Abstract
Aim
The aim of this phase II study was to determine the efficacy and tolerability of the bimonthly, pharmacokinetically intensified LV5FU2 regimen in the treatment of metastatic colorectal cancers.
Methods
A total of 53 patients (23% second-line; 25 male/28 female; mean age 67 years; WHO performance status 0 in 38, 1 in 10 and 2 in 5) were treated in cycle 1 with the standard LV5FU2 regimen (leucovorin 200 mg/m2 per day followed by a 5-FU bolus 400 mg/m2 per day and a 22-h 5-FU continuous infusion 600 mg/m2 per day for two consecutive days every 2 weeks), and the AUC in mg·h/l·m2 was calculated. For cycle 2, according to a predefined schedule depending on the cycle-1 AUC value, in the absence of grade 3 toxicity, the 5-FU infusion dose was increased by 150% for AUC ≤5, by 100% for AUC >5–10, by 50% for AUC >10–15, and by 25% for AUC >15–20. 5-FU plasma concentrations were determined using high-performance liquid chromatography. A Bayesian methodology was used to assess individual pharmacokinetic parameters using the NONMEM computer program.
Results
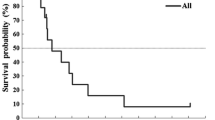
Among the 53 eligible patients, 87% (per-protocol population) received an increased dose in cycle 2 and 72% received the same dose. The median relative dose intensity was 1.28 (range 0.5–1.54) compared with the non-adapted theoretical total 5-FU dose. The objective response rate was 37% (95% CI 23–50%) in the intention-to-treat population and 47% (95% CI 29–65%) in the first-line per-protocol population. The median response duration was 10.4 months. The median progression-free survival (PFS) and overall survival (OS) were, respectively, 7 and 18.6 months. PFS and OS in first-line per-protocol patients were, respectively, 9.2 and 20 months. No deaths were attributed to toxicity of 5-FU despite the high doses administered. Of the 53 patients, 19% experienced gastrointestinal and 30% haematological grade 3/4 toxicities. Hand-foot syndrome was common but mild (grade 3 in one patient).
Conclusions
This strategy could be compared in a phase III trial with the standard LV5FU2 regimen.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite the use of adjuvant chemotherapy, nearly 50% of all newly diagnosed patients with colorectal cancer will develop metastatic disease. For these patients, 5-FU combined with folinic acid is still considered one of the best first-line chemotherapy options because it enhances the response rate. However, overall survival (OS) is not necessarily improved [1]. Various therapeutic schedules have been proposed, particularly monthly [24] or weekly [4, 18] regimens. One of the most effective and less-toxic schedules compared with the Mayo Clinic regimen is the bimonthly combination of high infusion folinic acid and 5-FU over 48 h as a bolus and continuous infusion (LV5FU2) [7] which compared with the Mayo Clinic regimen has produced significantly better objective response rates (32.6% versus 14.5%) and a lower incidence of grade 3/4 toxicity (11.1% versus 23.9% in the monthly arm). When our study was initiated, this bimonthly regimen was considered the standard first-line chemotherapy in several European countries for patients with advanced colorectal cancer.
The efficacy of 5-FU has been shown to increase in combination with oxaliplatin [2, 3, 8] or irinotecan [9]. Nevertheless, the benefit in OS was moderate with the irinotecan combination [9] (17.4 vs 14.1 months, P<0.031) and significance was not reached with the oxaliplatin combination (16.2 vs 14.7 months) [8]. Other studies have shown that efficacy can be improved by individually modulating the doses of 5-FU according to the circadian rhythm (chronomodulated schedules) [19, 20, 21] or by dose adaptation based on pharmacokinetic parameters.
A 5-FU dose-effect relationship has been indicated in various studies using different schedules [4, 16, 18, 27]. However, in these studies the dose of 5-FU was calculated only according to body surface area (BSA) and this can lead to severe toxicities. In addition, significant intra- and interpatient variations in pharmacokinetic parameters have been observed in some recent studies, in particular the steady-state 5-FU plasma concentration, and the area under the curve (AUC). These studies have shown a relationship between these pharmacokinetic parameters and 5-FU efficacy and toxicity [10, 12, 13, 25, 28]. More recently, individual 5-FU dosage optimization by pharmacokinetic monitoring has been developed, using weekly increases in 8-h 5-FU infusion. A phase III randomized trial [14] comparing a pharmacokinetically intensified schedule with a standard regimen has shown superiority of the intensified schedule with a response rate of 39% as compared to 19%. OS was also improved (16 vs 13 months), but the difference was not significant. At the present time, however, the weekly 8-h 5-FU infusion used as the control arm in this study, is not considered a standard treatment.
In a recent feasibility study, a 5-FU dose intensification strategy was developed within the standard bimonthly LV5FU2 regimen [31]. This study showed that this schedule could be adapted from cycle 2 onwards according to a range of AUC values obtained from five blood samples obtained during cycle 1 with a control for toxicity. The aim of the present study was to confirm the results of this approach in terms of efficacy and tolerability in a phase II trial.
