Abstract
Purpose
To evaluate the response to lapatinib, an inhibitor of epidermal growth factor receptors 1 and 2, in patients with advanced bilary tree cancer (BTC) and hepatocellular cancer (HCC).
Methods
Lapatinib was dosed at 1,500 mg/day orally continuously.
Results
Fifty-seven patients were accrued (BTC 17, HCC 40). Therapy was well tolerated. The response in BTC was 0% and in HCC was 5%. The progression free survival (PFS) for BTC and HCC patients was 1.8 (95% CI: 1.7–5.2) months and 2.3 (95% CI: 1.7–5.6) months. The median survival for BTC and HCC patients was 5.2 (95% CI 3.3–∞) months and 6.2 (95% CI: 5.1–∞) months. EGFR genotyping indicated HCC patients with <20 repeats have the lowest PFS. The occurrence of any skin rash significantly prolonged PFS and survival.
Conclusions
Lapatinib was well-tolerated. There was evidence of activity in HCC, but therapy with lapatinib did not meet the predefined efficacy rate.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There are few effective therapies for patients with advanced hepatocellular (HCC) or biliary tree cancer (BTC). HCC is a common malignancy worldwide, with an estimated incidence of 667,000 cases/year [1]. In the US (estimated incidence 17,500/year), the incidence has increased, probably due to hepatitis C exposure [1]. The overall prognosis for HCC is poor with mortality similar to incidence rates. HCC typically presents at an advanced stage with only a minority of cases suitable for surgical resection, liver ablative procedures or liver transplantation [2]. In patients who have surgically unresectable HCC, few treatment options exist. Transarterial chemoembolization (TACE) may benefit selected patients with localized diseases, however systemic chemotherapy has been largely ineffective [2]. Administration of systemic chemotherapy is also limited, as patients with HCC, frequently have multiple comorbidities, liver dysfunction and cirrhosis. Advanced BTC (ampullary, bile duct and gallbladder) similarly have a poor prognosis and are resistant to standard chemotherapeutic regimens [3, 4].
Hepato-carcinogenesis is a multistep process and multiple molecular pathways are involved [5]. In particular, growth factor receptor activation and angiogenesis may play a significant role in carcinogenesis. The epidermal growth factor receptor (EGFR) family, especially EGFR1 (EGFR) is commonly overexpressed in HCC [5]. Though over expression of EGFR2 (Her2/neu) is less common in HCC, it may play an important role as Her2/neu somatic mutations, which may predict response to EGFR targeted agents have been reported [6]. Genetic alterations in BTC are less well characterized, but similar molecular pathways to HCC have been identified [7, 8].
Two phase 2 studies have reported on the activity of erlotinib, an oral inhibitor of EGFR in patients with advanced HCC. Philip et al. [9] evaluated erlotinib in 38 patients. EGFR overexpression was detected in 88% of specimens by immunohistochemistry (IHC). Therapy with erlotinib was well tolerated and a radiological partial response was seen in 3 patients (8%), with median overall survival of 13 months. Thomas et al. evaluated the agent in 40 patients with HCC. No objective responses were noted, and the median survival of patients was 10.8 months [10]. Erlotinib was also evaluated in 42 patients with BTC; EGFR expression was detected in 81% of patients, with response seen in 3 patients. The median survival was 7.5 months [11].
In this study, we evaluated lapatinib, an oral dual kinase inhibitor of EGFR and Her-2/neu in patients with biliary tree and HCC based on the hints of activity from the erlotinib clinical trials and the preclinical observation that dual inhibition of the EGF family appears to have a greater inhibitory effect on downstream signaling pathways than inhibition of either receptor alone [12]. The primary objective of this study was to determine the objective response rate to lapatinib in patients with BTC and HCC. Secondary objectives were to determine the overall survival (OS), progression free survival (PFS) and toxicities. Exploratory molecular and pharmacogenomic correlative studies were done on blood and archived tumor specimens to identify specific patient subsets that may benefit from lapatinib therapy.
