Abstract
Purpose
To characterize and compare pharmacokinetic parameters in children and adults treated with temozolomide (TMZ) administered for 5 days in three doses daily, and to evaluate the possible relationship between AUC values and hematologic toxicity.
Methods
TMZ pharmacokinetic parameters were characterized in pediatric and adult patients with primary central nervous system tumors treated with doses ranging from 120 to 200 mg/m2 per day, divided into three doses daily for 5 days. Plasma levels were measured over 8 h following oral administration in a fasting state. A total of 40 courses were studied in 22 children (mean age 10 years, range 3–16 years) and in 8 adults (mean age 30 years, range 19–54 years).
Results
In all patients, a linear relationship was found between systemic exposure (AUC) and increasing doses of TMZ. Time to peak concentration, elimination half-life, apparent clearance and volume of distribution were not related to TMZ dose. No differences were seen among TMZ Cmax, t1/2, Vd or CL/F in children compared with adults. Intra- and interpatient variability of systemic exposure were limited in both children and adults. No statistically significant differences were found between the AUCs of children who experienced grade 4 hematologic toxicity and children who did not.
Conclusions
No difference appears to exist between pharmacokinetic parameters in adults and children when TMZ is administered in three doses daily. Hematologic toxicity was not related to TMZ AUC. AUC measurement does not appear to be of any use in optimizing TMZ treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Temozolomide (TMZ) is a DNA-methylating agent of the imidazotetrazine class that has demonstrated antitumor activity and a relatively safe toxicity profile in phase I and II trials in adult patients with melanoma and brain tumors [2, 4, 7, 9, 13]. TMZ is rapidly adsorbed after oral administration and is spontaneously converted at physiologic pH into its active metabolite 5-(3-methyltriazen-1-y1)imidazole-4-carboxamide (MTIC). Conversion of TMZ into MTIC occurs at a constant rate and MTIC parallels the TMZ disappearance curve, accounting for 2.5% of TMZ plasma levels [17]. MTIC acts by alkylation of DNA at both the N7 position and the O6 position of guanine, generating O6-methylguanine DNA adducts [10]. In the presence of O6-methylguanidine the cellular repair enzyme O6-alkylguanine DNA alkyltransferase (AGT) removes the methyl groups, irreversibly inactivating itself. It has been shown that the depletion of AGT enhances toxicity of TMZ in human tumor cell lines.
The antitumor activity of TMZ has been shown to be strongly schedule-dependent [21] and a higher response rate compared with a single-day dose [13] has been found when the total dose is administered over 5 days [2, 3, 13, 15]. The recommended dose for phase II studies in previously untreated adult patients is 200 mg/m2 per day for 5 days every 28 days. In pretreated adult patients, to prevent rare but severe thrombocytopenia, the first dose is usually given at 150 mg/m2 per day for 5 days, with escalation of the second dose to 200 mg/m2 per day for 5 days.
TMZ's good brain penetration made this drug particularly attractive for the treatment of central nervous system (CNS) tumors. For this reason, a large number of patients with CNS tumors, mainly high-grade gliomas, were enrolled in early clinical trials. TMZ demonstrated efficacy mainly in both recurrent anaplastic astrocytoma and glioblastoma multiforme [9]. These studies prompted the evaluation of TMZ in pediatric patients with brain tumors. A study by the Children Cancer Group [14] defined the maximum tolerated dose (MTD) of TMZ for children without prior craniospinal irradiation (CSI) as 215 mg/m2 and for those with prior CSI as 180 mg/m2 daily for 5 days every 28 days. Estlin et al. [8] have indicated 200 mg/m2 once a day for five consecutive days as the recommended dose for phase II studies in pediatric patients who had not received prior CSI or nitrosourea therapy. Estlin et al., in 19 children, found a lower clearance of TMZ that resulted in an increase of approximately 40% in systemic exposure compared with reported adult values.