Patients and methods
Patients
Inclusion criteria were as follows: histologically proven metastatic adenocarcinoma of the colon or rectum, unresectable locally advanced disease, more than one bidimensionally measurable target lesion at least 15 mm in diameter, WHO performance status (PS) of 2 or less, age 18–75 years, no more than one previous adjuvant or palliative treatment, a wash-out period of 4 weeks from the last course of chemotherapy, and provision of written informed consent. Laboratory data requirements for each patient before study entry were as follows: absolute neutrophil count ≥1.5×103/mm3, platelet count ≥100×103/mm3, prothrombin time ≥50%, and total bilirubin level not more than 1.5 times the institutional upper normal limit. Exclusion criteria included the following: other concomitant cancer, major organ failure (cardiac, renal or hepatic disease), known contraindications to 5-FU, and poor venous access which would prevent the pharmacokinetic study from being carried out. The trial was approved by the Ethics Review Committee of Montpellier.
Study design and chemotherapy regimen adjustment
In cycle 1 patients received the bimonthly LV5FU2 regimen according to the schedule described by de Gramont et al. [7]: folinic acid (200 mg/m2 per day) by i.v. infusion over 2 h followed by a 5-FU bolus (400 mg/m2 per day) and immediately after by continuous 5-FU infusion (600 mg/m2 per day) administered via a battery-operated pump over the next 22 h for two consecutive days, i.e. 2000 mg/m2 per cycle.
Pharmacokinetic parameters (particularly AUC) were determined during cycles 1 and 2. For cycle 2, 5-FU continuous infusion doses were adapted in the absence of grade 3 or worse toxicity according to a predefined adaptation schedule [31] depending on the value of the AUC. The continuous infusion dose was increased by 150% for AUC ≤5 mg·h/l·m2, by 100% for AUC between 5 and 10 mg·h/l·m2, by 50% for AUC between 10 and 15 mg·h/l·m2, by 25% for AUC between 15 and 20 mg·h/l·m2 and no increase for AUC >20 mg·h/l·m2. Doses were to remain constant during the subsequent cycles if toxicity grades remained less than 3. For grade 3/4 toxicity, the infusion dose was to be reduced by 25%. The regimen was to be discontinued if disease progression or non-tolerable toxicity occurred.
The dose intensity for the first six cycles, expressed in milligrams per metre squared per week, was calculated as the ratio of the total dose administered to the total treatment time. Relative dose intensity was calculated in two ways: as the ratio of the observed dose intensity to the dose intensity of the standard 5-FU regimen (1000 mg/m2 per week) with and without dose adaptation.
Pharmacokinetic study
A pharmacokinetic study was performed for all patients during the first two cycles using at least five 3-ml blood samples collected into heparinized tubes before treatment (T0), 20 min after the i.v. bolus which lasted less than 2 min (T1 and T3), and 4 h before the end of each 22-h 5-FU continuous infusion (T2 and T4) (Fig. 1). Since most of the patients were ambulatory, the last blood sample was taken 4 h before the end of 5-FU continuous infusion to avoid the possibility of a missing sample due to the late arrival of the patient. Indeed, if a patient were delayed in returning for removal of the pump, the pump could have stopped before the sample could be taken. That is why the exact timing of the blood sample was systematically noted and taken into account in the modelization curve. This modelization uses all five sampling points in calculating the pharmacokinetic parameters.
Immediately after collection, blood samples were centrifuged (2000 g) at +4°C for 10 min, then the plasma was stored at −40°C until assay. The cycle-1 AUC was calculated in order to adapt the 5-FU continuous infusion dose for cycle 2. The pharmacokinetic study was also performed during cycle 2 in order to validate the AUC values after 5-FU dose adaptation.
The 5-FU plasma concentrations were determined in the Val d'Aurelle Oncopharmacology Laboratory using high-performance liquid chromatography with ultraviolet absorbance detection as described elsewhere [17]. Precision, expressed as percent coefficient of variation, ranged from 2.7% to 13% and the accuracy ranged from 94% to 105%. The limits of quantification and detection were 20 and 10 ng/ml, respectively. Quality control tests were performed during every analysis sequence.
A methodology to calculate individual 5-FU pharmacokinetic parameters after i.v. infusion has previously been developed [6]. An open one-compartment pharmacokinetic model with zero-order input rate was used to describe the kinetics of 5-FU, and circadian time-dependent changes in 5-FU concentrations were also taken into account in the model. The circadian model was defined as the sum of two cyclic components. The amplitude of the first cyclic component (over 24 h) was about 30% of the average clearance and the amplitude of the second cyclic component (over 12 h) was about 50% of the amplitude of the first component. The acrophase (peak) times of the first and the second periodic component were 4.2 and 0.41 h A.M., respectively. In that study, a non-linear mixed-effects procedure was used to estimate the population parameters and to explore the interindividual variability in relation with the following covariates: gender, BSA, age, body weight, height, liver enzymes and serum creatinine. Bayesian methodology developed in the previous study enabled the estimation of individual 5-FU pharmacokinetic parameters with good precision and without bias from sparse data (two blood sampling times over a 24-h period, i.e. 20 min after the start and 4 h before the end of each continuous infusion). Thus, in the present study, Bayesian methodology was used to assess individual pharmacokinetic parameters (total plasma clearance, CL, and volume of distribution, V, using the NONMEM computer program version 5.1) [5] through the Visual-NM graphical interface (Visual-NM program, version 5.1, 1998. RDPP, Montpellier, France). Such an approach combines prior knowledge of the means and dispersions of pharmacokinetic parameters in the population to which the selected individual belongs, and the individual data. The area under the plasma concentration-time curve (AUC, mg·h/l·m2) was computed from dose/CL.
