Abstract
In spite of recent development in the treatment armamentarium for multiple myeloma, overall survival (OS) still depends on risk status and sensitivity to treatment of each patient. We have evaluated the clinical relevance of the Revised International Staging System (R-ISS) by comparing it with the original ISS in 718 Japanese patients. The distribution of patients according to response was similar between the ISS and R-ISS stages. Treatment response was greatly influenced by initial treatment modalities and deeper response was observed more frequently in transplanted patients. The R-ISS discriminated the difference in OS between the stages more distinctly than the ISS (p = 9.0 × 10−15 and p = 4.0 × 10−10, respectively). Differences in OS were clarified by both R-ISS and ISS in non-transplanted patients (p = 2.4 × 10−12 and p = 1.4 × 10−8, respectively), but the ISS failed to distinguish the difference between the stages in transplanted patients (p = 0.13). In contrast, the R-ISS could at least discriminate the excellent prognosis of stage I patients whereas the distinction between stage II and III was not that clear (p = 0.033). The R-ISS stage II encompassed a large number of patients, and the prognosis was heterogeneous depending on the fulfillment of prognostic factors such as LDH and adverse cytogenetics. These results suggest that treatment factors and prognostic factors greatly affect the therapeutic response and outcome, and the R-ISS is superior to ISS in prognostication of both transplant-eligible and -ineligible patients in our current clinical practice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Multiple myeloma (MM) is a plasma cell neoplasm characterized by the presence of monoclonal immunoglobulin in serum and/or urine and clinical symptoms related to the end organ damage by MM comprising hypercalcemia, renal insufficiency, anemia, and bone complications (CRAB symptoms) [1]. Recently, the International Myeloma Working Group (IMWG) has updated the diagnostic criteria to address a change in the diagnosis of a subset of patients without CRAB symptoms but with biomarkers of malignancy (called SLiM, derived from an acronym of the biomarkers) from smoldering MM (SMM) to MM requiring treatment [2]. As such, MM is a heterogeneous disease and survival outcome varies considerably depending on the risk status of each patient, determined by patient-related factors such as age and frailty, disease-related factors such as clinical stage and cytogenetic abnormalities (CA), and treatment-related factors such as the use of novel agents and autologous stem cell transplantation (ASCT).
Survival of patients with MM has shown a continued improvement over the past decade by the emerging novel therapies comprising initially bortezomib and thalidomide, followed by next-generation proteasome inhibitors, carfilzomib and ixazomib, and next-generation immunomodulatory drugs (IMiDs), lenalidomide and pomalidomide, as well as other new class of agents such as elotuzumab and daratumumab classified as monoclonal antibodies [3]. However, MM patients are not always treated with the novel therapies because of comorbidities and/or socio-economical reasons [4].
To evaluate tumor burden of MM, the IMWG initially developed the International Staging System (ISS) based on the serum levels of albumin and β2-microglobulin in 2005 [5]. Subsequently, several investigators have reported the importance of LDH and CA in the prognostication of MM [6, 7]. Recently, the IMWG has proposed the Revised ISS (R-ISS) as a more potent prognostic system than the original ISS by incorporating both LDH as a function of tumor burden and the presence of high-risk CA as a function of disease biology into the ISS [8]. Although the R-ISS was constructed based on the data obtained solely from patients enrolled in clinical trials testing novel agents, its validity has also been evaluated in patients of routine clinical practice in many countries including North America, Europe, and Asia [9,10,11]. However, there have been no studies evaluating the relevance of the R-ISS in the context of different treatment modalities in clinical practice.
In the present study, we have evaluated and compared the clinical relevance of the R-ISS with the original ISS in Japanese MM patients treated with different modalities in routine clinical practice.
Patients and methods
Patients
The Japanese Society of Myeloma has conducted two pivotal retrospective studies. The first study was carried out based on the collected data from 1383 patients diagnosed and treated in clinical practice between January 1990 and December 2000, before the era of novel agents, to disclose the real figure of the management of MM at that time in Japan [12]. The second study was to analyze changes in outcome by comparing the data of the previous study with more recent data involving 2234 patients diagnosed and treated between January 2001 and December 2012 [13]. Subsequently, the second study was further extended to a total of 3270 patients from 38 affiliated hospitals. Among these, a sufficient data set including FISH (fluorescence in situ hybridization) analysis and details of initial treatment was obtained in 718 patients and served as the basis of the present study.
