Abstract
Monomorphic epitheliotropic intestinal T-cell lymphoma (MEITL) is a provisional entity in the 2017 World Health Organization classifications. To further elucidate the clinicopathologic features of this new disease, we carried out a retrospective, multicenter analysis of 42 patients with MEITL. The median age of the patients was 59 years (range, 20–84 years), and 27 patients (64 %) were male. Thirty-two patients (76 %) were Ann-Arbor stages I–II and 28 (67 %) were Lugano stages I–II1&2. The most frequent site of involvement was the jejunum (N = 21). Most cases expressed CD8 (79 %) and CD56 (95 %) and did not express CD30 (5 %) or EBER (0 %). The median progression-free survival was 6.9 months (95 % CI 4.3–9.6); the median OS was 14.8 months (2.4–27.2). Thirty-two patients (76 %) underwent surgery and 37 (88 %) received chemotherapy. A complete response (CR) rate was 38 %. Sixteen patients had undergone autologous stem cell transplantation (ASCT). Relapse or progression was documented in 24 cases, most frequently in the primary site (N = 23). Four cases showed central nervous system relapse. Age over 55 years, poor performance scale, advanced Lugano stage (IIE–IV), not achieving CR, and not receiving ASCT were associated with inferior OS. While the optimal management of MEITL remains undetermined, achieving CR and consolidative ASCT seem essential. As CHOP might be insufficient for achieving CR, more efficient combinations should be investigated. Additionally, considering the frequent local failure and CNS relapse, novel therapeutic approaches are required to improve survival.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
While the gastrointestinal tract (GIT) is the most common site of primary extranodal non-Hodgkin’s lymphoma (NHL), peripheral T-cell lymphoma (PTCL) arising from the GIT is relatively rare [1, 2]. Until the 1990s, intestinal PTCLs were considered to be sequelae of long-standing celiac disease. However, reports of intestinal PTCLs without prior enteropathy [3] suggested the presence of another subtype with different morphologic features lacking prior enteropathy. In the 2008 World Health Organization (WHO) classification, the disease was named enteropathy-associated T-cell lymphoma (EATL). Among them, 10–20 % of cases show monomorphic small- to medium-sized cells without enteropathy and were classified as a type II variant [4]. Subsequent studies have reported that, in addition to the absence of prior enteropathy and predominance in Asia and South America, there were differences in immunophenotypes and molecular features compared with type I EATL [5,6,7,8,9,10]. These studies have shown that type II EATLs present monomorphic small-to-medium-sized cells expressing CD8 and CD56, which are usually of γδ T-cell origin. In addition, these tumors were enriched in JAK2 and SETD2 mutations. Based on these observations, the revised 4th WHO classification in 2017 proposed monomorphic epitheliotropic intestinal T-cell lymphoma (MEITL) as a new disease entity, instead of type II EATL [11].
Although these two diseases have been segregated, one of their common features is their very poor prognosis. If untreated, patients will invariably die within several months due to multifocal intestinal perforation or bleeding. Currently, induction chemotherapy followed by autologous stem cell transplantation (ASCT) is considered the standard strategy, as with other subtypes of PTCLs. However, owing to the paucity of cases, the roles of induction therapy, ASCT, and surgery have not been evaluated in large-scale analyses. This multicenter, retrospective analysis involving 12 tertiary institutes in Korea was performed to further elucidate the clinicopathologic features and clinical outcomes of MEITL.
Patients and methods
Patients
Patients diagnosed with MEITL (type II EATL) from 2002 to 2017 were included in the analysis. The inclusion criteria were as follows: (1) histologically confirmed diagnosis; (2) availability of full medical and pathologic reports for central review. Patient medical records, including age; sex; stage; presenting symptoms; laboratory findings including complete blood count, lactate dehydrogenase (LDH), and C-reactive protein (CRP) levels; primary sites; treatment modalities; and treatment outcomes were collected.
Compliance with ethical standards
All authors declare that he or she has no conflict of interest. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The exemptions of obtaining the informed consents were approved by the institutional review board of each institute. This study was supported by the Chung-Ang University Research Grants in 2017.
