Abstract
The International Myeloma Working Group has proposed the Revised International Staging System (R-ISS) for risk stratification of multiple myeloma (MM) patients. There are a limited number of studies that have validated this risk model in the autologous stem cell transplant (ASCT) setting. In this retrospective study, we evaluated the applicability and value for predicting survival of the R-ISS model in 134 MM patients treated with new agents and ASCT at the Mayo Clinic in Arizona and the University Hospital of Salamanca in Spain. The patients were reclassified at diagnosis according to the R-ISS: 44 patients (33%) had stage I, 75 (56%) had stage II, and 15 (11%) had stage III. After a median follow-up of 60 months, R-ISS assessed at diagnosis was an independent predictor for overall survival (OS) after ASCT, with median OS not reached, 111 and 37 months for R-ISS I, II and III, respectively (P < 0.001). We also found that patients belonging to R-ISS II and having high-risk chromosomal abnormalities (CA) had a significant shorter median OS than those with R-ISS II without CA: 70 vs. 111 months, respectively. Therefore, this study lends further support for the R-ISS as a reliable prognostic tool for estimating survival in transplant myeloma patients and suggests the importance of high-risk CA in the R-ISS II group.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since the introduction of proteasome inhibitors (PIs), immunomodulatory (IMIDs) drugs, and other novel agents, a continuous improvement of survival has been shown in multiple myeloma (MM), predominantly in younger patients [1, 2]. Novel agent-based induction, followed by high-dose melphalan and autologous stem cell transplantation (ASCT) is the standard of care for newly diagnosed, transplant-eligible, MM patients based on randomized trials showing improved progression-free survival (PFS) and overall survival (OS) [3,4,5,6,7].
MM is a heterogeneous disease with variability in response to treatment and survival due to the interaction between host factors and those intrinsic to disease biology [8]. In this setting, there is a continued need for and interest in devising reliable prognostic tools, not only in order to provide the accurate prognostic information possible to patients but also for adopting risk-adapted strategies to improve their survival and quality of life.
Several risk-stratification models have been developed. In 2005, the International Staging System (ISS) emerged, based solely on the serum albumin and β2-microglobulin concentration. Although the ISS has been widely validated, its prognostic value in the era of novel agents and transplant settings needs to be readdressed [9,10,11]. Evaluation of cytogenetic abnormalities (CA) and levels of lactate dehydrogenase (LDH) are important as these reflect disease biology, and both markers have independent prognostic value [12, 13]. Several studies have shown that high-risk CA, such as the presence of chromosome 14 translocations or 17p abnormalities, are the most important prognostic biomarkers in MM patients [14,15,16]. Other studies proposed a risk stratification model based on the combination of both host and disease factors: ISS and CA [17, 18] or LDH [19]. Finally, a new Revised-International Staging System (R-ISS) was developed by the International Myeloma Working Group (IMWG) that integrates the aforementioned ISS, LDH, and high-risk CA, resulting in three groups with different outcomes [20].
However, there is limited data on the applicability of this prognostic model in the setting of novel induction therapy followed by ASCT. Thus, the aim of this study is to determine whether the new R-ISS is a valid risk model for predicting survival in a cohort of unselected transplant MM patients, treated at Mayo Clinic in Arizona and the University Hospital of Salamanca in Spain. We show that R-ISS model allows a more accurate stratification of the transplant patients, identifying a high-intermediate risk group based on the presence of high-risk CA in the R-ISS II group.
Methods
We retrospectively studied a cohort of newly diagnosed MM patients who underwent ASCT and were followed up at Mayo Clinic in Arizona and University Hospital of Salamanca in Spain from January 2005 to December 2014. Both Institutional Review Boards approved the study and it was performed in accordance with the Declaration of Helsinki. Informed consent was obtained from all individual participants included in the study. To be eligible, we required a complete dataset including ability to determine ISS, LDH, and CA. Since these institutions are tertiary referral centers many patients were excluded as fluorescence in situ hybridization (FISH) was either not done, done without selection of plasma cells by cell separation or cIg technique, or LDH or β2-microglobulin data were lacking. Patients were often seen after induction therapy had been initiated by the referring physicians and obtaining testing for FISH at that point is not informative. All patients were required to have PI- or IMIDs-based therapies as induction treatment.