The diagnosis of MM was made according to the IMWG criteria [1] and the clinical stage of MM was determined based on the Durie and Salmon staging system [14]. Patients with asymptomatic (smoldering) MM and primary amyloidosis were excluded. Baseline demographic, clinical, and laboratory data, and details regarding treatment and response to treatment were collected retrospectively during the period between January 2014 and May 2014. This study has involved unselected patients treated consecutively in routine clinical practice in the participating hospitals of the Japanese Society of Myeloma. This study was conducted in accordance with the institutional guidelines with approval of the Ethics Committee/Institutional Review Board of Tokushima Prefectural Central Hospital.
Stages according to the ISS and R-ISS
The ISS and the R-ISS stages in each patient were determined according to the respective IMWG criteria previously published [5, 8]. Abnormal LDH was defined as a serum level greater than the upper limit of normal. CA was analyzed on the CD138 selected bone marrow plasma cells by FISH method. The cut-off level of ≥ 10% was used to determine the presence of CA in each FISH analysis. High-risk CA was defined as the presence of any of t(4;14), t(14;16), or del(17p) according to the R-ISS criteria [8].
Treatment
Treatment for each patient was determined by the respective physician-in-charge. The short-term use of dexamethasone for the emergency purpose was not considered as a therapeutic line. Initial therapy was classified based on the use of novel agents incorporating thalidomide, lenalidomide, and bortezomib or the use of ASCT. ASCT was conducted with high- or intermediate-dose melphalan conditioning followed by peripheral blood stem cell transplantation according to the institutional protocol and was regarded as initial therapy only when it was performed upfront after induction therapy. Treatment response was assessed according to the uniform response criteria by the IMWG [15].
Statistical analysis
Fisher’s exact test was used to compare differences between categorical variables, whereas the Mann-Whitney U test was used for continuous or nominal values. Kaplan-Meier method was used to create the overall survival (OS) curves, and differences between the curves were analyzed by the log-rank test and the Holm test for multiple comparisons when applicable. Statistical analyses were performed with EZR version 1.30 (Saitama Medical Center, and Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria) [16].
Results
Patient characteristics
In the present study, we collected the clinical data of 3270 patients diagnosed and treated in the period of between 2001 and 2012 (whole group). A sufficient data set including FISH analysis and the information of initial therapy was evaluable in 718 patients of them and was subjected to the analysis (study group). The follow-up periods of these patients ranged from 0.4 to 152.8 months (median, 29.5 months).
Baseline characteristics of the patients are summarized in supplementary Table 1. There were 374 males and 344 females. The median age was 67 years old (range, 31–89). The isotype of M protein was IgG in 433 patients (60.2%), IgA in 138 (19.2%), IgD in 17 (2.4%), and Bence Jones type in 111 (15.5%). The Durie and Salmon stages I, II, and III were distributed in 9.0%, 26.2%, and 64.8% of the patients, respectively. The ISS stages I, II, and III were distributed in 31.8%, 35.7%, and 32.5%, respectively. Patient baseline features including age, gender, M protein isotype, Durie and Salmon stage, and ISS stage were not significantly different between the whole patient group and the study patient group. The percentage of patients with high β2-microglobulin levels was similar in both groups (51.1% vs 54.7%). The percentage of patients with abnormal serum LDH (>normal upper limit) was significantly lower in the study patient group (19.4% vs 26.5%, p < 0.0001). Among the 718 patients, those with adverse CA such as t(4;14), t(14;16), and del(17p) were observed in 142 (19.8%), 14 (1.9%), and 63 (8.8%) patients, respectively. The percentage of patients with abnormal karyotype was slightly higher in the study patient group (33.3% vs 28.2%, p = 0.0219). As for the initial therapy, the proportion of patients treated with conventional chemotherapy alone was lower (33.1% vs 48.7%), whereas that of the patients treated with novel therapy alone was higher in the study patient group than in the whole patient group (36.3% vs 18.7%). ASCT was performed in 30.6% of the study patient group and in 32.6% and the whole patient group, respectively.
Initial therapy
Treatment regimens of the initial therapy for the 718 study patients are shown in supplementary Table 2. Two hundred and thirty-eight patients (33.1%) received conventional chemotherapy alone including melphalan + prednisolone (MP) reaching to 21.1%, followed by vincristine + doxorubicin + dexamethasone (VAD) and high-dose dexamethasone. Two hundred and sixty patients (36.3%) received novel agent-based regimens alone including bortezomib + dexamethasone ± cyclophosphamide (BD/VCD), bortezomib + MP (VMP), and thalidomide + MP (MPT). A total of 220 patients underwent upfront ASCT either after the induction therapy with conventional chemotherapy alone represented by VAD and high-dose dexamethasone in 69 (9.6%) patients or after the induction with novel agents including BD, VCD, and bortezomib + doxorubicin + dexamethasone (PAD) in another 151 patients (21.0%). Maintenance therapy was given to 125 transplanted patients and to 240 non-transplanted patients in different doses and schedules.