Analysis
For staging, the results of standard procedures including computed tomography (CT), 18F-fluorodeoxyglucose positron emission tomography (PET)-CT, and bone marrow examinations were centrally reviewed. The stages were defined by the Ann-Arbor [12] and Lugano systems for gastrointestinal lymphoma [13]. The histopathologic diagnoses of MEITL were centrally reviewed by two experienced lymphoma histopathologists. Immunohistochemical staining for Ki-67, CD3, CD4, CD8, CD30, CD56, Epstein-Barr virus-encoded RNA (EBER), and T-cell intracellular antigen-1 (TIA1) was carried out according to the protocols of the institutes. The T-cell receptor (TCR) gene clonal rearrangement was examined in available cases.
The primary endpoint of the analysis was overall survival (OS), which was calculated from the date of diagnosis to the date of death using the Kaplan-Meier method. The secondary endpoint was the complete response (CR) rate, as defined by complete metabolic and radiologic responses by Lugano classification [12]. The OS of each prognostic subgroup was compared using log-rank tests. CRs were compared by Pearson’s λ2 tests. For all statistical analyses, p < 0.05 was considered significant, and the analyses were performed using the IBM SPSS Statistics for Windows, version 21.0 (IBM Corp., USA).
Results
Patient characteristics
A total of 42 patients were analyzed. Their median age was 59 years (range 20–84 years), and 27 patients (64 %) were male. The Eastern Cooperative Oncology Group performance scales (ECOG-PS) were 0–1 in 23 patients (55 %) and 2–4 in 19 patients (45 %). None of the patients had a history of celiac disease. By the Ann-Arbor staging system, 11 (26 %), 21 (50 %), and 10 patients (24 %) were classified as stage I, II, and IV, respectively. By the Lugano staging system, eight (19 %), 19 (45 %), one (2 %), five (12 %), and nine patients (21 %) were classified as stages I, II1, II2, IIE, and IV, respectively. Two patients with multiple, non-contiguous involvement confined to the GIT were classified as stage IV by the Ann-Arbor system and as stage I by the Lugano system.
The most commonly involved primary site was the jejunum (N = 21, 50 %), followed by the ileum (N = 19, 45 %), large intestine (N = 13, 31 %), and stomach (N = 2, 5%). Fifteen patients (36 %) had a multiple-site involvement. Extranodal involvements outside of the GIT, such as the liver (N = 1), spleen (N = 1), or lung (N = 2), were relatively rare; among 21 patients with central nervous system (CNS) imaging, none was involved. Further, of the 38 patients who had undergone bone marrow biopsy, only one (3 %) had involvement. The presenting symptoms included abdominal pain in 36 (86 %), diarrhea in 14 (33 %), perforation in 12 (29 %), bleeding in seven (17 %), and poor oral intake in seven patients (17 %). B symptoms were present in 20 patients (48 %), and bulky disease, defined as the largest diameter greater than 5 cm, was present in 12 patients (29 %). LDH elevation was noted in 11 of 38 patients (29 %), and the median CRP value was 2.8 mg/dL (range, 0.01–325.0). The details are described in Table 1.
Pathologic features
All 42 cases expressed CD3. CD8 staining was performed in 39 cases, 31 cases (79 %) of which were positive, while only four of 37 cases (11 %) expressed CD4. Two representative markers of MEITL CD56 and TIA1 were highly expressed in our cohort, with positivity in 39 of 41 (95 %), and 14 out 17 examined cases (82 %), respectively. Only one of 21 examined cases expressed CD30 and none of the 30 cases in which EBER was examined was positive. TCR gene clonal rearrangement was examined in seven cases; four of which were of γδ T-cell origin (57 %). The details are described in Table 2.