Baseline data were collected by searching at medical records database of Mayo Clinic and University Hospital of Salamanca and included data required for the assignment of the R-ISS described by Palumbo et at. [20]. Serum LDH level was classified as normal or high according to the normal range given by the local laboratory. High LDH was defined as higher than the upper limit of normal range and normal LDH was defined as a serum level lower than the upper limit of normal. Cytogenetic evaluation was performed by FISH as previously published [12, 14]. Response to treatment was evaluated according to International uniform response criteria for MM 2006 [21].
The primary and secondary endpoints were OS and PFS from ASCT, respectively. OS was considered the time from date of transplantation to death. PFS was defined as the time from date of transplantation to relapse, progression or death, regardless of cause. Patients without a recorded progression or death date were censored for PFS or OS at their last follow-up. Survival curves were plotted by Kaplan-Meier method, with differences assessed with the log-rank test. Effects of potential risk factors of progression were analyzed in a Cox proportional hazards model. The chi-square, t student and Mann-Whitney U tests were used to estimate the statistically significant differences among baseline characteristics. P values were considered at the conventional 5% significance level. All statistical analyses were performed using IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corp.
Results
Patient characteristics
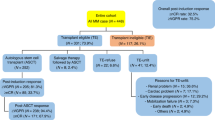
In all, 134 patients had complete data and subsequently were included in the study. A total of 92 out of 509 patients who consecutively underwent ASCT at Mayo Clinic and 42 out of 107 from the University Hospital of Salamanca. The baseline characteristics are summarized in Table 1. There were 70 (52%) men and 64 (48%) women. The median age at diagnosis was 62 years (range, 29–78 years), and 41 (45%) patients were older than 65 years at the time of ASCT. Fifteen (11%) patients presented with renal impairment (RI) at the time of diagnosis.
According to ISS, 62 (46%) patients had stage I, 38 (28%) stage II, and the remaining 34 (25%) stage III disease at diagnosis. In addition, there were 39 patients (29%) with high-risk CA: 18 patients (14%) with del17p; 17 patients (13%) with t(4;14); and 7 (5%) with t(14;16). Seventeen patients (13%) had high LDH levels. Consequently, patients were re-staged at diagnosis according to the R-ISS, resulting 44 patients (33%) with stage I, 75 (56%) with stage II, and 15 (11%) with stage III. Thus, 18 patients previously categorized as having low risk (ISS I) and 15 patients as high risk (ISS III) were reclassified as intermediate risk (R-ISS II), according to the new revised staging system (Table 2).
ASCT features and treatment response
Median time from diagnosis to ASCT was 6.7 months (range 4–69 months, Table 3). All patients received induction therapy before ASCT; 104 (78%) patients received PI-based therapy, with CyBorD (cyclophosphamide, bortezomib, and dexamethasone) as the preferred choice. One hundred and eleven (83%) patients responded to first line of induction and 23 (17%) were initially refractory and received more than one line of induction treatment (Table 4). In the majority of cases, high-doses of melphalan were used as conditioning, followed by infusion of autologous stem cells, with the median CD34+ cells-dose infused over 2 × 106/kg. Median times to engraftment were 11 (range 5–19) and 17 (range 7–34) days for neutrophils and platelets, respectively. Only one case of graft failure was reported.
Most patients underwent ASCT in at least partial response (PR), and there was an improvement in response category after ASCT, with an increase of complete response (CR) rate from 43 (32%) patients to 72 (54%) patients in CR before and after ASCT, respectively.
Survival analysis
The median follow-up was 59.6 months (range, 7.3–135.4 months). Forty-one (31%) patients died and 93 (69%) patients progressed or died at any time after ASCT. The median OS was 110.9 months (95% CI 86.6–135.2 months) from ASCT and the median PFS was 34.5 months (95% CI 29.7–42.1 months) from ASCT.
In our series, there were no statistically significant differences in terms of OS or PFS among groups according to ISS classification. However, patients classified as having high-risk CA showed a statistically significant shorter median OS than those with standard-risk CA 57.4 months vs. median OS not reached (NR) (P = 0.001), respectively. Patients with high LDH levels had a significantly inferior OS, 36.1 vs. NR (P < 0.001), compared with patients with normal LDH levels at diagnosis.