Assessment criteria
The primary endpoints of this trial were the response rate and toxicity. The secondary endpoints were response duration, time to progression and OS.
Patients were evaluated for response by a CT scan after six treatment cycles using standard WHO response criteria. Complete response (CR) was defined as complete disappearance of all evaluable disease for at least 4 weeks. Partial response (PR) was characterized by a decrease of at least 50% in the sum of the products of the diameters of measurable lesions for at least 4 weeks. Stable disease (SD) required a <50% decrease or an increase of <25% in metastatic lesions. Progressive disease (PD) was an increase of at least 25% in the disease or the appearance of a new neoplastic lesion. Response duration was calculated from the date of inclusion until progression among partial responders and from the date of CR to progression among complete responders. Progression-free survival (PFS) and OS were calculated from the date of inclusion to progression and death from any cause, respectively. Patients who never progressed nor relapsed after a response were considered censored for PFS. Patients alive at the last known follow-up date were considered censored for OS. Toxicity was graded according to standard WHO criteria, and evaluated before each cycle with particular attention to diarrhoea, mucositis, hand-foot syndrome and neutropenia.
Statistical considerations
The sample size was calculated according to a three-stage design with a minimum of 14 patients in the first stage. If no objective responses were observed among the first 14 patients, the study was to be closed early and deemed negative. If at least one objective response was achieved, enrolment was to be extended to a minimum of 28 patients. In order to obtain complete data with regard to dose adaptation, it was planned to register an additional 12 patients if the response rate in the second stage was between 15% and 30%. A total sample size of at least 40 evaluable patients was needed in order to keep the type I and type II errors below 10%. Analyses of pharmacokinetic parameters are presented for the per-protocol population, defined as those patients who received an increased dose in cycle 2 according to protocol.
The distribution of patient characteristics are expressed as percentages or median values. Pharmacokinetic parameters are presented as means±SD or medians (range) and were compared between populations using the non-parametric Kruskal-Wallis test. Median follow-up was calculated according to the Kaplan-Meier method using death status as the censoring indicator [26]. The log-rank test was used to identify prognostic factors for PFS and OS. A backward stepwise multivariate Cox regression analysis was performed on the variables significant in the univariate analysis in order to define a prognostic index for PFS and OS using clinical variables. Separate prognostic models were tested for clinical and pharmacokinetic variables. The pharmacokinetic data were then evaluated for their potential role as a prognostic factor, adjusted on important clinical variables.
Results
Patient characteristics
Between May 1997 and December 1999, 54 patients from two centres were enrolled. One patient was considered ineligible since she had non-measurable disease and a PS of 3. The characteristics for the 53 eligible patients are presented in Table 1. Nine patients (17%) had a non-resected primary. Of 16 patients (30%) previously treated with chemotherapy, 4 had received 5-FU in the adjuvant setting and 12 had received chemotherapy for metastatic disease (CPT-11 8 patients, 5-FU 3 patients, raltitrexed+oxaliplatin 1 patient). These latter patients (23%) were considered in second-line treatment. Of the 53 patients, 25 (47%) had only one site involved, 26 (49%) had two sites involved and 2 (4%) had three sites involved. The most frequent sites were liver (91%), lung (40%) and lymph nodes (17%). Median follow-up was 38 months (range 4–43 months).
Drug exposure
Chemotherapy
A total of 435 chemotherapy cycles were administered during the study (301 for the first six cycles of treatment). Of the 53 patients, 45 (85%) received at least six cycles and 26 (49%) received more than six cycles.
All patients received 2000 mg/m2 of 5-FU in cycle 1. Table 2 indicates 5-FU dose adaptation compliance in cycle 2 according to the initial AUC value obtained after cycle 1. In cycle 2, depending on the value of the AUC in cycle 1, 46 patients (87%) received an increased dose according to protocol. Dose was not increased in cycle 2 for seven patients for the following reasons: toxicity in four patients (grade 4 neutropenia, grade 3 nausea/vomiting, fever, and thoracic pain), no AUC data for cycle 1 for one patient, and the AUC value was not taken into account by the clinician for two patients. The patient with grade 4 neutropenia discontinued treatment after cycle 1. Among these seven patients, four (7%) never received an increased dose during any of the following cycles due to toxicity.