Clinical outcomes
Surgery was performed in 32 cases (76 %); the reasons for the surgery included histologic confirmation (20 cases), management of initial complications (10 cases), and management of recurrent disease (two cases). Chemotherapy was administered to 37 patients (88 %) and the other 5 patients (12 %) underwent surgery alone. CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisolone) was the most frequently used frontline regimen (N = 30, 71 %), followed by CHOEP (CHOP plus etoposide, N = 3, 7 %), ICE (ifosfamide, carboplatin, and etoposide, N = 1, 2 %), IMVP-16 (ifosfamide, methotrexate, etoposide, and prednisone, N = 1, 2 %), EPOCH (etoposide, prednisolone, vincristine, cyclophosphamide, and doxorubicin, N = 1, 2 %), and ESHAP (etoposide, methylprednisolone, cytarabine, and cisplatin, N = 1, 2 %). With these combinations, 16 of 37 patients (43 %) achieved a CR, six achieved partial responses (PRs, 17 %), one had stable disease (2 %), and seven had progressive disease (19 %). There was a trend for a higher CR rate among patients who had received other than CHOP (5/7, 71 %) compared with those who had received CHOP (11/30, 37 %) (p = 0.095). Hematopoietic stem cell transplantation was performed in 16 patients, all of which were autologous stem cell transplantation (ASCT). Up-front and salvage ASCT was performed in 9 and 7 patients, respectively.
The median progression-free survival (PFS) of all patients was 6.9 months (95 % confidence interval [CI] 4.3–9.5). Among 24 patients with documented progressions, 23 (96 %) had progression in the primary gastrointestinal sites, six (25 %) had locoregional lymph node progression, and nine (38 %) had progression in distant organs. Of note, four cases had progression in the CNS. The median OS of all patients was 14.8 months (95 % CI 2.4–27.2). The 1- and 3-year OS rates were 57 % and 26 %, respectively. Among the 16 patients who had received ASCT, the 1- and 5-year OS rates were 100 % and 28 %, respectively. The details are described in Table 3.
Univariate analysis of OS
Patients aged 55 years or older showed a shorter OS compared to that in patients younger than 55 years (8.8 months, 95 % CI 0.0–18.0 vs. 25.7 months, 95 % CI 6.5–44.9, p = 0.012). Patients with a poor performance scale (ECOG-PS 2–4) were associated with a poor OS (6.5 months, 95 % CI 1.9–11.1 vs. 22.1 months, 95 % CI 2.3–41.9, p = 0.002). The Lugano system more accurately predicted OS than the Ann-Arbor system. By the Lugano system, the OS for patients classified as stages I–II1&2 was 18.8 months (95 % CI 9.6–28.0) and that of the patients classified as stages IIE–IV was 4.9 months (95 % CI 0.0–12.7) (p = 0.010). In contrast, the Ann-Arbor system was not able to discriminate the prognosis of patients between stages I–II (14.8 months, 95 % CI 4.5–25.1) and stages III–IV (8.7, 3.9–13.5) (p = 0.211). As expected, patients who received any chemotherapy were associated with a better OS compared to those who had not (18.5 months, 95 % CI 8.9–28.1) vs. 1.2 months (95 % CI 1.0–1.4), p < 0.001). Analysis of 37 patients who had received chemotherapy revealed that a CR (39.1 months, 95 % CI 15.5–62.7 vs. 8.7 months, 95 % CI 4.8–12.6; p = 0.001) and receiving ASCT (31.3 months, 95 % CI 17.2–45.4, vs. 6.8 months, 95 % CI 4.0–9.6; p = 0.001) were associated with a better OS (Fig. 1). While treatment other than CHOP showed a trend of a higher CR rate, it did not translate into a better OS. Whether the patient had proceeded to ASCT in CR vs. PR or in up-front vs. salvage setting did not significantly impact OS. OS of the 4 patients who had received ASCT in first CR and that of the 7 patients who had received ASCT as salvage treatment was not significantly different (39.1 months (95 % CI not estimable) vs. 21.4 months (95 % CI 18.2–24.6), p = 0.156).
The details are described in Table 4.
Discussion
The present study analyzed the clinicopathological features of 42 patients with MEITL. Despite the limited stages of most patients, their clinical outcomes were dismal, with a median OS barely exceeding 1 year. Several subsets, such as younger age (< 55 years), better performance status (ECOG 0–1), earlier Lugano stage (I–II1&2), chemotherapy, CR, and receiving ASCT, were associated with favorable outcomes. The short PFS (6.9 months) may have contributed to the frequent relapses in the GIT, with 23 of 24 (96 %) documented relapses noted in the GIT.