Materials and methods
Patient selection
Eligible patients were >18 years old, had histological or cytological confirmation of HCC or BTC and were not candidates for surgery or local ablative procedures. Patients were required to have measurable disease, ≤1 prior chemotherapy (including TACE) for metastatic or recurrent disease and the ability to swallow and retain oral medications. Other eligibility criteria were life expectancy of greater ≥12 weeks and an Eastern oncology performance status (ECOG) performance status of 0,1 or 2, adequate organ and marrow function defined as leukocytes ≥3,000/μL, absolute neutrophil count ≥1,500/μL, platelets ≥75,000/μL, total bilirubin <2 mg/dL, AST and ALT ≤5.0 X upper limit of institutional normal (ULN), prothrombin time <4 s above ULN (unless taking warfarin), creatinine ≤ULN or creatinine clearance ≥50 mL/min/1.73 m2 for patients with creatinine levels above ULN, left ventricular ejection fraction (LVEF) > lower limit of normal as measured by echocardiogram or multiple gated acquisition (MUGA) scan.
Ineligibility criteria included patients who were on concomitant medications classified as CYP3A4 inducers or inhibitors, prior treatment with EGFR agents, receiving combination anti-retroviral therapy for HIV, baseline Childs B or C scores, known brain metastases or were pregnant and lactating women.
All patients signed an informed consent prior to therapy, according to institutional and federal guidelines. The study was sponsored by the Cancer Therapy and Evaluation Program (CTEP) of the National Cancer Institute (NCI) and conducted by the phase II consortiums of California, Pittsburgh and the University of Chicago.
Study assessments and requirements
Prior to the start of treatment, a history and physical exam (H and P), complete blood count (CBC), chemistries including liver function tests, urine analysis, ECG and radiological scans to define extent of tumor were performed. LVEF was assessed prior to start of therapy and then every 8 weeks while on study. Following start of therapy, an H and P, CBC and chemistries was done every 2 weeks. Toxicity was graded according to the NCI common terminology criteria for adverse events (CTCAE) version 3.0.
Drug therapy: schedule and dose modification
Lapatinib was supplied as film coated capsules of 250-mg dosage strength by CTEP (Rockville, MD) under a clinical trials agreement with GlaxoSmithKline pharmaceuticals. Patients were instructed to take the required dose on an empty stomach (either 1 h before or 1 h after meals). Antiemetic therapy was at the discretion of the investigator, administered according to institutional guidelines. Patients were asked to complete a study medication diary on a daily basis, and the diary was reviewed every 2 weeks to assess patient-reported adherence. Each treatment cycle was 4 weeks.
Dose modifications
The starting dose was 1,500 mg/day continuously. Patients were required to meet pre-study laboratory requirements prior to dosing every 2 weeks. A maximum of three dose reductions and a 3-week delay for drug administration for toxicity was permitted. The dose reduction levels were 1,000, 750 or 500 mg/day. Patients requiring a fourth dose reduction were taken off study.
The management of specific agent-related adverse events were as follows: for cardiac toxicity: subjects who had a >20% decrease in LVEF from baseline had a repeat evaluation of LVEF, 1–2 weeks later while still receiving lapatinib. If there was grade ≥3 dysfunction or if the repeat LVEF value confirmed a ≥20% decrease, then lapatinib therapy was discontinued. For diarrhea: no intervention was required for grade 1. Loperamide at standard doses was started for grade 2 diarrhea, and lapatinib was continued, but could be held at the discretion of the investigator depending on the medical condition of patient. For grade ≥3 diarrhea, lapatinib was held until recovery to ≤grade 1 for up to 21 days. Subsequent dose of lapatinib was then reduced by 1 dose level. For skin rash: no intervention was required for grade 1; for grade 2 toxicity appropriate medical therapy was instituted, and therapy was not held unless it was unacceptable to patient or medically required. In that case lapatinib was held until recovery to ≤grade 1, up to 21 days, and then restarted at same dose. For grade ≥3 rash, lapatinib was held until recovery to ≤grade 1 for up to 21 days. Subsequent dose of lapatinib was then reduced by 1 dose level. For other toxicity: no intervention was necessary for grade 1 toxicity. In the case of grade 2 toxicity appropriate medical therapy was instituted, therapy was not held unless it was unacceptable to patient or medically required. In that case lapatinib was held until recovery to ≤grade 1, up to 21 days, and then restarted at same dose. In the case of grade ≥3 toxicity lapatinib was held until recovery to <grade 1 for up to 21 days. Subsequent dose of lapatinib was then reduced by 1 dose level.