Overall, 49 patients (93%) received an increased dose at least once during treatment. Eight patients (15%) discontinued treatment prematurely before six cycles for the following reasons: PD (four patients), toxicity (three patients) and one patient died from PD in cycle 5. Among the 46 patients who received an increased dose in cycle 2 (per-protocol population), 33 (72%) were able to maintain the same dose increase for the following five cycles. Three patients stopped treatment after five cycles due to PD and ten patients received a decreased dose due to toxicity: grade 4 neutropenia (two patients, cycle 5), grade 3 neutropenia (four patients, cycles 3, 4, 5 and 6), grade 3 diarrhoea (one patient, cycle 4), grade 3 nausea/vomiting (one patient, cycle 6), and grade 3 mucositis (two patients, cycles 3 and 5). During cycles 2 to 6, infusions were delayed by 7 days or more for toxicity in 11 patients (21%) and 12 cycles (4%).
Pharmacokinetics
A total of 101 pharmacokinetic 5-FU assays were performed on the 53 eligible patients. Pharmacokinetic data were available for 46 patients for both cycles 1 and 2 (87%). One patient did not have pharmacokinetic data calculated for cycle 1 and six other patients did not have pharmacokinetic data calculated for cycle 2. Mean AUC values for cycles 1 and 2 were 9.1±3.2 mg·h/l·m2 (range 2.8–18.7 mg·h/l·m2) and 14.7±3.9 mg·h/l·m2 (range 3.1–22.3 mg·h/l·m2), respectively. AUC values for cycle 2 were significantly greater than the values for cycle 1 (P<0.0001). AUC as a function of percentage dose increase is presented in Fig. 2. The median increases in AUC values between cycles 1 and 2 were 10%, 22% and 100% in the 25%, 50% and 100% dose increase groups, respectively.
AUC (mean±SD) measured during cycles 1 and 2 in relation to percent 5-FU dose increase in cycle 2. AUC cycle 1 (○) determined the percent 5-FU infusion dose increase according to the protocol schedule. AUC cycle 2 (△) after dose adaptation was roughly the same whatever the 5-FU infusion dose increase
The overall median dose intensity for the first six cycles was 1282 mg/m2 per week (range 497–1545 mg/m2 per week). The median relative dose intensity, calculated as the median of the ratio of the actual dose intensity and the expected dose intensity derived from the cycle-1 AUC, was 0.95 (range 0.4–1.03). Compared to the standard LV5FU2 regimen without a dose adaptation scheme, the median relative dose intensity was 1.28 (range 0.5–1.54). The median total dose during the first six cycles was 16,788 mg/m2 (range 2,000–18,444 mg/m2) compared to 12,000 mg/m2 for the standard LV5FU2 regimen.
In the per-protocol patient population, AUC, clearance and dose parameters as a function of two groups (25% and 50% versus 100% dose increase) are presented in Table 3. AUC and clearance in cycle 1 were significantly different between the two groups as defined by protocol. For cycle 2, no difference in AUC was observed. However, clearance, 5-FU dose, total dose and dose intensity were significantly greater in the group with 100% dose increase. Relative dose intensity calculated with respect to the dose intensity derived from the cycle-1 AUC was not significantly different between the two groups.
Toxicity
The incidence of grade 3/4 toxicities are listed in Table 4 for the first six cycles and per patient. No deaths were attributed to toxicity of 5-FU despite the high doses administered. One patient died from PD in cycle 5 during treatment. Two patients were hospitalized due to severe adverse events, one for chest oppression occurring during cycles 3 and 4 and leading to discontinuation of the treatment and one for febrile aplasia during cycle 1.
Grade 3/4 neutropenia occurred during 9% of cycles and in 25% of patients during the first six cycles of treatment (Table 4). Eight patients (15%) experienced grade 4 neutropenia. The main grade 1 or worse non-haematological toxicities were nausea/vomiting (24% of cycles, 57% of patients), diarrhoea (24%, 53%), asthenia (27%, 56%), mucositis (24%, 55%), alopecia (22%, 49%), and hand-foot syndrome (12%, 35%). Hand-foot syndrome was common but remained mild during the first six cycles with 21 grade 1 cycles (12 patients), 11 grade 2 cycles (5 patients) and 2 grade 3 cycles (1 patient). No grade 4 hand-foot syndrome was observed. Cardiac toxicity, frequently associated with high doses of 5-FU, affected four patients (three grade 1, and one grade 3) and six cycles including five cycles with grade 1. Few grade 4 haematological toxicities were observed (13 cycles, 8 patients). Two patients experienced grade 4 nausea/vomiting and mucositis during one cycle.
Toxicities beyond cycle 6 were infrequent with only three patients with grade 4 anaemia during cycle 10, one grade 3 hand-foot syndrome during cycle 9 and one grade 3 asthenia during cycle 10.