The classification of NHLs continues to evolve owing to recent advances in our understanding of their molecular pathogenesis. In this regard, several new entities were proposed in the revised 4th WHO classification, including double-hit lymphoma or nodal T-cell lymphomas with T-follicular helper phenotype [11]. MEITL, formerly considered a type II EATL, is another provisional entity in this classification. In addition to their distinct morphologic and immunophenotypic findings, several molecular features differed significantly, which facilitated its segregation. Using the comparative genomic hybridization technique, Tomita et al. reported that the genomic profiles differed between types I and II EATL, as the latter showed a frequent gain of 8q24 [14]. Roberti et al. carried out comparative analyses of types I and II EATL, reporting that SETD2, STAT5B, or JAK3 mutations were exclusively found in type II EATL [9]. Küçük et al. also found enrichment of STAT5B and STAT3 mutations in type II EATL [15]. Currently, none of the agents targeting these pathways has been evaluated in MEITL. Interestingly, recent in vitro studies showed that bortezomib, AZD1775 (WEE1 inhibitor), and midostaurin inhibited the growth of SETD2 mutant cell lines [16, 17].
Owing to its paucity and recent identification, the incidence, natural course, and clinical outcomes of MEITL are poorly understood. Several studies have reported that EATL (types I and II) accounts for approximately 1 % of all NHLs [18,19,20]. These studies did not analyze type II EATL separately; however, if we assume the Asian population or those without prior celiac disease as having MEITL, approximately 20 % of patients in the cohort were expected to have MEITL. However, in Asia, where celiac disease is exceptionally rare, almost all cases of intestinal T-cell lymphoma are considered to be MEITL.
Regardless of subtype, the prognosis of EATL is dismal. While most studies reported OS of less than 1 year, several studies have demonstrated the benefit of ASCT [20,21,22,23]. The Scotland and Newcastle Lymphoma Group (SNLG) compared the efficacy of IVE/MTX (ifosfamide, vincristine, etoposide/methotrexate) followed by ASCT to that of a historical group treated with anthracycline-based chemotherapy [20]. The 5-year PFS and OS rates were significantly higher in the IVE/MTX-ASCT arm than in those treated with anthracycline-based chemotherapy (PFS, 52 % vs. 22 %, p = 0.01; OS, 60 % vs. 22 %, p = 0.03). A retrospective study by the EBMT reported 4-year PFS and OS rates of 54 % and 59 %, respectively, in 44 patients with EATL who received ASCT [22]. In a Dutch study including 61 patients with EATL [23], the 1- and 5-year OS rates were 40 % and 11 %, respectively. However, in five patients who had received ASCT, the rates were 100 % and 33 %, respectively. As all of these studies were performed in Europe, where type I EATL predominates, these results cannot be adapted to MEITL. In our study, 16 patients received ASCT; their 1- and 5-year OS rates were 100 % and 28 %, respectively; these numerical data are less favorable than those of the aforementioned European studies. Whether this is the consequence of the differences between EATL and MEITL or of differences in ethnicity requires further study. Of note, a SEER data analysis that did not distinguish EATL and MEITL demonstrated a significantly worse prognosis in Asian patients with EATL than that in Caucasian patients, despite similar ages at diagnosis and stages [19].
Successful induction treatment is a prerequisite for ASCT. CHOP has been the most widely used standard induction regimen for PTCL, as in our study. However, the CR rate of CHOP was non-significantly lower than that of non-CHOP (37 % vs. 71 %, p = 0.095). This was also observed in the SNLG report, with the CR rate of IVE/MTX slightly higher than that of anthracycline-based chemotherapy (65 % vs. 42 %, p = 0.06) [20]. Tse et al. also reported a lower CR rate of CHOP or anthracycline-based regimen (7/20 = 35 %) than that of L-asparaginase-based regimens (3/5 = 60 %) [7]. Interestingly, we have already experienced this in the treatment of NK/T-cell lymphoma (NKTCL), for which CHOP is no longer a standard of care. Iqbal et al. showed that, when comparing gene expression profiles of NKTCLs, non-hepatosplenic γδ, hepatosplenic, and αβ-PTCLs, non-hepatosplenic γδ-PTCLs had molecular features that were strikingly similar to those of NKTCLs rather than αβ-PTCLs [24]. Thus, NKTCL and MEITL may share the same mechanisms for the ineffectiveness of CHOP. Several studies have reported that CD56, the commonly expressed antigen of both diseases, is frequently associated with a higher expression of P-glycoprotein [25,26,27]. Further investigations are needed to address the proper induction regimens for MEITL (or EATL).