Given the short plasma half-life of TMZ, once-daily administration could allow the restoration of the DNA repair protein AGT, thereby reducing TMZ antitumor activity [20]. For this reason we designed a multicenter phase II study in children in which the daily dose was divided into three doses daily for five consecutive days repeated every 28 days. Eight adults with brain tumors were treated with TMZ on the same schedule. The aim of the study was to characterize the pharmacokinetics of TMZ given in three doses daily, to compare pharmacokinetic parameters in children and adults and to evaluate possible relationships between AUC and hematologic toxicity.
Patients and methods
Patients
The patient characteristics are summarized in Table 1. All the patients, except two with a radiologic diagnosis of an intrinsic brain stem glioma, had histologically proven malignancy. All patients had normal renal and hepatic function. Hematologic values were normal in all but six heavily pretreated patients. These patients, with baseline values below the normal range, received a lower initial dosage. Written informed consent to participate in the study was obtained from patients, their parents, or both.
Treatment and toxicity evaluation
TMZ was purchased from Schering-Plough (Milan, Italy) as either 20-mg or 100-mg hard gelatin capsules. The drug was administered in the morning after an overnight fast; patients were not allowed to eat for at least 2 h after TMZ treatment. Patients who had received prior CSI or who were heavily pretreated (second- and third-line treatment including high-dose chemotherapy with peripheral blood stem cell rescue) received a starting dose of 180 mg/m2 per day. The initial dose was further reduced in six patients with persistently low platelet counts (two treatments at 120 mg/m2 per day; one treatment at 135 mg/m2 per day; three treatments at 150 mg/m2 per day). Previously untreated patients received 200 mg/m2 per day. The daily dose was divided into three doses daily, one dose every 8 h, for five consecutive days. Cycles were repeated at 28-day intervals. The calculated doses of TMZ were rounded up to the nearest 20 mg to accommodate the capsule sizes. Antiemetics (usually ondansetron) were allowed on a prophylactic basis.
Complete blood counts and serum biochemistry tests were performed weekly, or more frequently if indicated. Hematologic toxicities were graded using the WHO scoring system. When significant myelosuppression was observed, the dosage in the subsequent course was reduced by 10%.
Plasma sampling and assay
Samples were obtained from all patients at the time of the first dose of the first cycle of treatment. In ten patients, a further pharmacokinetic analysis was performed at the time of the first dose of the second cycle. Blood samples (5 ml) were collected into prechilled heparinized tubes prior to TMZ administration and at 0.5, 1, 1.5, 2, 3, 4, 6 and 8 h after administration. Specimens were immediately separated by centrifugation for 10 min at 4°C and the plasma acidified with 1 M HCl (100 μl/ml), separated into two equal portions and stored at −20°C. Plasma TMZ concentrations were determined by a high-performance liquid chromatography (HPLC) assay according to the method of Shen et al. [19], with a slightly modified extraction procedure. Briefly, plasma samples were mixed with ethazolastone solution (IS) kindly donated by Dr. D'Incalci and extracted on Oasis HLB extraction cartridges (Waters). Matrix components were eliminated with 750 μl 0.5% acetic acid. Samples were then eluted with 1 ml methanol, completely evaporated under nitrogen at room temperature, reconstituted in 200 μl 0.5% acetic acid and centrifuged for 5 min. The recovery of TMZ with this modified extraction procedure was 86–90%. A volume of 30 μl of supernatant was injected into the HPLC system. The samples were analyzed using a Waters HPLC system (510 HPLC pump, 996 photodiode array detector and 717plus autosampler). Chromatographic separation was achieved using a reversed-phase HP ODS-Hypersil 5-μm column (100×4.6 mm ID) equipped with a guard column. The mobile phase was methanol/0.5% acetic acid (10:90, v/v). The limit of quantitation of the method (0.1 μg/ml) was sensitive enough to detect the 8-h plasma concentration.