Evaluation of response
Radiological tests were performed at baseline and every 8 weeks to assess response by RECIST criteria [13]. Progression free survival was calculated from the day of starting therapy till progression or death. Overall survival was calculated from day of starting therapy till death.
Trial design and statistics
The primary endpoint of this study was response rate. Patients with BTC and HCC were accrued and assessed in separate strata. A Simon optimal two-stage design was used [14]. In the first stage 17 patients were enrolled separately to each group. At least >1 response was required to proceed to the second stage, for a total of 37 patients in each cohort. Lapatinib would be considered active if ≥4 responses (≥11%) were observed among the 37 evaluable patients in a cohort (90% power to detect a true response rate of ≥20%).
Correlative studies
Paraffin embedded tumor blocks and/or at least 16 unstained paraffin-embedded slides of tumor tissue were collected at study entry. EGFR staining was performed using Clone E30 (Biogenex, San Ramon CA). Briefly, 4 μ paraffin sections were deparaffinized and exposed to microwave heating for uniform antigen retrieval. The primary antibody was applied for 60 min followed by appropriate rinsing and application of a secondary antibody of biotinylated horse anti-mouse IgG (Vector, Burlingame CA) at 1:200 dilution. After rinsing, the Vector Elite ABC reagent was used. After rinsing, DAB was applied and the color development monitored by light microscopy. The pathologist (RGE) interpreted the sections by light microscopy for percentage of tumor cells having a distinct membrane staining pattern. Detection of KRAS codon 12 mutations was carried out as previously described using a sensitive two-step restriction fragment-length polymorphism–polymerase chain reaction (RFLP–PCR) assay that enriches for the presence of mutations [15]. Blood samples for EGFR genotyping were collected in two EDTA tubes (8–10 ml each) pre study and prior to cycle 2 and 3. One tube was stored as whole blood; the other was centrifuged and aliquoted for analysis.
EGFR genotyping
The EGFR dinucleotide polymorphism was determined with 5′-end 33p γATP labeled PCR protocol with a few modifications. In summary: DNA template, dNTPs, 5′-end 33p γATP labeled primer, unlabelled complementary primer, Taq polymerase (Perkin Elmer Inc, Connecticut, USA) and PCR buffer were used together in a final PCR. The reaction was carried out and the reaction products were separated using a 6% denaturing polyacrylamide DNA sequencing gel, which then was vacuum blotted for 1 h at 80°C and exposed to an XAR film (Eastman-Kodak Co., New York, USA) overnight. The exact number of repeats was confirmed by direct sequencing. The EGFR intron 1 (CA) n16–23 repeat in each allele was categorized at the sample median, 20 (CA) n , as described previously [16]. Other polymorphisms including significant polymorphisms in genes involved in the Her1/Her2 pathway (Tissue Factor A-603G, Cox-2 T+8473C, EGF A+61G, Cyclin D1 A+870G, IL-8 T-251A, VEGF-936 C+936T, HER2_neu codon 655 A/G, EGFR G+497A) were determined.
Results
Patient characteristics
Fifty-seven patients were entered between November 2004 and February 2006. There were 17 patients with BTC and 40 with HCC. On review, 3 treated patients with HCC were ineligible for study due to having Child B scores and were not evaluable for response, but included in the toxicity analysis. Patient characteristics are given in Table 1. A total of 186 cycles were administered (median 2 cycles, range 1–12).
Toxicity
Therapy was well tolerated in both BTC and HCC subjects (Table 2). Twenty-four of 57 patients (47%) required at least one dose modification, and 4 patients (7%) discontinued therapy due to toxicity. Most dose reductions occurred in patients who had received ≥4 cycles of therapy.
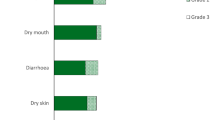
Hematological toxicities were uncommon with grade 3/4 anemia and thrombocytopenia seen in 4 and 2% of patients, respectively. The most common grade 3/4 toxicities were diarrhea (7%), fatigue (6%) and elevations of AST/ALT (9%). Common grade 1–2 toxicities were diarrhea (46%), nausea (32%) and fatigue (46%). Elevations of liver function tests were also seen, but these were mostly grade 1 or 2. A grade 1/2 skin rash was observed in 19 patients (35%) and one patient developed a grade 3 rash. There were no changes in LVEF following therapy.
