m2) the median T max was 3.5 h (Table 3). The disappearance of temozolomide from plasma was monoexponential with a mean half-life of 2 h. There was no accumulation of temozolomide over the 5 days of dosing interval as evidenced by the pre-dose and 24 h post-dose day 5 plasma concentrations, which were below the 2 ng/mL lower limit of quantification in most patients.
Temozolomide plasma pharmacokinetic parameters for the 21 patient sample sets are presented in Table 3. Temozolomide plasma concentrations 24 and 48 h after the fifth dose were below the 2 ng/mL lower limit of quantification for the assay in a majority of the patients; therefore, these time points were not included in the calculation of pharmacokinetic parameters. Inter-patient variability for the pharmacokinetic parameters was minimal. The coefficient of variation for the AUC0–∞ across the 5 dose levels ranged from 9.8 to 22% (median, 20%).
The relationship between temozolomide dose and AUC0–∞ is shown in Fig. 2. The AUC0–∞ in the four patients treated at the 100 mg/m2 per day dose level falls below the line of proportionality, suggesting that absorption may be saturable at this dose level. The C max also failed to increase in proportion to the dose at the higher dose levels (Table 3), but this is explained in part by the more prolonged absorptive phase at the 75 and 100 mg/m2 per day dose levels.
Relationship between the administered dose per m2 of body surface area and the AUC0–∞ of temozolomide. The line of proportionality was generated by normalizing all of the AUCs to a dose of 100 mg/m2, taking the mean of the normalized AUCs, and plotting a line from 0,0 through 100, mean normalized AUC
The mean (±SD) apparent clearance (CL/F) of temozolomide across the five dose levels was 96 ± 26 mL/min/m2 (Table 3). The apparent clearance was higher at the 75 and 100 mg/m2 per day dose levels (mean, 113 mL/min/m2) compared to the clearance in patients treated at the three lower dose levels (mean, 85 mL/min/m2) (P < 0.01). Apparent clearance was age independent (Fig. 3) and was similar (P = NS) in males (mean, 102 mL/min/m2) and females (mean, 85 mL/min/m2).
Pharmacodynamic correlation
The dose and AUC0–∞ of temozolomide were not predictive of the percent decrease in the neutrophil count from baseline to nadir (Fig. 4). Severe neutropenia (ANC < 500/mcL, closed symbols in Fig. 4) occurred at all dose levels and across the range of AUC0–∞s, although severe neutropenia was more common at the higher doses and AUC0–∞s.
Relationship between the administered dose normalized to body surface area (a) and the AUC0–∞ (b) and the percent decrease in the absolute neutrophil count (ANC) on cycle 1 of therapy. Closed symbols represent patients who had a nadir ANC <500/mcL and open symbols represent patients who had a nadir ANC >500/mcL
Discussion
We studied the pharmacokinetics of temozolomide in patients who received concurrent O6BG, which was administered to modulate AGT-mediated resistance to temozolomide. The tolerable dose of temozolomide (75 mg/m2 per day) administered in combination with 120 mg/m2 per day of O6BG on this phase I trial was less than half of the standard 200 mg/m2 per day single agent dose due to dose limiting neutropenia [17]. The enhanced hematological toxicity of temozolomide does not appear to be the result of a pharmacokinetic interaction. The apparent clearance of temozolomide in our patients (96 mL/min/m2) is comparable to the apparent clearance in children on prior single studies (91 mL/min/m2) [8, 11, 12].
This study evaluated a lower dosage range (28–100 mg/m2 per day) of temozolomide than prior studies. Drug concentration was quantifiable in plasma for 9.5 h after the dose with the sensitive LC/MS/MS assay, but by 24 h post-dose (12 half-lives), drug could not be consistently measured. The time points out to 9.5 h post-dose accounted for most of the drug exposure from an oral dose of temozolomide as evidenced by the fact that the extrapolated portion of the AUC (AUClast–∞) accounted for only a median of 9% of the total AUC0–∞.
The relationship between dose and AUC0–∞ appeared to be non-linear at the 100 mg/m2 per day dose level (Fig. 2), possibly from saturation of absorption. This is supported by the more prolonged absorptive phase at the two highest dose levels. However, saturation of absorption is not supported by data from other pharmacokinetic studies evaluating the standard 200 mg/m2 per day dose level. Panetta et al. [11] reported an AUC0–∞ for temozolomide in children of 37,700 ng h/mL at the 200 mg/m2 dose. This value is exactly twofold higher than the mean of the AUC0–∞ normalized to a dose of 100 mg/m2 (18,600 ng h/mL) for the 21 patients on this study. Therefore, the absorption and clearance of temozolomide appears to be linear and proportional to the dose over the dosage range of 28–200 mg/m2 per day.
The limited inter-patient variability in the AUC0–∞ and the half-life are a reflection, in part, of the mechanism of drug elimination, which occurs primarily by spontaneous chemical decomposition to MTIC in solution at physiological pH. This is a first order chemical reaction that is not dependent on drug metabolizing enzymes and accounts for the monoexponential disappearance of the drug from plasma. This chemical reaction will continue to occur in blood samples ex vivo unless samples are rapidly cooled and the plasma is acidified [9, 13]. These steps are critical to stabilizing temozolomide in plasma samples and to ensure accurate measurement of temozolomide.
Although severe neutropenia was more frequent at the highest dose levels on this phase I trial, it also occurred in patients treated at lower dose levels (28 and 55 mg/m2 per day) in combination with O6BG. We evaluated the relationship between temozolomide dose and drug exposure (AUC) and the degree of neutropenia. Drug exposure was not more predictive of the severity of neutropenia than the temozolomide dose, as has been reported by others [12]. The absence of a relationship between temozolomide AUC0–∞ and severity of neutropenia in our study may be related to variable sensitization of bone marrow stem cells from inhibition of DNA repair by O6BG.