Pharmacokinetic analysis
Pharmacokinetic parameters refer to the analysis of plasma levels over 8 h following administration of approximately one-third of the daily dose of TMZ. The maximum plasma concentration (Cmax) and time to maximum plasma concentration (Tmax) were derived from the plasma concentration-time curves. The terminal phase rate constant (k) was calculated as the negative of the slope of the log-linear terminal portion of the plasma concentration-time curve using linear regression. The area under the concentration-time curve (AUC) was calculated using the linear trapezoidal method from the start of treatment to the 8-h plasma concentration. The elimination half-life was calculated as 0.693 divided by the elimination phase-rate constant (k). The apparent clearance (CL/F), normalized for surface area, was calculated as the dose per meter squared divided by AUC0–8 h (F, fraction of oral dose absorbed, assumed to be 1.0). The apparent volume of distribution (Vd) was obtained by dividing CL/F by k. The dose administered in the 8-h fraction ranged from 60 to 80 mg/m2, depending on the patient's stratification in 180 and 200 mg/m2 per day doses, on subsequent dose reduction for toxicity and on accommodation to capsule size.
Statistical analysis
Statistical analyses were performed using non-parametric tests. The degree of correlation between dose and AUC was determined by the Kruskal-Wallis (ANOVA by ranks) test, supplemented with post-hoc comparison by the Mann-Whitney U-test. P<0.05 was considered as the significant probability level. Statistical analysis was performed using Statistica software for Windows (StatSoft, 1997).
Results
Pharmacokinetics
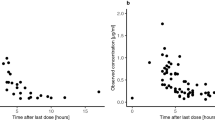
Relevant pharmacokinetic parameters as a function of dose and patient age are summarized as means±SD in Table 2. Maximum plasma concentrations were achieved approximately 1 h after drug administration and ranged from 1.53 to 7.22 μg/ml. No statistically significant increase was observed in Cmax as a function of dose (r 2=0.15, P>0.05). The values for TMZ Tmax, elimination half-life, apparent clearance and apparent volume of distribution were dose-independent. Figure 1 shows the trend of clearance in relation to age of the patient population. No difference between children and adult clearance was noted. The CL/F range was 77–148 ml/min per m2 in children and 85–117 ml/min per m2 in adults, with a coefficient of variation of 19% and 9%, respectively. The apparent volume of distribution ranged from 7.3 to 25.5 l/m2 in children and 12.5–18.7 l/m2 in adults. Interindividual variability for systemic exposure was limited, with a coefficient of variation in the range 7–24% in children and 7–13% in adults at different dose levels. Figure 2 shows the relationship between administered dose and AUC in children. A significant linear correlation was found between increasing doses of TMZ and AUC (P<0.01). The relatively low r 2 value (0.33) is related to the limited dose interval analyzed: 60 to 80 mg/m2. In eight children and two adults from whom samples were available for pharmacokinetic analysis during the second cycle of treatment, the AUC was similar to that found in the first cycle, with an intrapatient coefficient of variation of 13%. Taking into account all the pharmacokinetic parameters examined, no statistically significant differences between the pediatric and the adult group were found.
Toxicity
In the pediatric group one course was not evaluated for toxicity because of the death of the patient. Myelosuppression occurred after TMZ administration with a mean nadir value on day 25 for thrombocytopenia and on day 22 for neutropenia. Hematologic toxicities according to the WHO grading system are shown in Table 3. In children, grade 3/4 thrombocytopenia was observed in 9 out of 28 cycles (32%), and grade 3/4 neutropenia in 5 out of 28 cycles (18%). In adults, grade 3/4 thrombocytopenia was observed in 4 out of 11 cycles (36%). No adult patient experienced grade 3 or 4 neutropenia.
In order to evaluate the degree of correlation between TMZ plasma AUC and hematologic toxicity, we compared the AUC values obtained from pediatric patients who experienced grade 4 hematologic toxicities and those who did not (Fig. 3). No statistically significant differences were found between the two groups, indicating no relationship between AUC and hematologic toxicity under the experimental conditions utilized.