Efficacy
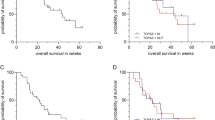
There were no objective responses in the group of BTC patients, stable disease (SD) was documented in 4 patients (26%) with duration of 3–5.5 months. In patients with HCC, 2 objective responses were noted (5%) and SD in 13 patients (35%) with duration of 3–14 months. The partial responses were in a patient with no prior therapy and in a other following TACE. PFS and OS are depicted in Fig. 1. The median PFS for patients with BTC and HCC were 1.8 months (95% CI 1.7–5.2) and 2.3 months (95% CI 1.7–5.6), respectively. The median OS for BTC and HCC patients were 5.2 months (95% CI 3.3–∞) and 6.2 months (95% CI 5.1–∞). The occurrence of any rash in BTC and HCC patients significantly correlated with prolonged PFS (increased from 2.0 to 5.0 months, P = 0.03) and OS (increased from 5.0 to 10.0 months, P = 0.004) (Fig. 2).
Correlative studies
The overexpression of EGFR was seen in 7 out 12 (58%) of specimens with sufficient archival tumor material for analysis. For BTC, 2 of 2 showed strong EGFR staining in 90–100% of tumor cells. In HCC, 5 of 10 showed staining in 30–100% of tumor cells. In all these 12 samples, KRAS wild type was detected and mutations were absent.
Genotyping information was available in 28 out of 40 (70%) HCC patients. To evaluate the effect of the number of (CA) n repeats on the clinical outcome among all study participants, we separated patients into two subgroups: 8 patients (29%) had EGFR (CA) n repeats <20 and 20 patients (71%) had any (CA) n repeats ≥20. Patients with (CA) n repeats <20 showed lower PFS compared to those with (CA) n repeats <20. (P = 0.016, log-rank test). We did not observe any statistical significant association between EGFR (CA) n repeats and response or OS. We did not observe statistically significant associations between other tested genes involved in the EGFR pathway (n = 8) and response, PFS or overall survival.
Discussion
Preclinical evidence supports the notion that progression of HCC can be significantly inhibited by EGFR-targeted agents in vitro and in vivo, enhance chemo-sensitivity [17, 18] and that dual inhibition of EGFR and HER2 may be a effective therapeutic strategy in ERB-driven tumors [12]. Oral lapatinib therapy in our study was well tolerated. In BTC, lapatinib did not show activity, however partial responses were seen in two patients with HCC. The response rate of 5% did not meet the predefined endpoint of 11% and thus we do not feel that further evaluation of single agent lapatinib in this disease is warranted in unselected patients. The response rate of 5% in HCC patients in our study is similar to published studies of single agent therapy with erlotinib, sorafinib and cetuximab [9, 10, 19, 20]. Base line characteristics of the etiology or viral titers in HCC patients was not collected, but our patient population can be expected to be similar to other studies [9, 10, 19]. However the median survival of 6.2 months (95% CI 5.1–∞) is lower than other studies, where overall survival of 8–13 months was reported [9, 10]. The low median survival in our study may be due to the small sample size, but it is likely that lapatinib did not have significant activity in HCC.
The occurrence of skin rash in about 1/3 of patients was associated with improved PFS and survival, suggesting an effect on the ERB1 pathway. Though occurrence of a skin rash is correlated to outcome in colon and pancreatic cancer patients treated with cetuximab and erlotinib [21], in HCC a similar association was not reported [9, 19]. In a randomized study of breast cancer patients who were treated with capecitabine or the combination of capecitabine and lapatinib, the incidence of skin rash was low and did not correlate to outcome [22]. Though in preclinical models lapatinib has dual inhibitory activity on both ERB1 and ERB2, the predominant inhibitory action may be via the ERB2 pathway [23]. In HCC, the incidence of ERB2 over expression and activating mutations is low, which might in part explain the disappointing results in our study [8]. Resistance to EGFR therapy may be dependant on KRAS status, with resistance seen in KRAS mutant tumors [24]. In HCC, KRAS appears to be mutated in about 30% of tumors and may be associated with vinyl chloride exposure [25]. In our study KRAS mutations were absent in all 12 samples studied.