As expected, patients with R-ISS III had a significantly shorter median OS from ASCT compared to patients with R-ISS II or R-ISS I (37.2 vs. 110.9 months vs. not reached, respectively, P = 0.0001) (Fig. 1). The probability for 5-year OS was 28, 68, and 86% per R-ISS III, II, and I, respectively. Although there were more cases with renal impairment and anemia at diagnosis in the R-ISS III group, no statistically significant differences in baseline characteristics were identified among groups to explain the differences in OS observed, as shown in Table 2. Patients who belonged to R-ISS III group had also a significantly shorter median PFS than the R-ISS II and R-ISS I groups: 19.2 vs. 36.1 vs. 35.2 months, respectively (P = 0.05) (Fig. 2).
Overall survival (OS) by R-ISS risk group. OS was defined as time from date of transplantation until date of death. There were statistically significant differences in OS with median OS not reached, 110.9 and 37.2 months in R-ISS risk groups I, II, and III, respectively. The probability of OS at 5 years was 28, 68, and 86% for R-ISS III, II, and I, respectively
In order to explore whether the presence of high-risk CA had an impact on the survival of the R-ISS II group, we re-classified R-ISS II into two subgroups according to the presence or not of high-risk CA and we compared both of them with either R-ISS I and III. As a result, we had four groups with significantly different OS: 44 (33%) patients with R-ISS I and a median OS NR; 50 (37%) patients with R-ISS IIa (not R-ISS stage I or III and absence of high-risk CA) with a median OS of 110.9 months; 25 (19%) patients with R-ISS IIb (not R-ISS stage I or III but presence of high-risk CA) with a median OS of 69.8 months; and 15 (11%) patients with R-ISS III and a median OS of 37.2 months, P < 0.001, (Fig. 3). Although there were no statistically significant differences among groups, there was a trend towards shorter PFS in the R-ISS IIb group compare with PFS in either R-ISS I or IIa: 32.9 vs. 35.2 vs. 48.2 months, respectively (P = 0.06).
Overall survival (OS) by R-ISS, incorporating new subclassification of R-ISS group II by presence or absence of high risk chromosomal abnormalities. R-ISS group II was subclassified into groups IIa (defined as not group I or III and lack of high risk chromosomal abnormalities (t(4;14), t(14;16) or del17p) and groups IIb (defined as not R-ISS group I or III and presence of high risk chromosomal abnormalities). Median OS was not reached, 110.9 months, 69.8 months and 37.2 months in R-ISS groups I, IIa, IIb, and III, respectively
Additional risk factors for OS were identified in the univariate analysis such as renal impairment at diagnosis (median OS 37.2 months; P < 0.001) and ≥ 2 lines of induction treatment (median OS 60.9 months; P = 0.005) (Table 5). All patients were included in the multivariate analysis and R-ISS was selected as an important independent predictor for OS. An increase HR of 6.9, 95% CI 2.4–20.3; P = 0.001 was observed between R-ISS III and R-ISS I, and a HR of 1.5, 95% CI 0.6–3.6; NS, between R-ISS II and R-ISS I. Another discriminating factor for OS was ≥ 2 lines of induction before ASCT (HR = 3.8, 95% CI 1.7–8.5; P = 0.001).
In addition, independent-risk factors for shorter PFS were identified in the multivariate analysis such as R-ISS III (HR = 2.9, 95% CI 1.3–6.6; P = 0.008) and having received more than 1 line of induction before ASCT (HR = 2.8, 95% CI 1.5–5.1; P = 0.001). The achievement of CR on day 100 after ASCT and having received maintenance treatment were independently associated with longer PFS, as shown in the multivariate analysis in Table 5.
Discussion
We showed in this study that R-ISS is a reproducible and applicable method to robustly predict survival in MM patients who underwent ASCT. This is one of the first evaluations and validations of the R-ISS in transplant MM patients.
We present a representative series of exclusively transplant patients who received novel agents with outcomes comparable to those recently reported in prospective and randomized controlled studies, with 80% of patients achieving very good partial response or better after ASCT, median PFS of 45 months and 4 year-OS of 81% [3, 4, 6, 7].