Tumour response
One patient was not evaluable for response since he discontinued treatment for toxicity after cycle 1. There were 19 objective responses (2 complete and 17 partial), 17 patients in SD and 16 patients with PD at the first evaluation (Table 5). The objective response rate for the 52 eligible and evaluable patients was 37%. More favourable response rates were observed for patients treated in first-line chemotherapy (objective response rate 40%, 95% CI 24%–56%) and for first-line patients who respected the dose increase according to protocol. In this latter group (per-protocol and first-line population), the objective response rate was 47% (95% CI 29%–65%). The median response duration was 10.4 months, 11.2 and 7.2 months for patients in first- and second-line therapy, respectively.
Survival
Progression-free survival
Median PFS was 7 months with a 1-year PFS rate of 32% (95% CI 20.1%–44.7%). Among the patients who responded or were in SD, four had been surgically treated with complete resection of metastasis (three liver and one lung resection). At the time of analysis, these four patients were still alive without disease at 25, 38, 41 and 44 months. Univariate analyses showed that survival curves were significantly poorer for patients with more than one metastatic site vs only one site (1-year PFS 26% vs 36%, P=0.027), second-line vs first-line treatment (1-year PFS 17% vs 35%, P=0.015), weight ≤65 kg vs >65 kg (1-year PFS 19% vs 48%, P=0.063), BSA ≤1.7 m2 vs >1.7 m2 (1-year PFS 19% vs 42%, P=0.083), initial haemoglobin level <11 g/dl vs ≥11 g/dl (1-year PFS 13% vs 35%, P=0.017), and poor PS (1-year PFS 32%, 44%, and 0% for PS 0, 1 and 2, respectively, P<0.0001). Weight and BSA were at the limit of statistical significance (P=0.063 and P=0.083), whereas all the pharmacokinetic variables were non-significant.
Overall survival
At the time of analysis, 43 patients (81%) had died. Ten patients were still alive at a median follow-up of 38 months (range 4–43 months). Median survival of the entire population was 18.6 months with a 2-year survival rate of 32% (95% CI 20%–45%). Univariate analysis showed that survival rates were significantly poorer for patients with more than one metastatic site compared to only one site (2-year OS 17% vs 48%, P=0.003), second-line vs first-line treatment (2-year OS 8% vs 40%, P=0.012), initial haemoglobin level <11 g/dl vs ≥11 g/dl (2-year OS 15% vs 40%, P=0.005) and poor PS (2-year OS 37%, 30% and 0% for PS 0, 1 and 2, respectively, P<0.0001). All the pharmacokinetic variables were non-significant.
Multivariate analysis
Clinical variables
A backward stepwise multivariate Cox regression analysis was performed on the variables significant in the univariate analysis (Table 6). As expected, PS was the most important prognostic factor and the multivariate analysis was performed on patients with PS 0 or 1, since PS 2 already defined a poor prognostic category. For patients with PS 0 or 1, a prognostic score was obtained from the sum of the following three indices: second-line treatment (+1), two or more metastatic sites (+1), and initial haemoglobin level <11 g/dl (+1). Good-prognosis patients (31 patients) had a score of 0 or 1 and PS 0 or 1. Poor-prognosis patients (19 patients) had a score of 2 or 3, or PS 2. From this classification, good and poor prognostic groups had a median PFS of 10.4 and 4.1 months and a median OS of 24.7 and 7.6 months, respectively. The objective response rates were not significantly different (48% and 22%, respectively, P=0.07).
Pharmacokinetic variables
A statistically significant interaction was found between AUC attained in cycle 2 (≤15 vs >15 mg·h/l·m2) and BSA (≤1.7 vs >1.7 m2) indicating that patients with a small body area (≤1.7 m2) and who did not attain an AUC of at least 15 mg·h/l·m2 in cycle 2 had an unfavourable PFS as compared to the other patients (median PFS 4.4 vs 9.2 months, P=0.016). However, this interaction was not significant when stratified on the prognostic index status (P=0.071), but remained significant in the good-prognosis group (P=0.018). No pharmacokinetic variables were significant for OS.
Discussion
The present phase II trial confirms part of the preliminary results of a feasibility study conducted in our institution [31], demonstrating that individual 5-FU continuous dosing, adapted to the 5-FU plasma concentration in cycle 1, could be performed according to a predefined protocol. In our present study, the planned infusion dose increase was applicable across the next cycles for most of the patients (87%) without inducing severe toxic effects or deaths as it seemed to improve efficacy.
Despite the fact that 5-FU is a prodrug that undergoes a series of intracellular conversions to active cytotoxic species, there is an increasing body of evidence that relates plasma 5-FU concentrations to toxicity and efficacy. Gamelin et al. [13] and Hillcoat et al. [15] have shown a dose-response relationship between 5-FU plasma level and response, but the serious toxicities observed restricted the application of intensive dose strategies. Moreover, a great variability in 5-FU plasma levels during cycle 1 has been noted previously [23, 30]. These data justified 5-FU dose adjustment depending on pharmacokinetic parameters, as previously shown by several investigators [11, 12, 22]. Gamelin et al. [13] investigated 5-FU therapeutic intensification with individual dose adjustment in the treatment of patients with colorectal metastatic cancer. A weekly dose of 5-FU was administered as an 8-h infusion with leucovorin. The dose of 5-FU was adapted weekly according to 5-FU plasma levels in order to reach 5-FU plasma levels in the range 2000 to 3000 μl/l.