Distribution of the ISS and R-ISS stages
The distribution of patients according to the ISS and the R-ISS stages in the study patients is shown in Table 1 and comprises 228 patients (31.8%) assigned to the ISS stage I, 257 (35.7%) to the stage II, and remaining 233 (32.5%) to the stage III, whereas 154 patients (21.4%) were assigned to the R-ISS stage I, 438 (61.1%) to the stage II, and remaining 126 (17.5%) to the stage III, respectively. It was noted that the distribution of the 718 patients according to the R-ISS stages showed a higher predilection for the stage II although the same 718 study patients were equally distributed by the ISS stages.
Best response according to the ISS and R-ISS stages
Best response according to the ISS and R-ISS is shown in Table 2. The overall response rates (≥partial response, PR) were as follows: 71.5% for the ISS stage I, 64.6% for the ISS stage II, and 61.2% for the ISS stage III (p = 0.14); and 70.4% for the R-ISS stage I, 64.6% for the R-ISS stage II, and 61.4% for the R-ISS stage III (p = 0.45), respectively. The rate of deep response such as stringent complete response (sCR) and CR differed by the stages; 18.7% for the ISS stage I, 11.8% for the ISS stage II, and 7.6% for the ISS stage III (p = 0.01); and 18.4% for the R-ISS stage I, 10.9% for the R-ISS stage II, and 10.5% for the R-ISS stage III (p = 0.12), respectively. Thus, the percentage of patients achieving PR or better was similar between the ISS stages and the R-ISS stages. Although the overall response rate and deep response rate were higher in stage I than in other stages by the ISS and R-ISS, it seemed difficult to predict therapeutic responses with these classifications.
Best response according to initial therapy
In contrast to the similar distribution of patients achieving best response according to the ISS and R-ISS stages, the distribution of patients achieving best response differed considerably according to the initial therapy categories as shown in Table 3. The overall response rate (≥PR) was 41.6% for the conventional chemotherapy group, 68.8% for the novel agent group, 76.1% for the conventional chemotherapy + ASCT group, and 91.7% for the novel agents + ASCT group, respectively (p = 1.07 × 10−17). The deep response rate (sCR and CR) according to initial therapies was 1.8%, 12.3%, 4.5%, and 34.0%, respectively, and was the highest in the novel agent + ASCT group (p = 5.1 × 10−15).
OS according to the ISS and R-ISS stages by treatment modalities
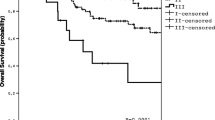
We then analyzed OS of the study patients according to the ISS and R-ISS stages. Figure 1 and Fig. 2 show the survival curves according to respective ISS stages and R-ISS stages. In both figures, survival curves of total patient population are shown in A, those of patients treated without novel agents in B, with novel agents in C, those treated without ASCT in D, and with ASCT in E. The difference between stages was evaluated by the p value of three-stage comparison by the log-rank test. The p values between each stage were analyzed by the Holm test as shown in supplementary Table 3.
The median OS of the study patients according to the ISS was 100.7 months for stage I, 65.2 months for stage II, and 50.9 months for stage III, respectively (p = 4.0 × 10−10, Fig. 1a). In the analysis evaluating the effect of the use of novel agents as initial therapy, the difference in OS was more pronounced in the novel agent group (p = 4.1 × 10−6, Fig. 1c) compared with the conventional chemotherapy group (p = 4.8 × 10−4, Fig. 1b). In the comparison according to the use of transplantation, survival curves between the stages were clearly separated in the non-transplant group (p = 1.4 × 10−8, Fig. 1d), but not in the transplant group (p = 0.13, Fig. 1e).
According to the R-ISS, the corresponding median OS for each stage was 152.8, 65.2, and 45.3 months, respectively (p = 9.0 × 10−15, Fig. 2a). The difference in survival curves between the stages was significant whether the initial therapy was novel agents-based (p = 3.4 × 10−7, Fig. 2c) or conventional chemotherapy-based (p = 2.3 × 10−7, Fig. 2b). When comparing the survival curves of patients treated with ASCT, the R-ISS clearly distinguished the difference between the stages in the non-transplant group (p = 2.4 × 10−12, Fig. 2d). Although the R-ISS could show a superb survival of the stage I patients treated with upfront ASCT, the survival curve for stage II overlapped with that of stage III (p = 0.033, Fig. 2e).