According to the results recently reported by several studies which evaluate the R-ISS in myeloma patients [20, 22,23,24,25,26], the distribution of patients is quite similar, particularly stage III (10–17% in these studies), with a slightly higher proportion of R-ISS I cases in our series (33% vs. 18–28%). This could be explained by the fact that the aforementioned studies included elderly population as well, with potentially more cases of hypoalbuminemia and chronic renal disease [27]. Regarding survival, although our R-ISS stage III patients displayed a shorter OS than patients with stage III in the study of Palumbo et al. [20], it is important to point out that the original study was developed in a selected cohort of patients enrolled in clinical trials, and our results are closer to those reported by the Greek and the British group in unselected MM patients cohorts [23, 25].
Although only 134 patients had complete data, they were precisely those who were consecutively diagnosed and treated at Mayo Clinic in Arizona and University Hospital of Salamanca, whereas the remaining patients were referred to these centers after induction treatment for the transplant procedure and diagnosis data were not completely available. Thus, our series may be considered a representative sample of newly diagnosed transplant patients.
An immediate consequence of re-staging myeloma patients with the R-ISS model is that patients belonging to either ISS I with additional high-risk features (high LDH or high-risk CA) or ISS III with low-risk features (normal LDH and standard-risk CA) are now reclassified as R-ISS II, with accurate identification of low and high-risk patients according to their OS: median NR and 37 months, respectively. As showed in the above studies, there is a higher proportion of patients categorized as R-ISS II, including patients with high-risk CA belonging to either ISS II or ISS I. Several studies have shown that genetic events have a determinant role in prognosis in myeloma patients [12, 14, 19]. Based on this, we explored the impact of the presence of high-risk CA on R-ISS II survival. This group of patients was split into IIa and IIb groups, according to the absence or presence of high-risk CA, respectively. Interestingly, statistically significant differences in survival were found between IIa and IIb stages: median OS 111 and 70 months, respectively. These findings need to be validated in independent and prospective studies, and perhaps will allow improvement of the risk assessment of not only the low and high-risk transplant myeloma patients, but also the intermediate risk group, identifying low-intermediate and a high-intermediate risk subgroups according to the presence or not of del17p, t(4;14) or t(14;16).
In addition, we compared the prognostic value of R-ISS with other potential predictors of survival in our series of transplant patients and we showed that R-ISS is an independent prognostic marker. R-ISS model employs a feasible evaluation of both host factors and disease biology, encompassing ISS staging, LDH, and genetics. All of these factors should be assessed in the diagnosis work-up according to the IMWG recommendations [28, 29]. ISS was not selected as a predictor of survival in this cohort of patients exclusively treated with novel agents; this result is consistent with other studies [10, 11, 26] and could be explained by the fact that ISS model was developed before 2002 when only a minority of patients had received IMID- or PI-based therapies.
Interestingly, all of the patients were treated with a novel agent-based induction and most of them achieved partial response or better before ASCT; however, those patients who were refractory to the first line of induction and needed at least a second line had a 3-fold higher risk of progression or fourfold higher risk of death after ASCT. In fact, treatment response is a well-known surrogate marker of PFS and, most importantly, OS [30,31,32]. Indeed, response 100 days after ASCT was an independent predictor for PFS, with 60% reduction in risk of progression or death in those who achieved CR after ASCT, as shown in the multivariate analysis performed in our study.
In summary, R-ISS assessed at diagnosis was an independent predictor for OS after ASCT in our series, with median OS for the different R-ISS groups comparable to those reported by Palumbo et al. in their subgroup of younger patients [20]. Thus, this study lends further support for the R-ISS as a reliable prognostic tool for estimating OS in transplant-eligible MM patients with one contribution: the identification of R-ISS II subgroups according to the presence or not of high-risk CA. If this observation is validated in other independent studies, this approach might help to improve the risk-stratification of transplant patients and thus, accurately predict survival and define tailored treatment strategies.