Discussion
Treatment of high-grade gliomas represents a real challenge to neuro-oncologists, mainly because of the high recurrence rate of these tumors even after radical excision, which is possible only in a limited number of patients. Currently available chemotherapy (nitrosoureas, procarbazine and vincristine or the PCV protocol) is now at least 25 years old and even when combined with radiotherapy has not changed the fatal prognosis for patients with high-grade glioma, and the progression-free survival in patients with glioblastoma multiforme has not greatly improved. Therefore, the initial reports of a substantial antitumor activity of TMZ in brain tumors demonstrated by Newlands et al. [13] prompted a number of clinical studies both in children and adults. The most recent reports indicate that TMZ is active in relapsed oligodendroglioma and has a definite, although modest, activity in high-grade glioma both at relapse and in newly diagnosed patients [22]. In addition, we have recently shown that TMZ induces a high response rate in patients with relapsed medulloblastoma [18]. The treatment with TMZ was well tolerated, with less than 10% of patients experiencing grade 3 or 4 hematologic toxicity. In this small subset of patients severe hematologic toxicity was apparently unrelated to clinical characteristics and to other factors.
The schedule of administration appears to be crucial to determining the clinical effectiveness of TMZ and the schedule of daily TMZ for five consecutive days per 28-day cycle is the most widely adopted mode of administration, although different schedules have been suggested. TMZ schedule-dependence has been demonstrated both in preclinical models and in the original phase I trial conducted by Newlands et al. [13]. As well as by schedule, TMZ activity may be modulated by high levels of AGT. Lee et al. [12] have shown AGT depletion in peripheral blood mononuclear cells following oral TMZ administration within 4 h from the first dose.
Pharmacokinetics of TMZ may give some indication of the above-mentioned aspects, so we incorporated a pharmacokinetic study in the phase II study with TMZ administered for 5 days in three oral doses daily, with the goal of achieving an adequate circulating MTIC level and prolonged AGT depletion over 24 h. A similar attempt to deplete AGT activity was also used in a study by Spiro et al. [20] with a twice-daily regimen of TMZ 200 mg/m2 per day for 5 days in adults with metastatic solid tumors. They found that TMZ is able to deplete AGT in the peripheral blood cells, but only partially and variably depletes AGT levels in different visceral tumors. The study indicated no clear advantage of the fractionated dose over single administration.
In the present study, we examined the plasma pharmacokinetics in children treated with TMZ at doses ranging from 600 to 1000 mg/m2 administered every 8 h for five consecutive days, and compared the plasma pharmacokinetics with those in adult patients under identical experimental conditions. To our knowledge this is the first study in which TMZ pharmacokinetics have been investigated under the same experimental conditions in adults and children. For all the pharmacokinetic parameters examined, no statistically significant differences were noted between the pediatric and the adult group. The clearance in children (100±19 ml/min per m2, n=29) overlapped the data obtained in adult patients in our study (104±9 ml/min per m2, n=11). In a number of phase I trials [1, 5, 6, 7, 11] involving adult patients, matching values for clearance (104, 119, 106, 115 and 115 ml/min per m2, respectively) have been found, including two studies in which TMZ was administered also at lower single daily doses (50, 75 and 85 mg/m2 [6] and 50 mg/m2 [7]). Estlin et al. [8], in a phase I study in pediatric patients, found that the apparent clearance was approximately 72 ml/min per m2. This low value resulted in a 40% increase in TMZ AUC in children compared with adult patients. A higher clearance rate (85 ml/min per m2) has been reported by Panetta et al. [16] in 26 children who received doses ranging from 145 to 200 mg/m2 per day. We found a low intra- and interpatient variability in children, comparable to the behavior of TMZ described in adults. Hematologic toxicity was a rare event in adults as compared to children with severe thrombocytopenia occurring in only 1 course out of 11. In children a relatively higher incidence of grade 4 neutropenia and thrombocytopenia as compared to adults was found. However, the intensity of previous treatment may account at least in part for this difference. Our results are similar to those reported by Estlin et al. who found an incidence higher than 30% of grade 3/4 neutropenia and thrombocytopenia at the 1000 mg/m2 dose level.