The difference between survival curves according to each stage was more significant in the R-ISS than in the ISS (supplementary Table 3). However, there was a tendency that the difference between stages II and III was smaller than that between stages I and II, especially in patients treated with novel agents (Figs. 1c and 2c) and those with transplantation (Figs. 1e and 2e).
Comparison of the OS within the R-ISS stages
When comparing R-ISS with ISS, more patients were assigned to stage II of R-ISS than to stage II of ISS, and patients with the R-ISS stage II were heterogeneous in terms of prognostic factors such as elevated LDH and adverse CA (Table 1). Therefore, we further evaluated OS of each constituent of the R-ISS stage II according to the risk factors (Table 1). The R-ISS stage II is composed of six subgroups: (i) ISS stage I with 1 risk factor, (ii) ISS stage I with 2 risk factors, (iii) ISS stage II without risk factor, (iv) ISS stage II with 1 risk factor, (v) ISS stage II with 2 risk factors, and (vi) ISS stage III without risk factor. We have confirmed that the outcome of the six subgroups of the R-ISS stage II patients was extremely variable than we had anticipated; median OS for the subgroup (i); either 52.6 months or 100.7 month depending on which of the two risk factors was met, subgroup (ii): 34.0 months, subgroup (iii); 130.8 months, subgroup (iv): either 49.5 or 53.8 months depending on which of the two factors was met, subgroup (v): 46.9 months, and subgroup (vi): 61.8 months, respectively (p = 6.8 × 10−6, and p values between each subgroup are shown in supplementary Table 4). These findings would implicate that the presence of abnormal LDH and/or high-risk CA defined in the R-ISS might have a much stronger impact on OS than the two factors defined in the original ISS (i.e., serum albumin and β2-microglobulin).
Discussion
In the present study, we have evaluated the clinical relevance of the original ISS and R-ISS based on the long-term follow-up data of Japanese MM patients treated initially with conventional chemotherapy or with novel agents ± ASCT in routine clinical practice.
We first assessed the treatment response according to the ISS and R-ISS stages. The percentage of patients achieving PR or better was similar between the ISS stages and the R-ISS stages. The percentage of patients achieving deep response (sCR and CR) was significantly higher in stage I patients than in stage II and III patients in the ISS, but such a difference was not observed in the R-ISS stages. Next, we assessed the response according to the treatment modality. It was found that the use of novel agents and ASCT in initial therapy significantly yielded a better response with higher deep responses. Accordingly, the treatment modalities such as the use of novel agents and ASCT are more important in treatment response than the ISS and R-ISS stages. Our previous study in routine clinical practice has shown that initial treatment with novel agents and/or ASCT achieved a deep response such as sCR and CR, which was associated with a long-term survival [13]. In this context, deep response achieved by treatment is more important as a prognostic factor for patient outcome. Recently, meta-analyses of clinical trials have shown that minimal residual disease (MRD) negativity was one of the most powerful prognostic factors in OS regardless of treatment or risk status [17, 18]. Thus, we need to develop treatment regimens incorporating the next-generation novel agents that would achieve a higher MRD negativity status [19].
We evaluated the potential of the two staging systems in association of survival outcome of 718 patients treated upfront with either conventional chemotherapy or novel therapy followed by ASCT or not. It was found that separation between stages was more distinct in the R-ISS than the ISS in accordance with the previous reports [9,10,11], particularly in non-transplanted patients (Figs. 1d and 2d). However, the usefulness of the ISS and R-ISS was limited in the groups of patients treated with novel agents (Figs. 1c and 2c) or with ASCT (Figs. 1e and 2e). According to the original report, the median OS of R-ISS stage III was reported to be 42 months in the transplanted patients [8]; however, it was extended to 73.8 months in the corresponding patients in our study. We understand the comparison itself is not pertinent, but as a possible explanation for this outstanding result, we would presume that the prognosis of the stage III patients could well be improved by the use of upfront ASCT and novel salvage therapies in transplant-eligible patients. In fact, another Japanese study group has indicated that recent induction therapy with novel agents followed by ASCT would be able to overcome the poor prognosis of the ISS stage III disease and has implicated that the ISS would no longer be able to stratify the prognosis of the transplanted patients [20]. Importantly, bortezomib has been shown to improve renal insufficiency and survival of patients with renal failure having a high level of β2-microglobulin to be diagnosed with ISS stage III disease [21]. Recent large-scale clinical phase III studies on upfront ASCT after induction therapy including bortezomib have demonstrated a significant impact of ASCT on deep responses associated with MRD negativity and prolongation of progression-free survival [22, 23]. In addition, recent clinical studies have disclosed a favorable effect of proteasome inhibitors such as bortezomib and carfilzomib to improve the response and OS in high-risk patients harboring t(4;14) and del(17p) [24]. Taken together, frontline treatment with novel agents + ASCT and probably subsequent novel salvage therapy would likely to improve outcome even in patients with risk features such as renal failure and adverse CA. Of interest, the R-ISS could clarify a superb outcome of the stage I patients treated with upfront ASCT (Fig. 2e), which was not detected by the original ISS. Thus, the R-ISS could also be beneficial for identifying a good prognosis group without abnormal LDH and adverse CA, and these patients would mostly benefit from recent advances in treatment with novel agents and ASCT.