References
Kumar SK, Rajkumar SV, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK, Zeldenrust SR, Dingli D, Russell SJ, Lust JA, Greipp PR, Kyle RA, Gertz MA (2008) Improved survival in multiple myeloma and the impact of novel therapies. Blood 111(5):2516–2520. https://doi.org/10.1182/blood-2007-10-116129
Fonseca R, Abouzaid S, Bonafede M, Cai Q, Parikh K, Cosler L, Richardson P (2017) Trends in overall survival and costs of multiple myeloma, 2000-2014. Leukemia 31:1915–1921. https://doi.org/10.1038/leu.2016.380
Palumbo A, Cavallo F, Gay F, Di Raimondo F, Ben Yehuda D, Petrucci MT, Pezzatti S, Caravita T, Cerrato C, Ribakovsky E, Genuardi M, Cafro A, Marcatti M, Catalano L, Offidani M, Carella AM, Zamagni E, Patriarca F, Musto P, Evangelista A, Ciccone G, Omede P, Crippa C, Corradini P, Nagler A, Boccadoro M, Cavo M (2014) Autologous transplantation and maintenance therapy in multiple myeloma. N Engl J Med 371(10):895–905. https://doi.org/10.1056/NEJMoa1402888
Gay F, Oliva S, Petrucci MT, Conticello C, Catalano L, Corradini P, Siniscalchi A, Magarotto V, Pour L, Carella A, Malfitano A, Petro D, Evangelista A, Spada S, Pescosta N, Omede P, Campbell P, Liberati AM, Offidani M, Ria R, Pulini S, Patriarca F, Hajek R, Spencer A, Boccadoro M, Palumbo A (2015) Chemotherapy plus lenalidomide versus autologous transplantation, followed by lenalidomide plus prednisone versus lenalidomide maintenance, in patients with multiple myeloma: a randomised, multicentre, phase 3 trial. Lancet Oncol 16(16):1617–1629. https://doi.org/10.1016/s1470-2045(15)00389-7
Biran N, Jacobus S, Vesole DH, Callander NS, Fonseca R, Williams ME, Abonour R, Katz MS, Rajkumar SV, Greipp PR, Siegel DS (2016) Outcome with lenalidomide plus dexamethasone followed by early autologous stem cell transplantation in patients with newly diagnosed multiple myeloma on the ECOG-ACRIN E4A03 randomized clinical trial: long-term follow-up. Blood Cancer J 6(9):e466. https://doi.org/10.1038/bcj.2016.68
Cavo M, Palumbo A, Zweegman S, Dimopoulos M, Hajek R, Pantani L (2016) Upfront autologous stem cell transplantation (ASCT) versus novel agent-based therapy for multiple myeloma (MM): a randomized phase 3 study of the European Myeloma Network (EMN02/HO95 MM trial). J Clin Oncol Off J Am Soc Clin Oncol 34:15
Attal M, Lauwers-Cances V, Hulin C, Facon T, Caillot D, Escoffre M, Arnulf B, Macro M, Belhadj K, Garderet L (2015) Autologous transplantation for multiple myeloma in the era of new drugs: a phase III study of the Intergroupe Francophone du Myelome (IFM/DFCI 2009 trial). Blood 126(23):391–391
Fonseca R, San Miguel J (2007) Prognostic factors and staging in multiple myeloma. Hematol Oncol Clin North Am 21(6):1115–1140, ix. https://doi.org/10.1016/j.hoc.2007.08.010
Kastritis E, Zervas K, Symeonidis A, Terpos E, Delimbassi S, Anagnostopoulos N, Michali E, Zomas A, Katodritou E, Gika D, Pouli A, Christoulas D, Roussou M, Kartasis Z, Economopoulos T, Dimopoulos MA (2009) Improved survival of patients with multiple myeloma after the introduction of novel agents and the applicability of the International Staging System (ISS): an analysis of the Greek Myeloma Study Group (GMSG). Leukemia 23(6):1152–1157. https://doi.org/10.1038/leu.2008.402
Takamatsu H, Honda S, Miyamoto T, Yokoyama K, Hagiwara S, Ito T, Tomita N, Iida S, Iwasaki T, Sakamaki H, Suzuki R, Sunami K (2015) Changing trends in prognostic factors for patients with multiple myeloma after autologous stem cell transplantation during the immunomodulator drug/proteasome inhibitor era. Cancer Sci 106(2):179–185. https://doi.org/10.1111/cas.12594