In our study, we investigated 5-FU individual dose adjustment with the LV5FU2 schedule, which is one of the most frequently used schedules in Europe for the treatment of colorectal metastatic cancer. The objective response rates, 37% in evaluable and 47% in first-line patients, were higher than those seen with the classical LV5FU2 regimen (32.6%) [7], and even with the simplified bimonthly regimen (37%) using doses ranging from 2800 to 4000 mg/m2 depending on the number of cycles and tolerability of the total 5-FU dose [9, 29]. These response rates are consistent with the 43% response rate observed by Gamelin et al. [13], and were not far from those obtained in phase III trials with first-line bimonthly regimens, 39% with irinotecan [9] and 51% with oxaliplatin [8], respectively. The median PFS of 7.6 months in first-line patients observed in our study compares well with the best observed median PFS of 8.2 months reported by de Gramont et al. with the Folfox 4 regimen [8]. The median OS, 19.2 months among first-line patients, is among the best reported [13] and is slightly higher than that observed with 5-FU combined with irinotecan (17.4 months) (keeping in mind, however, that patient characteristics may be different between phase II and phase III trials) [9]. These encouraging results for OS may be explained by the fact that new drugs such as irinotecan or oxaliplatin could be used in case of progression in second-line treatment and thus have an impact on extending survival.
Considering the high doses of 5-FU administered, the incidence of grade 3/4 toxic events remained low, particularly neutropenia, diarrhoea and hand-foot syndrome, compared with various other studies in which intensified 5-FU doses or combined regimens were used [8, 9, 22]. This adaptation schedule avoided early life-threatening toxicities: four patients had an initial AUC value ≥15 mg·h/l·m2; the 5-FU dose was increased by 25% in three patients and decreased in one patient due to toxicity. Among these patients, important but transient grade 3/4 haematological toxicities were observed in two patients. Persistent but tolerable hand-foot syndrome was observed from cycle 2 onwards in three patients who received a 25% dose increase, and who in spite of this toxicity, continued their treatment for 12 cycles and showed SD (one patient developed cardiac pain during cycle 2). If we had used the simplified bimonthly regimen of 5-FU high-dose continuous infusion right from the start with 2800 mg/m2 5-FU total dose, these four patients certainly would have been exposed to much higher levels of toxicity, which may have compromised the continuation and the relative efficacy of the treatment.
Thus, this individual pharmacokinetically adapted regimen represents a good compromise between maximum efficacy and controlled tolerability because it allowed a higher plasma level of 5-FU in patients with lower initial AUC values, thus potentially enhancing its efficacy with tolerable toxicity. It avoided using high levels of 5-FU which could have induced severe toxicities in patients with higher AUC during cycle 1. This is consistent with the assertion of Gamelin et al. that dose calculation based on BSA alone [13] is inappropriate and will not lead to optimal levels of 5-FU, and thus would inevitably reduce the efficacy of the treatment. Our study showed that the 5-FU dose administered with the standard LV5FU2 regimen is not appropriate because the 5-FU continuous infusion dose used in our regimen from cycle 2 onwards compared with that of cycle 1 (by the classical bimonthly schedule) could be doubled in 34 of 53 patients (64%) without necessarily compromising tolerability.
The adaptation schedule described here was derived from the wide variability in AUC data observed during cycle 1 in our previous study [31]. As expected, the interindividual variability of AUC was smaller during cycle 2, after 5-FU dose adjustment, than during cycle 1.
As expected, the results of the univariate analysis indicated that a good PS, first-line treatment, one metastatic site and elevated haemoglobin levels were the most important clinical prognostic factors in terms of response, PFS and OS. Also, the pharmacokinetic parameters (AUC) and BSA interacted to isolate a potential subgroup of patients with a poor prognosis for PFS, which included patients with a small BSA (≤1.7 m2) who did not attain an AUC value of at least 15 mg·h/l·m2. This result may suggest that the adaptation schedule used in this protocol may need to be adapted differently according to BSA, for example by using a different 5-FU dose increase pattern for patients with a BSA ≤1.7 m2. In the multivariate analysis, this interaction was marginally significant when stratified on prognostic status but held up in good-prognosis patients. Overall, these results show that 5-FU dose adjustment can be correctly performed, but there may still be some room for improvement in patients with a small BSA.
In practice, this pharmacokinetic follow-up with measurements of a few plasma samples appeared to be a feasible method. It helped to detect dihydropyrimidine dehydrogenase (DPD) partial deficiencies and prevent toxicity. For patients with high levels of DPD activity, that can lead to an under-dosage with conventional regimens, the individual dose adjustment allows more accurate increases in 5-FU dose. Patients with reduced fluorouracil catabolism are more likely to be exposed to severe toxicity. The measurement of DPD activity in patients cannot be considered fully predictive, and the role of DPD gene variants in this syndrome has yet to be clarified. Thus, such pharmacokinetic monitoring appears to be more reliable and easy to perform.