Notably, according to the definition of R-ISS, more patients would be obliged to be assigned to the R-ISS stage II. We have found that a higher number of patients were classified as R-ISS stage II, and more importantly, we have also found that the outcome of these patients was quite heterogeneous depending on the presence or absence of risk factors comprising elevated LDH and adverse CA. This heterogeneity of the R-ISS stage II has also been pointed out by other groups [25, 26], indicating that elevated LDH and adverse CA were more powerful prognostic factors than albumin and β2-microglobulin under the current treatment. Moreover, a recent genome-wide analysis of the largest set of patients has identified an extremely poor outcome group that is called as Double-Hit MM, either with bi-allelic TP53 inactivation or with amplification of CKS1B in ISS stage III patients [27]. Thus, the importance of genomic abnormality in prognostication is being further clarified in patients with MM, and the clinical relevance of the prognostic factors for a staging system needs to be reevaluated along with advances in research and treatment in the future.
In conclusion, this real-life, multicenter, retrospective study has demonstrated that the R-ISS is a valid and reliable tool for prognostication in both transplant-eligible and -ineligible MM patients regardless of initial treatment either with conventional chemotherapy or with novel agents. Notably, the R-ISS is useful for the discrimination of the stage I disease with very good prognosis when treated with upfront ASCT. However, the R-ISS still has a challenge because R-ISS stage II patients are composed of quite heterogeneous population in terms of OS due to variable fulfillment of prognostic factors and also because the prognosis of a proportion of the stage III has become improved due to advances in novel therapies and ASCT. Our results would provide an important basis for evaluating outcome achieved by the progress of treatment in the future clinical practice of MM.
References
International Myeloma Working Group (2003) Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: a report of the international myeloma working group. Br J Haematol 121(5):749–757
Rajkumar SV, Dimopoulos MA, Palumbo A, Blade J, Merlini G, Mateos MV, Kumar S, Hillengass J, Kastritis E, Richardson P, Landgren O, Paiva B, Dispenzieri A, Weiss B, LeLeu X, Zweegman S, Lonial S, Rosinol L, Zamagni E, Jagannath S, Sezer O, Kristinsson SY, Caers J, Usmani SZ, Lahuerta JJ, Johnsen HE, Beksac M, Cavo M, Goldschmidt H, Terpos E, Kyle RA, Anderson KC, Durie BGM, Miguel JFS (2014) International myeloma working group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol 15(12):e538–e548
Anderson KC (2016) Progress and paradigms in multiple myeloma. Clin Cancer Res 22(22):5419–5427
Fonseca R, Abouzaid S, Bonafede M, Cai Q, Parikh K, Cosler L, Richardson P (2017) Trends in overall survival and costs of multiple myeloma, 2000-2014. Leukemia 31(9):1915–1921
Greipp PR, San Miguel J, Durie BG, Crowley JJ, Barlogie B, Blade J et al (2005) International staging system for multiple myeloma. J Clin Oncol 23(15):3412–3420
Avet-Loiseau H, Durie BG, Cavo M, Attal M, Gutierrez N, Haessler J et al (2013) Combining fluorescent in situ hybridization data with ISS staging improves risk assessment in myeloma: an international myeloma working group collaborative project. Leukemia 27(3):711–717
Moreau P, Cavo M, Sonneveld P, Rosinol L, Attal M, Pezzi A, Goldschmidt H, Lahuerta JJ, Marit G, Palumbo A, van der Holt B, Bladé J, Petrucci MT, Neben K, san Miguel J, Patriarca F, Lokhorst H, Zamagni E, Hulin C, Gutierrez N, Facon T, Caillot D, Benboubker L, Harousseau JL, Leleu X, Avet-Loiseau H, Mary JY (2014) Combination of international scoring system 3, high lactate dehydrogenase, and t(4;14) and/or del(17p) identifies patients with multiple myeloma (MM) treated with front-line autologous stem-cell transplantation at high risk of early MM progression-related death. J Clin Oncol 32(20):2173–2180