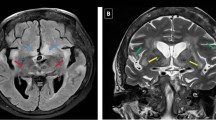
The patients in our cohort frequently experienced local relapses. Of the 24 cases of documented relapse or progression, 23 were locoregional GIT. As shown in Fig. 2, malignant T cells widely spread under normal-appearing gastrointestinal mucosa and were involved in the resection margins. Along with refractoriness to chemotherapy, this may be the cause of the frequent local relapse. Considering the limited role of chemotherapy in MEITL, more effective local control may have a role, especially in cases of bulky disease or with a remnant lesion [28, 29]. At the time of diagnosis, no CNS involvement was found, but four cases (10 %) of CNS involvements were noted during treatment. While the incidences of CNS relapse in PTLCs range from 2.1 to 8.8 % [30,31,32], that of EATL seems to be slightly higher, with CNS relapse reported in 4 of 56 patients (7 %) and 1 of 8 patients (13 %) by Ellin et al. and Yi et al., respectively. Although initial CNS evaluation and prophylaxis is not generally recommended in patients with PTCL, these data suggest their consideration in cases of MEITL.
In line with the previous studies [6, 7, 33], CD8 (79 %) and CD56 (95 %) were frequently expressed, whereas CD4 (11 %), CD30 (5 %), and EBER (0 %) were not. In terms of TCR gene analysis, three and four patients expressed TCRαβ and TCRγδ, respectively. Although MEITL is thought to originate from γδ T cells, there are considerable differences according to the reports [6, 7, 34], suggesting the heterogeneity of the disease.
Some of the pitfalls of this study include its retrospective design. Thus, there are limitations in evaluating the prognostic factors, including the role of ASCT. The favorable outcomes of 16 patients who had received ASCT may have contributed to a selection bias as they were young, with good performance status, and showed a good response to chemotherapy. Given that MEITL is a rare and newly proposed disease, international and multicenter efforts are required to carry out a prospective study. Another limitation is that the responses were examined by individual investigators, although the radiologic reports were centrally reviewed. However, as we assessed OS, we believe the conclusive data have validity. Finally, the current study did not assess the molecular features of MEITL.
In conclusion, our analysis of the clinicopathologic features of 42 MEITL patients revealed poor outcomes, in agreement with the findings of previous studies. Young age, good performance status, early Lugano stage, CR, and receiving ASCT were associated with improved OS. CHOP regimen was associated with a lower CR rate, and more efficacious combinations should be investigated. Considering the frequent local failure as well as CNS relapse, development of novel approaches is essential to improving survival. In addition, emerging understanding of molecular pathogenesis including JAK/STAT or epigenetic pathways in MEITL will lead to more precise approaches to this disease.