References
Baer JC, Freeman AA, Newlands ES, Watson AJ, Rafferty JA, Margison GP (1993) Depletion of O6-alkylguanine-DNA alkyltransferase correlates with potentiation of temozolomide and CCNU toxicity in human tumour cells. Br J Cancer 67:1299–1302
Bull VL, Tisdale MJ (1987) Antitumour imidazotetrazines-XVI. Macromolecular alkylation by 3-substituted imidazotetrazinones. Biochem Pharmacol 36:3215–3220
D’Atri S, Tentori L, Lacal PM, Graziani G, Pagani E, Benincasa E, Zambruno G, Bonmassar E, Jiricny J (1998) Involvement of the mismatch repair system in temozolomide-induced apoptosis. Mol Pharmacol 54:334–341
Danson SJ, Middleton MR (2001) Temozolomide: a novel oral alkylating agent. Expert Rev Anticancer Ther 1:13–19
Dolan ME, Mitchell RB, Mummert C, Moschel RC, Pegg AE (1991) Effect of O6-benzylguanine analogues on sensitivity of human tumor cells to the cytotoxic effects of alkylating agents. Cancer Res 51:3367–3372
Dolan ME (1997) Inhibition of DNA repair as a means of increasing the antitumor activity of DNA reactive agents. Adv Drug Deliv Rev 26:105–118
Estlin EJ, Lashford L, Ablett S, Price L, Gowling R, Gholkar A, Kohler J, Lewis IJ, Morland B, Pinkerton CR, Stevens MCG, Mott M, Stevens R, Newell DR, Walker D, Dicks-Mireaux C, McDowell H, Reidenberg P, Statkevich P, Marco A, Batra V, Dugan M, Pearson ADJ (1998) Phase I study of temozolomide in pediatric patients with advanced cancer. Br J Cancer 78:652–661
Horton TM, Thompson PA, Berg SL, Adamson PC, Ingle AM, Dolan ME, Delaney SM, Hedge M, Weiss HL, Wu MF, Blaney SM (2007) Phase I pharmacokinetic and pharmacodynamic study of temozolomide in pediatric patients with refractory or recurrent leukemia: a Children’s Oncology Group Study. J Clin Oncol 25:4922–4928
Kim H, Likhari P, Parker D, Statkevich P, Marco A, Lin CC, Nomeir AA (2001) High-performance liquid chromatographic analysis and stability of anti-tumor agent temozolomide in human plasma. J Pharm Biomed Anal 24:461–468
Newlands ES, Stevens MF, Wedge SR, Wheelhouse RT, Brock C (1997) Temozolomide: a review of its discovery, chemical properties, pre-clinical development and clinical trials. Cancer Treat Rev 23:35–61
Panetta JC, Kirstein MN, Gajjar A, Nair G, Fouladi M, Heideman RL, Wilkinson M, Stewart CF (2003) Population pharmacokinetics of temozolomide and metabolites in infants and children with primary central nervous system tumors. Cancer Chemother Pharmacol 52:435–441
Riccardi A, Mazzarella G, Cefalo G, Garre ML, Massimino M, Barone C, Sandri A, Ridola V, Ruggiero A, Mastrangelo S, Lazzareschi I, Caldarelli M, Maira G, Madon E, Riccardi R (2003) Pharmacokinetics of temozolomide given three times a day in pediatric and adult patients. Cancer Chemother Pharmacol 52:459–464
Shen F, Decosterd LA, Gander M, Leyvraz S, Biollax J, Lejeune F (1995) Determination of temozolomide in human plasma and urine by high-performance liquid chromatography after solid-phase extraction. J Chromatogr B Biomed Appl 667:291–300
Stevens MF, Hickman JA, Langdon SP, Chubb D, Vickers L, Stone R, Baig G, Goddard C, Gibson NW, Slack JA, Newton C, Lunt E, Fizames C, Lavelle F (1987) Antitumor activity and pharmacokinetics in mice of 8-carbamoyl-3-methyl-imidazo[5, 1-d]-1, 2, 3, 5-tetrazin-4(3H)-one (CCRG 81045; M & B 39831), a novel drug with potential as an alternative to dacarbazine. Cancer Res 47:5846–5852
Tisdale MJ (1987) ntitumor imidazotetrazines-XV. Role of guanine O6 alkylation in the mechanism of cytotoxicity of imidazotetrazinones. Biochem Pharmacol 36:457–462
US Department of Health and Human Services FaDA (2001) Guidance for Industry, Bioanalytical Method Validation
Warren KE, Aikin AA, Libucha M, Widemann BC, Fox E, Packer RJ, Balis FM (2005) Phase I study of O6-benzylguanine and temozolomide administered daily for 5 days to pediatric patients with solid tumors. J Clin Oncol 23:7646–7653
Wedge SR, Newlands ES (1996) O6-benzylguanine enhances the sensitivity of a glioma xenograft with low O6-alkylguanine-DNA alkyltransferase activity to temozolomide and BCNU. Br J Cancer 73:1049–1052
Wedge SR, Porteous JK, Newlands ES (1996) 3-aminobenzamide and/or O6-benzylguanine evaluated as an adjuvant to temozolomide or BCNU treatment in cell lines of variable mismatch repair status and O6-alkylguanine-DNA alkyltransferase activity. Br J Cancer 74:1030–1036
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Meany, H.J., Warren, K.E., Fox, E. et al. Pharmacokinetics of temozolomide administered in combination with O6-benzylguanine in children and adolescents with refractory solid tumors. Cancer Chemother Pharmacol 65, 137–142 (2009). https://doi.org/10.1007/s00280-009-1015-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-009-1015-8