Individual 5-FU dose adaptation based on pharmacokinetic parameters within a LV5FU2 regimen enables therapeutic drug monitoring and could then be used as first-line treatment in patients with non-resectable metastases. This strategy could be compared in phase III trial with standard LV5FU2 regimen.
References
Advanced Colorectal Cancer Meta-Analysis Project (1992) Modulation of fluorouracil by leucovorin in patients with advanced colorectal cancer: evidence in terms of response rate. J Clin Oncol 10:896
Andre T, Louvet C, Raymond E, Tournigand C, de Gramont A (1998) Bimonthly high-dose leucovorin, 5-fluorouracil infusion and oxaliplatin (FOLFOX3) for metastatic colorectal cancer resistant to the same leucovorin and 5-fluorouracil regimen. Ann Oncol 9:1251
Andre T, Bensmaine MA, Louvet C, Francois E, Lucas V, Desseigne F, Beerblock K, Bouche O, Carola E, Merrouche Y, Morvan F, Dupont-Andre G, de Gramont A (1999) Multicenter phase II study of bimonthly high-dose leucovorin, fluorouracil infusion, and oxaliplatin for metastatic colorectal cancer resistant to the same leucovorin and fluorouracil regimen. J Clin Oncol 17:3560
Ardalan B, Chua L, Tian EM, Reddy R, Sridhar K, Benedetto P, Richman S, Legaspi A, Waldman S, Morrell L, et al (1991) A phase II study of weekly 24-hour infusion with high-dose fluorouracil with leucovorin in colorectal carcinoma. J Clin Oncol 9:625
Beal SL (1992) NONMEM user's guide. University of California at San Francisco
Bressolle F, Joulia JM, Pinguet F, Ychou M, Astre C, Duffour J, Gomeni R (1999) Circadian rhythm of 5-fluorouracil population pharmacokinetics in patients with metastatic colorectal cancer. Cancer Chemother Pharmacol 44:295
de Gramont A, Bosset JF, Milan C, Rougier P, Bouche O, Etienne PL, Morvan F, Louvet C, Guillot T, Francois E, Bedenne L (1997) Randomized trial comparing monthly low-dose leucovorin and fluorouracil bolus with bimonthly high-dose leucovorin and fluorouracil bolus plus continuous infusion for advanced colorectal cancer: a French intergroup study. J Clin Oncol 15:808
de Gramont A, Figer A, Seymour M, Homerin M, Hmissi A, Cassidy J, Boni C, Cortes-Funes H, Cervantes A, Freyer G, Papamichael D, Le Bail N, Louvet C, Hendler D, de Braud F, Wilson C, Morvan F, Bonetti A (2000) Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol 18:2938
Douillard JY, Cunningham D, Roth AD, Navarro M, James RD, Karasek P, Jandik P, Iveson T, Carmichael J, Alakl M, Gruia G, Awad L, Rougier P (2000) Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet 355:1041
Erlichman C, Fine S, Elhakim T (1986) Plasma pharmacokinetics of 5-FU given by continuous infusion with allopurinol. Cancer Treat Rep 70:903
Fety R, Rolland F, Barberi-Heyob M, Merlin JL, Conroy T, Hardouin A, Riviere A, Milano G (1994) Clinical randomized study of 5FU monitoring versus standard dose in patients with head and neck cancer: preliminary results. Anticancer Res 14:2347
Gamelin EC, Danquechin-Dorval EM, Dumesnil YF, Maillart PJ, Goudier MJ, Burtin PC, Delva RG, Lortholary AH, Gesta PH, Larra FG (1996) Relationship between 5-fluorouracil (5-FU) dose intensity and therapeutic response in patients with advanced colorectal cancer receiving infusional therapy containing 5-FU. Cancer 77:441
Gamelin E, Boisdron-Celle M, Delva R, Regimbeau C, Cailleux PE, Alleaume C, Maillet ML, Goudier MJ, Sire M, Person-Joly MC, Maigre M, Maillart P, Fety R, Burtin P, Lortholary A, Dumesnil Y, Picon L, Geslin J, Gesta P, Danquechin-Dorval E, Larra F, Robert J (1998) Long-term weekly treatment of colorectal metastatic cancer with fluorouracil and leucovorin: results of a multicentric prospective trial of fluorouracil dosage optimization by pharmacokinetic monitoring in 152 patients. J Clin Oncol 16:1470
Gamelin E, Jacob J, Danquechin-dorval E, Pezet D, Delva R, Raoul J (1998) Multicentric randomized trial comparing in weekly treatment of advanced colorectal cancer (CRC) intensified 5-fluorouracil and folinic acid (FA) with 5-FU pharmacokinetic monitoring to a constant dose calculated with body surface area (Meeting abstract). American Society of Clinical Oncology, Alexandria, VA, p 270
Hillcoat BL, McCulloch PB, Figueredo AT, Ehsan MH, Rosenfeld JM (1978) Clinical response and plasma levels of 5-fluorouracil in patients with colonic cancer treated by drug infusion. Br J Cancer 38:719