References
Koch P, del Valle F, Berdel WE, Willich NA, Reers B, Hiddemann W, Grothaus-Pinke B, Reinartz G, Brockmann J, Temmesfeld A, Schmitz R, Rube C, Probst A, Jaenke G, Bodenstein H, Junker A, Pott C, Schultze J, Heinecke A, Parwaresch R, Tiemann M (2001) Primary gastrointestinal non-Hodgkin’s lymphoma: I. Anatomic and histologic distribution, clinical features, and survival data of 371 patients registered in the German Multicenter Study GIT NHL 01/92. J Clin Oncol 19(18):3861–3873. https://doi.org/10.1200/jco.2001.19.18.3861
Kim SJ, Choi CW, Mun YC, Oh SY, Kang HJ, Lee SI, Won JH, Kim MK, Kwon JH, Kim JS, Kwak JY, Kwon JM, Hwang IG, Kim HJ, Lee JH, Oh S, Park KW, Suh C, Kim WS (2011) Multicenter retrospective analysis of 581 patients with primary intestinal non-hodgkin lymphoma from the Consortium for Improving Survival of Lymphoma (CISL). BMC Cancer 11:321. https://doi.org/10.1186/1471-2407-11-321
Chott A, Dragosics B, Radaszkiewicz T (1992) Peripheral T-cell lymphomas of the intestine. Am J Pathol 141(6):1361–1371
Swerdlow SH CE, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW (2008) WHO classification of tumours of haematopoietic and lymphoid tissues. WHO Classification of Tumours, 4th Edition, Volume 2. IARC
Tan SY, Ooi AS, Ang MK, Koh M, Wong JC, Dykema K, Ngeow J, Loong S, Gatter K, Tan L, Lim LC, Furge K, Tao M, Lim ST, Loong F, Cheah PL, Teh BT (2011) Nuclear expression of MATK is a novel marker of type II enteropathy-associated T-cell lymphoma. Leukemia 25(3):555–557. https://doi.org/10.1038/leu.2010.295
Chan JK, Chan AC, Cheuk W, Wan SK, Lee WK, Lui YH, Chan WK (2011) Type II enteropathy-associated T-cell lymphoma: a distinct aggressive lymphoma with frequent γδ T-cell receptor expression. Am J Surg Pathol 35(10):1557–1569. https://doi.org/10.1097/PAS.0b013e318222dfcd
Tse E, Gill H, Loong F, Kim SJ, Ng SB, Tang T, Ko YH, Chng WJ, Lim ST, Kim WS, Kwong YL (2012) Type II enteropathy-associated T-cell lymphoma: a multicenter analysis from the Asia Lymphoma Study Group. Am J Hematol 87(7):663–668. https://doi.org/10.1002/ajh.23213
Wilson AL, Swerdlow SH, Przybylski GK, Surti U, Choi JK, Campo E, Trucco MM, Van Oss SB, Felgar RE (2013) Intestinal γδ T-cell lymphomas are most frequently of type II enteropathy-associated T-cell type. Hum Pathol 44(6):1131–1145. https://doi.org/10.1016/j.humpath.2012.10.002
Roberti A, Dobay MP, Bisig B, Vallois D, Boechat C, Lanitis E, Bouchindhomme B, Parrens MC, Bossard C, Quintanilla-Martinez L, Missiaglia E, Gaulard P, de Leval L (2016) Type II enteropathy-associated T-cell lymphoma features a unique genomic profile with highly recurrent SETD2 alterations. Nat Commun 7:12602. https://doi.org/10.1038/ncomms12602
Baumgartner AK, Zettl A, Chott A, Ott G, Muller-Hermelink HK, Starostik P (2003) High frequency of genetic aberrations in enteropathy-type T-cell lymphoma. Lab Invest 83(10):1509–1516
Swerdlow SH CE, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW (2017) WHO classification of tumours of haematopoietic and lymphoid tissues. WHO Classification of Tumours, Revised 4th Edition, Volume 2
Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, Lister TA (2014) Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol 32(27):3059–3068. https://doi.org/10.1200/jco.2013.54.8800
Rohatiner A, d'Amore F, Coiffier B, Crowther D, Gospodarowicz M, Isaacson P, Lister TA, Norton A, Salem P, Shipp M, et al. (1994) Report on a workshop convened to discuss the pathological and staging classifications of gastrointestinal tract lymphoma. Annals of oncology : official journal of the European Society for Med Oncol 5 (5):397–400