In order to evaluate a possible correlation in pediatric patients between TMZ plasma AUC and grade 4 hematologic toxicity, a comparison was made between the AUC values of children who did and did not develop grade 4 hematologic toxicities. No statistically significant difference was found between the two groups, indicating that no relationship existed between systemic exposure and hematologic toxicity. These results in children seem to be in contrast with the findings of Hammond et al. [11] who reported a statistically significantly higher median AUC value (32 vs 20 μg·h/ml, P=0.0019) in adult patients who experienced dose-limiting grade 4 neutropenia and thrombocytopenia. The different schedule utilized and, again, the intensity of previous treatment may in part explain this difference.
Our results suggest that the toxicity of TMZ is independent of pharmacokinetic parameters, and routine TMZ pharmacokinetic studies in patients undergoing treatment with the 5-day schedule does not seem to be useful in optimizing TMZ treatment results.
References
Baker SD, Wirth M, Statketich P, Reidenberg P, Alton K, Sartorius SE, Dugan M, Cutler D, Batra V, Grochow LB, Donehower RC, Rowinsky EK (1999) Absorption, metabolism and excretion of14C-temozolomide following oral administration to patients with advanced cancer. Clin Cancer Res 5:309
Bleehen NM, Newlands ES, Lee SM, Thatcher LN, Selby P, Calvert AH, Rustin GJ, Brampton M, Stevens MF (1995) Cancer research campaign phase II trial of temozolomide in metastatic melanoma. J Clin Oncol 13:910
Bower M, Newlands ES, Lee SM, Thatcher LN, Selby P, Calvert AH, Rustin GJ, Brampton M, Stevens MF (1997) Multicentre CRC phase II trial of temozolomide in recurrent or progressive high-grade glioma. Cancer Chemother Pharmacol 40:484
Brada M, Judson I, Beale P, Moore S, Reidenberg P, Statkevich P, Dugan M, Batra V, Cutler D (1999) Phase I dose-escalation and pharmacokinetic study of temozolomide (SCH 52365) for refractory or relapsing malignancies. Br J Cancer 81:1022
Britten CD, Rowinsky EK, Baker SD, Agarwala SS, Eckardt JR, Barrington R, Diab SG, Hammond LA, Johnson T, Villalona-Calero M, Fraass U, Statkevich P, Von Hoff DD, Eckhardt SG (1999) A phase I and pharmacokinetic study of temozolomide and cisplatin in patients with advanced solid malignancies. Clin Cancer Res 5:1629
Brock CS, Newlands ES, Wedge SR, Bower M, Evans H, Colquhoun I, Roddie M, Glaser M, Brampton MH, Rustin GJ (1998) Phase I trial of temozolomide using an extended continuous oral schedule. Cancer Res 58:4363
Dhodapkar M, Rubin J, Reid JM, Burch PA, Pitot HC, Buckner JC, Ames MM, Summan V (1997) Phase I trial of temozolomide (NSC 362856) in patients with advanced cancer. Clin Cancer Res 3:1093
Estlin EJ, Lashford L, Ablett S, Price L, Gowing R, Gholkar A, Kohler J, Lewis IJ, Morland B, Pinkerton CR, Stevens MCG, Mott M, Stevens R, Newell DR, Walker D, Dicks-Mireaux C, McDowell H, Reidenberg P, Statkevich P, Marco A, Batra V, Dugan M, Pearson ADJ (1998) Phase I study of temozolomide in pediatric patients with advanced cancer. United Kingdom Children's Cancer Study Group. Br J Cancer 78:652
Friedman HS, Kerby T, Calvert H (2000) Temozolomide and treatment of malignant glioma. Clin Cancer Res 6:2585
Gibson NW, Hickman JA, Erickson LC (1985) DNA cross-linking and cytotoxicity in normal and transformed human cells treated in vitro with 8-carbamoyl-3-(2-chloroethyl) imidazo[5,1-d]1,2,3,5-tetrazin-4(3H)-one. Cancer Res 44:1772