Tumor and genomic biomarker collection was optional and thus assessment in our trial and correlation to efficacy is limited by the number of samples and limited activity. We focused on EGFR genotyping based on the following observations: overexpression of EGFR mRNA and protein has been associated with tumor aggressiveness and poor clinical outcome in a variety of epithelial malignancies, including HCC [26, 27]. In addition, a highly polymorphic region in intron 1 of the EGFR gene is associated with transcription levels of EGFR in vitro and in vivo [28, 29]. The length of this (CA) n dinucleotide polymorphism correlated inversely with the transcriptional activity of the gene. In vitro studies showed that the transcriptional activity in cell lines containing a prolonged polymorphic region (>20 CA repeats) was markedly reduced compared with cells containing a shorter allele [18]. These findings were confirmed in human breast cancer samples. A constant decline of intratumoral EGFR protein expression was associated with increase in allele length. Furthermore, hemizygote tumors showed higher EGFR expression if the longer allele was lacking compared with tumors with the longer allele remaining [28]. To date, EGFR polymorphisms have not been reported to be causatively linked to clinical outcome in HCC patients. However, in our study, short repeat alleles of EGFR (CA) n, which code for increased EGFR gene expression, were found to be significantly associated with PFS (P = 0.016, log-rank test).
Based on our results and other recently published trials, there is a continued need for evaluation of novel molecularly targeted agents for patients with HCC and biliary tree cancer. Sorafenib [20], a small molecule inhibitor of the VEGF and the RAF kinase pathway is the first agent to be approved by the FDA for HCC, based on improved survival compared to best supportive care [20]. Sorafinib is now being evaluated in combination with other agents which target the signal transduction pathways. Understanding the molecular mechanisms and individualizing therapy for patients is the key to improve the outcome of patients with HCC and BTC [30].
References
El-Serag HB, Rudolph KL (2007) Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology 132:2557–2576
Alberts SR, Gores GJ, Kim GP et al (2007) Treatment options for hepatobiliary and pancreatic cancer. Mayo Clin Proc 82:628–637
Shimoda M, Kubota K (2007) Multi-disciplinary treatment for cholangiocellular carcinoma. World J Gastroenterol 13:1500–1504
Bartlett D, Ramanathan RK, Ben-Joseph E (2008) Gallbladder and bilary cancers. In: Devita VT, Hellman S, Rosenberg SA (eds) Cancer: principles and practice of oncology, 8th edn. Lippincott, Philadelphia
Berasain C, Castillo J, Prieto J et al (2007) New molecular targets for hepatocellular carcinoma: the ErbB1 signaling system. Liver Int 27:174–185
Bekaii-Saab T, Williams N, Plass C et al (2006) A novel mutation in the tyrosine kinase domain of ERBB2 in hepatocellular carcinoma. BMC Cancer 6:278
Yoon JH, Gwak GY, Lee HS et al (2004) Enhanced epidermal growth factor receptor activation in human cholangiocarcinoma cells. J Hepatol 41:808–814
Sirica A (2005) Cholangiocarcinoma: molecular targeting strategies for chemoprevention and therapy. Hepatology 41:5–15
Philip PA, Mahoney MR, Allmer C et al (2005) Phase II study of Erlotinib (OSI-774) in patients with advanced hepatocellular cancer. J Clin Oncol 23:6657–6663
Thomas MB, Chadha R, Glover K et al (2007) Phase 2 study of erlotinib in patients with unresectable hepatocellular carcinoma. Cancer 110:1059–1067
Philip PA, Mahoney MR, Allmer C et al (2006) Phase II study of erlotinib in patients with advanced biliary cancer. J Clin Oncol 24:3069–3074
Reid A, Vidal L, Shaw H et al (2007) Dual inhibition of ErbB1 (EGFR/HER1) and ErbB2 (HER2/neu). Eur J Cancer 43:481–489
Therasse P, Arbuck SG, Eisenhauer EA et al (2000) New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst 92:205–216