Tomita S, Kikuti YY, Carreras J, Kojima M, Ando K, Takasaki H, Sakai R, Takata K, Yoshino T, Bea S, Campo E, Nakamura N (2015) Genomic and immunohistochemical profiles of enteropathy-associated T-cell lymphoma in Japan. Modern Pathol 28(10):1286–1296. https://doi.org/10.1038/modpathol.2015.85
Kucuk C, Jiang B, Hu X, Zhang W, Chan JK, Xiao W, Lack N, Alkan C, Williams JC, Avery KN, Kavak P, Scuto A, Sen E, Gaulard P, Staudt L, Iqbal J, Zhang W, Cornish A, Gong Q, Yang Q, Sun H, d'Amore F, Leppa S, Liu W, Fu K, de Leval L, McKeithan T, Chan WC (2015) Activating mutations of STAT5B and STAT3 in lymphomas derived from γδ-T or NK cells. Nat Commun 6:6025. https://doi.org/10.1038/ncomms7025
Pfister SX, Markkanen E, Jiang Y, Sarkar S, Woodcock M, Orlando G, Mavrommati I, Pai CC, Zalmas LP, Drobnitzky N, Dianov GL, Verrill C, Macaulay VM, Ying S, La Thangue NB, D'Angiolella V, Ryan AJ, Humphrey TC (2015) Inhibiting WEE1 selectively kills histone H3K36me3-deficient cancers by dNTP starvation. Cancer Cell 28(5):557–568. https://doi.org/10.1016/j.ccell.2015.09.015
Martinelli G, Mancini M, De Benedittis C, Rondoni M, Papayannidis C, Manfrini M, Meggendorfer M, Calogero R, Guadagnuolo V, Fontana MC, Bavaro L, Padella A, Zago E, Pagano L, Zanotti R, Scaffidi L, Specchia G, Albano F, Merante S, Elena C, Savini P, Gangemi D, Tosi P, Ciceri F, Poletti G, Riccioni L, Morigi F, Delledonne M, Haferlach T, Cavo M, Valent P, Soverini S (2018) SETD2 and histone H3 lysine 36 methylation deficiency in advanced systemic mastocytosis. Leukemia 32(1):139–148. https://doi.org/10.1038/leu.2017.183
Gale J, Simmonds PD, Mead GM, Sweetenham JW, Wright DH (2000) Enteropathy-type intestinal T-cell lymphoma: clinical features and treatment of 31 patients in a single center. J Clin Oncol 18(4):795–803. https://doi.org/10.1200/jco.2000.18.4.795
Karanam PK, Al-Hamadani M, Go RS (2016) Enteropathy-associated T-cell lymphoma in the US: higher incidence and poorer survival among Asians. Br J Haematol 172(6):990–992. https://doi.org/10.1111/bjh.13555
Sieniawski M, Angamuthu N, Boyd K, Chasty R, Davies J, Forsyth P, Jack F, Lyons S, Mounter P, Revell P, Proctor SJ, Lennard AL (2010) Evaluation of enteropathy-associated T-cell lymphoma comparing standard therapies with a novel regimen including autologous stem cell transplantation. Blood 115(18):3664–3670. https://doi.org/10.1182/blood-2009-07-231324
Rongey C, Micallef I, Smyrk T, Murray J (2006) Successful treatment of enteropathy-associated T cell lymphoma with autologous stem cell transplant. Dig Dis Sci 51(6):1082–1086. https://doi.org/10.1007/s10620-006-8013-z
Jantunen E, Boumendil A, Finel H, Luan JJ, Johnson P, Rambaldi A, Haynes A, Duchosal MA, Bethge W, Biron P, Carlson K, Craddock C, Rudin C, Finke J, Salles G, Kroschinsky F, Sureda A, Dreger P (2013) Autologous stem cell transplantation for enteropathy-associated T-cell lymphoma: a retrospective study by the EBMT. Blood 121(13):2529–2532. https://doi.org/10.1182/blood-2012-11-466839
Nijeboer P, de Baaij LR, Visser O, Witte BI, Cillessen SA, Mulder CJ, Bouma G (2015) Treatment response in enteropathy associated T-cell lymphoma; survival in a large multicenter cohort. Am J Hematol 90(6):493–498. https://doi.org/10.1002/ajh.23992