Hammond LA, Eckardt JR, Baker SD, Eckardt SG, Dugan M, Forral K, Reidenberg P, Statkevich P, Weiss GR, Rinaldi DA, Von Hoff DD, Rowinsky EK (1999) Phase I and pharmacokinetic study of temozolomide on a daily-for-5-days schedule in patients with advanced solid malignancies. J Clin Oncol 17:2604
Lee SM, Tatcher N, Crowther D, Margison GP (1994) Inactivation of O6-alkylguanine-DNA alkyltransferase in human peripheral blood mononuclear cells by temozolomide. Br J Cancer 69:452
Newlands ES, Blackledge GR, Slack JA, Rustin GJ, Smith DB, Stuart NS, Quarterman CP, Hoffman R, Stevens MFG, Brampton MH, Gibson AC (1992) Phase I trial of temozolomide (CCRG 81045:M&B39831: NSC362856). Br J Cancer 65:287
Nicholson HS, Krailo M, Ames MM, Seibel NL, Reid JM, Liu-Mares W, Vezina LG, Ettinger AG, Reaman GH (1998) Phase I study of temozolomide in children and adolescent with recurrent solid tumors: a report from the Children's Cancer Group. J Clin Oncol 16:3037
O'Reilly SM, Newlands ES, Glaser MG, Brampton M, Rice-Edwards JM, Illingworth RD, Richards PG, Kennard C, Colquhoun IR, Lewis P, Stevens MFG (1993) Temozolomide: a new oral cytotoxic chemotherapeutic agent with promising activity against primary brain tumors. Eur J Cancer 29A:940
Panetta JC, Kirstein MN, Gajjar AJ, Nair G, Fouladi M, Heideman RL, Wilkinson M, Stewart CF (2002) Population pharmacokinetics of temozolomide and MTIC in infants and children with primary central nervous system tumors (abstract). Proc AACR 43:1355
Patel M, McCully C, Godwin K, Balis F (1995) Plasma and cerebrospinal fluid pharmacokinetics of temozolomide (abstract). Proc ASCO 14:1485
Riccardi R, Cefalo G, Ruggiero A, Abate ME, Massimino M, Zucchetti P, Mascarin M, Garrè ML, Spreafico F, Mastrangelo S, Clerico A, Ridola V, Mazzarella G, Di Rocco C, Donfrancesco A, Perilongo G, Fossati F, Madon E (2002) High response rate to temozolomide in heavily pretreated children with medulloblastoma (abstract). Proc AACR 43:3711
Shen F, Decosterd LA, Gander M, Leyvraz S, Biollaz S, Lejeune F (1995) Determination of temozolomide in human plasma and urine by high-performance liquid chromatography after solid-phase extraction. J Chromatogr B 667:291
Spiro TP, Liu L, Majka S, Haaga J, Willson JKV, Gerson SL (2001) Temozolomide: the effect of once- and twice-a-day dosing on tumor tissue levels of the DNA repair protein O6-alkylguanine-DNA alkyltransferase. Clin Cancer Res 7:2309
Stevens MF, Hickman JA, Langdon SP, Chubb D, Vickers L, Stone R, Baig G, Goddard C, Gibson NW, Slack JA, Newton C, Lunt E, Fizames C, Lavelle F (1987) Antitumor activity and pharmacokinetics in mice of 8-carbamoyl-3-methyl-imidazo[5,1-d]-1,2,3,5-tetrazin-4(3H)-one (CCRG 81045; M & B 39831), a novel drug with potential as an alternative to dacarbazine. Cancer Res 47:5846
Stupp R, Gander M, Leyvraz S, Newlands E (2001) Current and future developments in the use of temozolomide for the treatment of brain tumors. Lancet Oncol 2:552
Acknowledgements
This work was supported by AIRC, FOP and MURST.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Riccardi, A., Mazzarella, G., Cefalo, G. et al. Pharmacokinetics of temozolomide given three times a day in pediatric and adult patients. Cancer Chemother Pharmacol 52, 459–464 (2003). https://doi.org/10.1007/s00280-003-0677-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-003-0677-x