Lu J, Liu A, Fu W, Du J, Huang X, Chen W, Hou J (2015) The applicability of the international staging system in Chinese patients with multiple myeloma receiving bortezomib or thalidomide-based regimens as induction therapy: a multicenter analysis. Biomed Res Int 2015:856704. https://doi.org/10.1155/2015/856704
Fonseca R, Blood E, Rue M, Harrington D, Oken MM, Kyle RA, Dewald GW, Van Ness B, Van Wier SA, Henderson KJ, Bailey RJ, Greipp PR (2003) Clinical and biologic implications of recurrent genomic aberrations in myeloma. Blood 101(11):4569–4575. https://doi.org/10.1182/blood-2002-10-3017
Avet-Loiseau H, Attal M, Moreau P, Charbonnel C, Garban F, Hulin C, Leyvraz S, Michallet M, Yakoub-Agha I, Garderet L, Marit G, Michaux L, Voillat L, Renaud M, Grosbois B, Guillerm G, Benboubker L, Monconduit M, Thieblemont C, Casassus P, Caillot D, Stoppa AM, Sotto JJ, Wetterwald M, Dumontet C, Fuzibet JG, Azais I, Dorvaux V, Zandecki M, Bataille R, Minvielle S, Harousseau JL, Facon T, Mathiot C (2007) Genetic abnormalities and survival in multiple myeloma: the experience of the Intergroupe Francophone du Myelome. Blood 109(8):3489–3495. https://doi.org/10.1182/blood-2006-08-040410
Gutierrez NC, Castellanos MV, Martin ML, Mateos MV, Hernandez JM, Fernandez M, Carrera D, Rosinol L, Ribera JM, Ojanguren JM, Palomera L, Gardella S, Escoda L, Hernandez-Boluda JC, Bello JL, de la Rubia J, Lahuerta JJ, San Miguel JF, Group GPS (2007) Prognostic and biological implications of genetic abnormalities in multiple myeloma undergoing autologous stem cell transplantation: t(4;14) is the most relevant adverse prognostic factor, whereas RB deletion as a unique abnormality is not associated with adverse prognosis. Leukemia 21(1):143–150. https://doi.org/10.1038/sj.leu.2404413
Kapoor P, Fonseca R, Rajkumar SV, Sinha S, Gertz MA, Stewart AK, Bergsagel PL, Lacy MQ, Dingli DD, Ketterling RP, Buadi F, Kyle RA, Witzig TE, Greipp PR, Dispenzieri A, Kumar S (2010) Evidence for cytogenetic and fluorescence in situ hybridization risk stratification of newly diagnosed multiple myeloma in the era of novel therapie. Mayo Clin Proc 85(6):532–537. https://doi.org/10.4065/mcp.2009.0677
Kaufman GP, Gertz MA, Dispenzieri A, Lacy MQ, Buadi FK, Dingli D, Hayman SR, Kapoor P, Lust JA, Russell S, Go RS, Hwa YL, Kyle RA, Rajkumar SV, Kumar SK (2016) Impact of cytogenetic classification on outcomes following early high-dose therapy in multiple myeloma. Leukemia 30(3):633–639. https://doi.org/10.1038/leu.2015.287
Neben K, Jauch A, Bertsch U, Heiss C, Hielscher T, Seckinger A, Mors T, Muller NZ, Hillengass J, Raab MS, Ho AD, Hose D, Goldschmidt H (2010) Combining information regarding chromosomal aberrations t(4;14) and del(17p13) with the International Staging System classification allows stratification of myeloma patients undergoing autologous stem cell transplantation. Haematologica 95(7):1150–1157. https://doi.org/10.3324/haematol.2009.016436
Avet-Loiseau H, Durie BG, Cavo M, Attal M, Gutierrez N, Haessler J, Goldschmidt H, Hajek R, Lee JH, Sezer O, Barlogie B, Crowley J, Fonseca R, Testoni N, Ross F, Rajkumar SV, Sonneveld P, Lahuerta J, Moreau P, Morgan G (2013) Combining fluorescent in situ hybridization data with ISS staging improves risk assessment in myeloma: an international myeloma working group collaborative project. Leukemia 27(3):711–717. https://doi.org/10.1038/leu.2012.282