Iqbal J, Weisenburger DD, Chowdhury A, Tsai MY, Srivastava G, Greiner TC, Kucuk C, Deffenbacher K, Vose J, Smith L, Au WY, Nakamura S, Seto M, Delabie J, Berger F, Loong F, Ko YH, Sng I, Liu X, Loughran TP, Armitage J, Chan WC (2011) Natural killer cell lymphoma shares strikingly similar molecular features with a group of non-hepatosplenic γδ T-cell lymphoma and is highly sensitive to a novel aurora kinase A inhibitor in vitro. Leukemia 25(2):348–358. https://doi.org/10.1038/leu.2010.255
Raspadori D, Damiani D, Lenoci M, Rondelli D, Testoni N, Nardi G, Sestigiani C, Mariotti C, Birtolo S, Tozzi M, Lauria F (2001) CD56 antigenic expression in acute myeloid leukemia identifies patients with poor clinical prognosis. Leukemia 15(8):1161–1164
Bing Xu XS, Pengcheng Shi, Pengnan Xiao, Zhengshan Yu, Shuyun Zhou (2009) Relationship between CD56 antigen expression and quantification of MDR1 gene expression in patients with de novo acute myeloid leukemia (AML). Paper presented at the Annual Meeting of American Society of Hematology,
Schaich M, Koch R, Soucek S, Repp R, Ehninger G, Illmer T (2004) A sensitive model for prediction of relapse in adult acute myeloid leukaemia with t(8;21) using white blood cell count, CD56 and MDR1 gene expression at diagnosis. Br J Haematol 125(4):477–479. https://doi.org/10.1111/j.1365-2141.2004.04939.x
Kassira N, Pedroso FE, Cheung MC, Koniaris LG, Sola JE (2011) Primary gastrointestinal tract lymphoma in the pediatric patient: review of 265 patients from the SEER registry. J Pediatr Surg 46(10):1956–1964. https://doi.org/10.1016/j.jpedsurg.2011.06.006
Aleman BM, Haas RL, van der Maazen RW (2010) Role of radiotherapy in the treatment of lymphomas of the gastrointestinal tract. Best Pract Res Clin Gastroenterol 24(1):27–34. https://doi.org/10.1016/j.bpg.2009.12.002
Ellin F, Landstrom J, Jerkeman M, Relander T (2015) Central nervous system relapse in peripheral T-cell lymphomas: a Swedish Lymphoma Registry study. Blood 126(1):36–41. https://doi.org/10.1182/blood-2014-12-616961
Gurion R, Mehta N, Migliacci JC, Zelenetz A, Moskowitz A, Lunning M, Moskowitz C, Hamlin P, Horwitz S (2016) Central nervous system involvement in T-cell lymphoma: a single center experience. Acta Oncologica (Stockholm, Sweden) 55(5):561–566. https://doi.org/10.3109/0284186x.2015.1118656
Yi JH, Kim JH, Baek KK, Lim T, Lee DJ, Ahn YC, Kim K, Kim SJ, Ko YH, Kim WS (2011) Elevated LDH and paranasal sinus involvement are risk factors for central nervous system involvement in patients with peripheral T-cell lymphoma. Ann Oncol 22(7):1636–1643. https://doi.org/10.1093/annonc/mdq645
Kikuma K, Yamada K, Nakamura S, Ogami A, Nimura S, Hirahashi M, Yonemasu H, Urabe S, Naito S, Matsuki Y, Sadahira Y, Takeshita M (2014) Detailed clinicopathological characteristics and possible lymphomagenesis of type II intestinal enteropathy-associated T-cell lymphoma in Japan. Hum Pathol 45(6):1276–1284. https://doi.org/10.1016/j.humpath.2013.10.038
Chott A, Haedicke W, Mosberger I, Fodinger M, Winkler K, Mannhalter C, Muller-Hermelink HK (1998) Most CD56+ intestinal lymphomas are CD8+CD5-T-cell lymphomas of monomorphic small to medium size histology. Am J Pathol 153(5):1483–1490. https://doi.org/10.1016/s0002-9440(10)65736-7
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yi, J.H., Lee, GW., Do, Y.R. et al. Multicenter retrospective analysis of the clinicopathologic features of monomorphic epitheliotropic intestinal T-cell lymphoma. Ann Hematol 98, 2541–2550 (2019). https://doi.org/10.1007/s00277-019-03791-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-019-03791-y