Palumbo A, Avet-Loiseau H, Oliva S, Lokhorst HM, Goldschmidt H, Rosinol L, Richardson P, Caltagirone S, Lahuerta JJ, Facon T, Bringhen S, Gay F, Attal M, Passera R, Spencer A, Offidani M, Kumar S, Musto P, Lonial S, Petrucci MT, Orlowski RZ, Zamagni E, Morgan G, Dimopoulos MA, Durie BGM, Anderson KC, Sonneveld P, San Miguel J, Cavo M, Rajkumar SV, Moreau P (2015) Revised international staging system for multiple myeloma: a report from international myeloma working group. J Clin Oncol 33(26):2863–2869
Jimenez-Zepeda VH, Duggan P, Neri P, Rashid-Kolvear F, Tay J, Bahlis NJ (2016) Revised international staging system applied to real world multiple myeloma patients. Clin Lymphoma Myeloma Leuk 16(9):511–518
Kastritis E, Terpos E, Roussou M, Gavriatopoulou M, Migkou M, Eleutherakis-Papaiakovou E, Fotiou D, Ziogas D, Panagiotidis I, Kafantari E, Giannouli S, Zomas A, Konstantopoulos K, Dimopoulos MA (2017) Evaluation of the revised international staging system (R-ISS) in an independent cohort of unselected patients with multiple myeloma. Haematologica 102(3):593–599
Cho H, Yoon DH, Lee JB, Kim SY, Moon JH, Do YR, Lee JH, Park Y, Lee HS, Eom HS, Shin HJ, Min CK, Kim JS, Jo JC, Kang HJ, Mun YC, Lee WS, Lee JJ, Suh C, Kim K, and the Korean Multiple Myeloma Working Party (2017) Comprehensive evaluation of the revised international staging system in multiple myeloma patients treated with novel agents as a primary therapy. Am J Hematol 92(12):1280–1286
Shimizu K, Nagura E, Takatsuki K (2004) Management of patients with multiple myeloma in Japan: data of 1,383 patients from 16 hospitals and 1 treatment group. Leuk Lymphoma 45(12):2465–2469
Ozaki S, Handa H, Saitoh T, Murakami H, Itagaki M, Asaoku H, Suzuki K, Isoda A, Matsumoto M, Sawamura M, Konishi J, Sunami K, Takezako N, Hagiwara S, Kuroda Y, Chou T, Nagura E, Shimizu K (2015) Trends of survival in patients with multiple myeloma in Japan: a multicenter retrospective collaborative study of the Japanese Society of Myeloma. Blood Cancer J 5:e349
Durie BG, Salmon SE (1975) A clinical staging system for multiple myeloma. Correlation of measured myeloma cell mass with presenting clinical features, response to treatment, and survival. Cancer 36(3):842–854
Durie BG, Harousseau JL, Miguel JS, Blade J, Barlogie B, Anderson K et al (2006) International uniform response criteria for multiple myeloma. Leukemia 20(9):1467–1473
Kanda Y (2013) Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant 48(3):452–458
Lahuerta JJ, Paiva B, Vidriales MB, Cordon L, Cedena MT, Puig N et al (2017) Depth of response in multiple myeloma: a pooled analysis of three PETHEMA/GEM clinical trials. J Clin Oncol 35(25):2900–2910
Munshi NC, Avet-Loiseau H, Rawstron AC, Owen RG, Child JA, Thakurta A, Sherrington P, Samur MK, Georgieva A, Anderson KC, Gregory WM (2017) Association of Minimal Residual Disease with Superior Survival Outcomes in patients with multiple myeloma: a meta-analysis. JAMA Oncol 3(1):28–35
Kumar S, Paiva B, Anderson KC, Durie B, Landgren O, Moreau P, Munshi N, Lonial S, Bladé J, Mateos MV, Dimopoulos M, Kastritis E, Boccadoro M, Orlowski R, Goldschmidt H, Spencer A, Hou J, Chng WJ, Usmani SZ, Zamagni E, Shimizu K, Jagannath S, Johnsen HE, Terpos E, Reiman A, Kyle RA, Sonneveld P, Richardson PG, McCarthy P, Ludwig H, Chen W, Cavo M, Harousseau JL, Lentzsch S, Hillengass J, Palumbo A, Orfao A, Rajkumar SV, Miguel JS, Avet-Loiseau H (2016) International myeloma working group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol 17(8):e328–e346