Simon R (1989) Optimal two-stage design for phase II clinical trials. Control Clin Trials 10:1–10
Gautschi O, Huegli B, Ziegler A et al (2007) Origin and prognostic value of circulating KRAS mutations in lung cancer patients. Cancer Lett 254:265–273
Zhang W, Park DJ, Lu B et al (2005) Epidermal growth factor receptor gene polymorphisms predict pelvic recurrence in patients with rectal cancer treated with chemoradiation. Clin Cancer Res 11:600–605
Matsuo M, Sakurai H, Saiki I (2003) ZD1839, a selective epidermal growth factor receptor tyrosine kinase inhibitor, shows antimetastatic activity using a hepatocellular carcinoma model. Mol Cancer Ther 2:557–561
Schiffer E, Housset C, Cacheux W et al (2005) Gefitinib, an EGFR inhibitor, prevents hepatocellular carcinoma development in the rat liver with cirrhosis. Hepatology 41:307–314
Zhu AX, Stuart K, Blaszkowsky LS et al (2007) Phase 2 study of cetuximab in patients with advanced hepatocellular carcinoma. Cancer 110:581–589
Llovet JM, Ricci S, Mazzaferro V, SHARP Investigators Study Group (2008) Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 359:378–390
Peréz-Soler R, Saltz L (2005) Cutaneous adverse effects with HER1/EGFR-targeted agents: is there a silver lining? J Clin Oncol 23:5235–5246
Geyer CE, Forster J, Lindquist D et al (2006) Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med 355:2733–2743
Press MF, Finn RS, Cameron D et al (2008) HER-2 gene amplification, HER-2 and epidermal growth factor receptor mRNA and protein expression, and lapatinib efficacy in women with metastatic breast cancer. Clin Cancer Res 14:7861–7870
Van Cutsem E, Lang I, D’haens G et al (2008) KRAS status and efficacy in the first-line treatment of patients with metastatic colorectal cancer (mCRC) treated with FOLFIRI with or without cetuximab: the CRYSTAL experience. J Clin Oncol 26(May 20 suppl), abstr 2
Weihrauch M, Benicke M, Lehnert G et al (2001) Frequent k-ras-2 mutations and p16INK4Amethylation in hepatocellular carcinomas in workers exposed to vinyl chloride. Br J Cancer 84:982–989
Mayoral R, Fernandez-Martinez A, Bosca L et al (2005) Prostaglandin E2 promotes migration and adhesion in hepatocellular carcinoma cells. Carcinogenesis 26:753–761
Mitchell C, Nivison M, Jackson LF et al (2005) Heparin-binding epidermal growth factor-like growth factor links hepatocyte priming with cell cycle progression during liver regeneration. J Biol Chem 280:2562–2568
Buerger H, Gebhardt F, Schmidt H et al (2000) Length and loss of heterozygosity of an intron 1 polymorphic sequence of egfr is related to cytogenetic alterations and epithelial growth factor receptor expression. Cancer Res 60:854–857
Gebhardt F, Zanker KS, Brandt B (1999) Modulation of epidermal growth factor receptor gene transcription by a polymorphic dinucleotide repeat in intron 1. J Biol Chem 274:13176–13180
Llovet JM, Di Bisceglie AM, Bruix J et al (2008) Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst 100:698–711
Acknowledgments
This study was sponsored by the Cancer Therapy and Evaluation Program of the National Cancer Institute, Bethesda, MD 20892. Presented in part at the 42nd annual meeting of the American Society of Clinical Oncology, Orlando, FL. June 2006. Supported in part by NCI-NO1-CM-57018-16 (California Consortium), Dhont Foundation (HJ Lenz). P30CA47904 and NIH/NCCR/GCRC #5M01 RR 00056 (University of Pittsburgh Cancer Institute and Medical Center). The authors wish to thank Christine Garcia and Stella Chen, CCC-P coordinators for data management and coordination of study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ramanathan, R.K., Belani, C.P., Singh, D.A. et al. A phase II study of lapatinib in patients with advanced biliary tree and hepatocellular cancer. Cancer Chemother Pharmacol 64, 777–783 (2009). https://doi.org/10.1007/s00280-009-0927-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-009-0927-7