Moreau P, Cavo M, Sonneveld P, Rosinol L, Attal M, Pezzi A, Goldschmidt H, Lahuerta JJ, Marit G, Palumbo A, van der Holt B, Blade J, Petrucci MT, Neben K, san Miguel J, Patriarca F, Lokhorst H, Zamagni E, Hulin C, Gutierrez N, Facon T, Caillot D, Benboubker L, Harousseau JL, Leleu X, Avet-Loiseau H, Mary JY (2014) Combination of international scoring system 3, high lactate dehydrogenase, and t(4;14) and/or del(17p) identifies patients with multiple myeloma (MM) treated with front-line autologous stem-cell transplantation at high risk of early MM progression-related death. J Clin Oncol 32(20):2173–2180. https://doi.org/10.1200/jco.2013.53.0329
Palumbo A, Avet-Loiseau H, Oliva S, Lokhorst HM, Goldschmidt H, Rosinol L, Richardson P, Caltagirone S, Lahuerta JJ, Facon T, Bringhen S, Gay F, Attal M, Passera R, Spencer A, Offidani M, Kumar S, Musto P, Lonial S, Petrucci MT, Orlowski RZ, Zamagni E, Morgan G, Dimopoulos MA, Durie BG, Anderson KC, Sonneveld P, San Miguel J, Cavo M, Rajkumar SV, Moreau P (2015) Revised international staging system for multiple myeloma: a report from international myeloma working group. J Clin Oncol 33(26):2863–2869. https://doi.org/10.1200/jco.2015.61.2267
Durie BG, Harousseau JL, Miguel JS, Blade J, Barlogie B, Anderson K, Gertz M, Dimopoulos M, Westin J, Sonneveld P, Ludwig H, Gahrton G, Beksac M, Crowley J, Belch A, Boccadaro M, Cavo M, Turesson I, Joshua D, Vesole D, Kyle R, Alexanian R, Tricot G, Attal M, Merlini G, Powles R, Richardson P, Shimizu K, Tosi P, Morgan G, Rajkumar SV (2006) International uniform response criteria for multiple myeloma. Leukemia 20(9):1467–1473. https://doi.org/10.1038/sj.leu.2404284
Jimenez-Zepeda VH, Duggan P, Neri P, Rashid-Kolvear F, Tay J, Bahlis NJ (2016) Revised international staging system applied to real world multiple myeloma patients. Clin Lymphoma Myeloma Leuk 16(9):511–518. https://doi.org/10.1016/j.clml.2016.06.001
Kastritis E, Terpos E, Roussou M, Gavriatopoulou M, Migkou M, Eleutherakis-Papaiakovou E, Fotiou D, Ziogas D, Panagiotidis I, Kafantari E, Giannouli S, Zomas A, Konstantopoulos K, Dimopoulos MA (2016) Evaluation of the Revised International Staging System (R-ISS) in an independent cohort of unselected patients with multiple myeloma. Haematologica 102:593–599. https://doi.org/10.3324/haematol.2016.145078
Tandon N, Rajkumar SV, LaPlant B, Pettinger A, Lacy MQ, Dispenzieri A, Buadi FK, Gertz MA, Hayman SR, Leung N, Go RS, Dingli D, Kapoor P, Lin Y, Hwa YL, Fonder AL, Hobbs MA, Zeldenrust SR, Lust JA, Gonsalves WI, Russell SJ, Kumar SK (2017) Clinical utility of the Revised International Staging System in unselected patients with newly diagnosed and relapsed multiple myeloma. Blood Cancer J 7(2):e528. https://doi.org/10.1038/bcj.2017.13
Walker I, Coady A, Neat M, Ladon D, Benjamin R, El-Najjar I, Kazmi M, Schey S, Streetly M (2016) Is the revised international staging system for myeloma valid in a real world population? Br J Haematol 180:451–454. https://doi.org/10.1111/bjh.14341
Cho H, Yoon DH, Lee JB, Kim SY, Moon JH, Do YR, Lee JH, Park Y, Lee HS, Eom HS, Shin HJ, Min CK, Kim JS (2017) Comprehensive evaluation of the revised international staging system in multiple myeloma patients treated with novel agents as a primary therapy. Am J Hematol 92(12):1280–1286. doi:https://doi.org/10.1002/ajh.24891