Takamatsu H, Honda S, Miyamoto T, Yokoyama K, Hagiwara S, Ito T, Tomita N, Iida S, Iwasaki T, Sakamaki H, Suzuki R, Sunami K (2015) Changing trends in prognostic factors for patients with multiple myeloma after autologous stem cell transplantation during the immunomodulator drug/proteasome inhibitor era. Cancer Sci 106(2):179–185
Dimopoulos MA, Delimpasi S, Katodritou E, Vassou A, Kyrtsonis MC, Repousis P, Kartasis Z, Parcharidou A, Michael M, Michalis E, Gika D, Symeonidis A, Pouli A, Konstantopoulos K, Terpos E, Kastritis E (2014) Significant improvement in the survival of patients with multiple myeloma presenting with severe renal impairment after the introduction of novel agents. Ann Oncol 25(1):195–200
Attal M, Lauwers-Cances V, Hulin C, Leleu X, Caillot D, Escoffre M, Arnulf B, Macro M, Belhadj K, Garderet L, Roussel M, Payen C, Mathiot C, Fermand JP, Meuleman N, Rollet S, Maglio ME, Zeytoonjian AA, Weller EA, Munshi N, Anderson KC, Richardson PG, Facon T, Avet-Loiseau H, Harousseau JL, Moreau P, IFM 2009 Study (2017) Lenalidomide, Bortezomib, and dexamethasone with transplantation for myeloma. N Engl J Med 376(14):1311–1320
Cavo M, Hájek R, Pantani L, Beksac M, Oliva S, Dozza L, Johnsen HE, Petrucci MT, Mellqvist U-H, Conticello C, Driessen C, Marzocchi G, Dimopoulos MA, Zweegman S, Wu KL, Gamberi B, Crippa C, van der Holt B, Offidani M, Wester R, Vincelli ID, Troia R, Cornelisse P, Boccadoro M, Sonneveld P (2017) Autologous stem cell transplantation versus bortezomib-melphalan-prednisone for newly diagnosed multiple myeloma: second interim analysis of the phase 3 EMN02/HO95 study. Blood 130(Suppl 1):397
Sonneveld P, Avet-Loiseau H, Lonial S, Usmani S, Siegel D, Anderson KC, Chng WJ, Moreau P, Attal M, Kyle RA, Caers J, Hillengass J, San Miguel J, van de Donk NWCJ, Einsele H, Blade J, Durie BGM, Goldschmidt H, Mateos MV, Palumbo A, Orlowski R (2016) Treatment of multiple myeloma with high-risk cytogenetics: a consensus of the international myeloma working group. Blood 127(24):2955–2962
Jung SH, Kim K, Kim JS, Kim SJ, Cheong JW, Kim SJ, Ahn JS, Ahn SY, Yang DH, Kim HJ, Lee JJ (2018) A prognostic scoring system for patients with multiple myeloma classified as stage II with the revised international staging system. Br J Haematol 181(5):707–710
Gonzalez-Calle V, Slack A, Keane N, Luft S, Pearce KE, Ketterling RP et al (2018) Evaluation of revised international staging system (R-ISS) for transplant-eligible multiple myeloma patients. Ann Hematol 97(8):1453–1462
Walker BA, Mavrommatis K, Wardell CP, Ashby TC, Bauer M, Davies F, Rosenthal A, Wang H, Qu P, Hoering A, Samur M, Towfic F, Ortiz M, Flynt E, Yu Z, Yang Z, Rozelle D, Obenauer J, Trotter M, Auclair D, Keats J, Bolli N, Fulciniti M, Szalat R, Moreau P, Durie B, Stewart AK, Goldschmidt H, Raab MS, Einsele H, Sonneveld P, San Miguel J, Lonial S, Jackson GH, Anderson KC, Avet-Loiseau H, Munshi N, Thakurta A, Morgan G (2019) A high-risk, double-hit, group of newly diagnosed myeloma identified by genomic analysis. Leukemia 33(1):159–170
Acknowledgments
The following Japanese Society of Myeloma (JSM) institutions and investigators participated in this study: Tadao Ishida, Department of Gastroenterology, Rheumatology, and Clinical Immunology, Sapporo Medical University School of Medicine, Sapporo; Takahiro Kobayashi, Takaya Yamashita, Naoto Takahashi, Department of Hematology, Nephrology and Rheumatology, Akita University Hospital, Akita; Hiroshi Handa, Takayuki Saitoh, Hirokazu Murakami, Department of Hematology, Gunma University Hospital, Maebashi; Atsushi Isoda, Morio Matsumoto, Morio Sawamura, Department of Hematology, National Hospital Organization Nishigunma National Hospital, Shibukawa; Eishi Nogiwa, Department of Internal Medicine, Usui Hospital, Yasunaka: Chiaki Nakaseko, Department of Hematology, Chiba University Hospital, Chiba; Fumihiko Nakamura, Department of