Hryniuk WM, Figueredo A, Goodyear M (1987) Applications of dose intensity to problems in chemotherapy of breast and colorectal cancer. Semin Oncol 14:3
Joulia JM, Pinguet F, Ychou M, Duffour J, Topart D, Grosse PY, Astre C, Bressolle F (1997) Pharmacokinetics of 5-fluorouracil (5-FUra) in patients with metastatic colorectal cancer receiving 5-FUra bolus plus continuous infusion with high dose folinic acid (LV5FU2). Anticancer Res 17:2727
Kohne CH, Schoffski P, Wilke H, Kaufer C, Andreesen R, Ohl U, Klaasen U, Westerhausen M, Hiddemann W, Schott G, Harstick A, Bade J, Horster A, Schubert U, Hecker H, Dorken B, Schmoll HJ (1998) Effective biomodulation by leucovorin of high-dose infusion fluorouracil given as a weekly 24-hour infusion: results of a randomized trial in patients with advanced colorectal cancer. J Clin Oncol 16:418
Levi F, Giacchetti S, Adam R, Zidani R, Metzger G, Misset JL (1995) Chronomodulation of chemotherapy against metastatic colorectal cancer. International Organization for Cancer Chronotherapy. Eur J Cancer 31A:1264
Levi F, Soussan A, Adam R, Caussanel JP, Metzger G, Jasmin C, Bismuth H, Smolensky M, Misset JL (1995) A phase I-II trial of five-day continuous intravenous infusion of 5- fluorouracil delivered at circadian rhythm modulated rate in patients with metastatic colorectal cancer. J Infus Chemother 5:153
Levi F, Zidani R, Misset JL (1997) Randomised multicentre trial of chronotherapy with oxaliplatin, fluorouracil, and folinic acid in metastatic colorectal cancer. International Organization for Cancer Chronotherapy. Lancet 350:681
Milano G, Roman P, Khater R, Frenay M, Renee N, Namer M (1988) Dose versus pharmacokinetics for predicting tolerance to 5-day continuous infusion of 5-FU. Int J Cancer 41:537
Milano G, Etienne MC, Cassuto-Viguier E, Thyss A, Santini J, Frenay M, Renee N, Schneider M, Demard F (1992) Influence of sex and age on fluorouracil clearance. J Clin Oncol 10:1171
Poon MA, O'Connell MJ, Wieand HS, Krook JE, Gerstner JB, Tschetter LK, Levitt R, Kardinal CG, Mailliard JA (1991) Biochemical modulation of fluorouracil with leucovorin: confirmatory evidence of improved therapeutic efficacy in advanced colorectal cancer. J Clin Oncol 9:1967
Santini J, Milano G, Thyss A, Renee N, Viens P, Ayela P, Schneider M, Demard F (1989) 5-FU therapeutic monitoring with dose adjustment leads to an improved therapeutic index in head and neck cancer. Br J Cancer 59:287
Shuster JJ (1991) Median follow-up in clinical trials. J Clin Oncol 9:191
Spicer DV, Ardalan B, Daniels JR, Silberman H, Johnson K (1988) Reevaluation of the maximum tolerated dose of continuous venous infusion of 5-fluorouracil with pharmacokinetics. Cancer Res 48:459
Thyss A, Milano G, Renee N, Vallicioni J, Schneider M, Demard F (1986) Clinical pharmacokinetic study of 5-FU in continuous 5-day infusions for head and neck cancer. Cancer Chemother Pharmacol 16:64
Tournigand C, A de Gramont, Louvet C, Andre T, Carola E, Gilles-Amar V, Maindrault-Goebel F, Lotz JP, Molitor JL, Izrael V, Ecstein-Fraisse E, Krulik M (1998) A simplified bi-monthly regimen with leucovorin (LV) and 5-fluorouracil (5FU) for metastatic colorectal cancer (MCRC) (Meeting abstract). American Society of Clinical Oncology, Alexandria, VA, p 274
Vokes EE, Ratain MJ, Mick R, McEvilly JM, Haraf D, Kozloff M, Hamasaki V, Weichselbaum RR, Panje WR, Wenig B, et al (1993) Cisplatin, fluorouracil, and leucovorin augmented by interferon alfa-2b in head and neck cancer: a clinical and pharmacologic analysis. J Clin Oncol 11:360
Ychou M, Duffour J, Pinguet F, Kramar A, Joulia JM, Topart D, Bressolle F (1999) Individual 5FU-dose adaptation schedule using bimonthly pharmacokinetically modulated LV5FU2 regimen: a feasibility study in patients with advanced colorectal cancer. Anticancer Res 19:2229
Acknowledgement
We thank the "Ligue contre le Cancer" for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ychou, M., Duffour, J., Kramar, A. et al. Individual 5-FU dose adaptation in metastatic colorectal cancer: results of a phase II study using a bimonthly pharmacokinetically intensified LV5FU2 regimen. Cancer Chemother Pharmacol 52, 282–290 (2003). https://doi.org/10.1007/s00280-003-0658-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-003-0658-0