Bataille R, Annweiler C, Beauchet O (2013) Multiple myeloma international staging system: “staging” or simply “aging” system? Clin Lymphoma Myeloma Leuk 13(6):635–637. https://doi.org/10.1016/j.clml.2013.07.003
Dimopoulos M, Kyle R, Fermand JP, Rajkumar SV, San Miguel J, Chanan-Khan A, Ludwig H, Joshua D, Mehta J, Gertz M, Avet-Loiseau H, Beksac M, Anderson KC, Moreau P, Singhal S, Goldschmidt H, Boccadoro M, Kumar S, Giralt S, Munshi NC, Jagannath S (2011) Consensus recommendations for standard investigative workup: report of the international myeloma workshop consensus panel 3. Blood 117(18):4701–4705. https://doi.org/10.1182/blood-2010-10-299529
Fonseca R, Bergsagel PL, Drach J, Shaughnessy J, Gutierrez N, Stewart AK, Morgan G, Van Ness B, Chesi M, Minvielle S, Neri A, Barlogie B, Kuehl WM, Liebisch P, Davies F, Chen-Kiang S, Durie BG, Carrasco R, Sezer O, Reiman T, Pilarski L, Avet-Loiseau H (2009) International myeloma working group molecular classification of multiple myeloma: spotlight review. Leukemia 23(12):2210–2221. https://doi.org/10.1038/leu.2009.174
Lahuerta JJ, Mateos MV, Martinez-Lopez J, Rosinol L, Sureda A, de la Rubia J, Garcia-Larana J, Martinez-Martinez R, Hernandez-Garcia MT, Carrera D, Besalduch J, de Arriba F, Ribera JM, Escoda L, Hernandez-Ruiz B, Garcia-Frade J, Rivas-Gonzalez C, Alegre A, Blade J, San Miguel JF (2008) Influence of pre- and post-transplantation responses on outcome of patients with multiple myeloma: sequential improvement of response and achievement of complete response are associated with longer survival. J Clin Oncol Off J Am Soc Clin Oncol 26(35):5775–5782. https://doi.org/10.1200/JCO.2008.17.9721
Harousseau JL, Attal M, Avet-Loiseau H (2009) The role of complete response in multiple myeloma. Blood 114(15):3139–3146. https://doi.org/10.1182/blood-2009-03-201053
Majithia N, Rajkumar SV, Lacy MQ, Buadi FK, Dispenzieri A, Gertz MA, Hayman SR, Dingli D, Kapoor P, Hwa L, Lust JA, Russell SJ, Go RS, Kyle RA, Kumar SK (2016) Early relapse following initial therapy for multiple myeloma predicts poor outcomes in the era of novel agents. Leukemia 30(11):2208–2213. https://doi.org/10.1038/leu.2016.147
Funding
Verónica González-Calle was supported by the Fundación Española de Hematología y Hemoterapia and Janssen (Beca Estancias de Investigación en el Extranjero Convocatoria 2015-2016).
Author information
Authors and Affiliations
Contributions
RF, VGC, and AS conceived and designed the work that led to the submission. VGC, AS, SL, KEP, and RPK acquired data. VGC analyzed, interpreted the data and drafted the manuscript and RF and NK revised the manuscript. All the authors approved the final version.
Corresponding author
Ethics declarations
Disclosures
Dr. Rafael Fonseca: Consulting with AMGEN, BMS, Celgene, Takeda, Bayer, Jansen, Novartis, Pharmacyclics, Sanofi and Merck. Member of the Scientific Advisory Board of Adaptive Biotechnologies. Mayo Clinic and Dr. Fonseca hold a patent for the prognostication of myeloma via FISH with annual income of about $2K dollars. There are no conflicts of interest declared by the rest of the authors.
Rights and permissions
About this article
Cite this article
González-Calle, V., Slack, A., Keane, N. et al. Evaluation of Revised International Staging System (R-ISS) for transplant-eligible multiple myeloma patients. Ann Hematol 97, 1453–1462 (2018). https://doi.org/10.1007/s00277-018-3316-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-018-3316-7