Hematology and Oncology, Graduate School of Medicine, The University of Tokyo, Tokyo; Go Yamamoto, Department of Hematology, Toranomon Hospital, Tokyo; Kenshi Suzuki, Department of Hematology, Japanese Red Cross Medical Center, Tokyo; Shotaro Hagiwara, Division of Hematology, Department of Internal Medicine, National Medical Center for Global Health and Medicine, Tokyo; Naoki Takezako, Department of Hematology, National Hospital Organization National Disaster Medical Center, Tokyo; Masao Hagihara, Department of Hematology, Eiju General Hospital, Tokyo; Shinya Okuda, Department of Hematology and Oncology, JR Tokyo General Hospital, Tokyo; Naoto Tomita, Department of Internal Medicine and Clinical Immunology, Yokohama City University Hospital, Yokohama; Tomonori Nakazato, Department of Hematology, Yokohama Municipal Citizen’s Hospital, Yokohama; Akiko Negoro, Department of Hematology, Japan Labor Health and Welfare Organization Yokohama Rosai Hospital, Yokohama; Takaaki Chou, Department of Medicine, Niigata Cancer Center Hospital, Niigata; Junji Ito, Department of Clinical Laboratory, Nagoya City Midori General Hospital; Nagoya; Hiroshi Kosugi, Department of Hematology, Ogaki Municipal Hospital, Ogaki; Hiroyuki Takamatsu, Department of Hematology, Kanazawa University Graduate School of Medical Science, Kanazawa; Hiroyuki Takamatsu, Department of Hematology, NTT WEST Kanazawa Hospital, Kanazawa; Tatsuharu Ohno, Division of Hematology and Immunology, Department of Internal Medicine, Ohtsu Red Cross Hospital, Ohtsu; Junya Kuroda, Department of Hematology and Oncology, Kyoto Prefectural University of Medicine, Kyoto; Chihiro Shimazaki, Department of Hematology, Japan Community Healthcare Organization Kyoto-Kuramaguchi Medical Center, Kyoto; Nobumasa Inoue, Department of Internal Medicine, National Hospital Organization Osaka Medical Center, Osaka; Toru Murayama, Department of Hematology, Hyogo Cancer Center, Akashi; Jun Konishi, Kazutaka Sunami, Department of Hematology, National Hospital Organization Okayama Medical Center, Okayama; Yoshiaki Kuroda, Department of Hematology, Hiroshima University Hospital, Hiroshima; Mitsuhiro Itagaki, Hideki Asaoku, Department of Hematology, Hiroshima Red Cross Hospital, Hiroshima; Takaaki Miyake, Department of Hematology, Shimane University Hospital, Izumo; Toshio Wakayama, Department of Hematology, Shimane Prefectural Central Hospital, Izumo; Takeshi Harada, Masahiro Abe, Department of Hematology, Tokushima University Hospital, Tokushima; Shuji Ozaki, Etsuko Sekimoto, Hironobu Shibata, Toshio Shigekiyo, Department of Hematology, Tokushima Prefectural Central Hospital, Tokushima; Toshihiro Hashimoto, Department of Internal Medicine, Tokushima Municipal Hospital, Tokushima; Yasushi Takamatsu, Division of Medical Oncology, Hematology, and Infectious Disease, Department of Internal Medicine, Fukuoka University, Fukuoka; Naokuni Uike, Department of Hematology, National Kyushu Cancer Center, Fukuoka; Hiroyuki Hata, Department of Immunology and Hematology, Faculty of Life Sciences, Graduate School of Health Sciences, Kumamoto University, Kumamoto; Naoko Harada, Department of Hematology, National Hospital Organization Kumamoto Medical Center, Kumamoto, Japan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Kazutaka Sunami has received research funding from Ono Pharmaceutical Co. Ltd., Takeda Pharmaceutical Co. Ltd., Novartis Pharmaceutical Co. Ltd., and Celgene Co. Ltd. All other authors declare no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ozaki, S., Handa, H., Saitoh, T. et al. Evaluation of the Revised International Staging System (R-ISS) in Japanese patients with multiple myeloma. Ann Hematol 98, 1703–1711 (2019). https://doi.org/10.1007/s00277-019-03702-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-019-03702-1