Abstract
The aim of this study was to explore the clinical features and prognostic significance of CSF3R mutations in AML patients with CEBPA double mutations (CEBPAdm). One hundred one AML patients with CEBPAdm were retrospectively analyzed in this study. Mutation status of CSF3R gene, clinical features, and long-term outcomes were analyzed. The frequency of CSF3R mutations in patients with CEBPAdm was 19.80% (20/101). Patients with CSF3R mutations were associated with a lower platelet (u = 2.728, P = 0.006) and higher leukocytes (u = 3.178, P = 0.001) compared with those with wide-type CSF3R gene. The 5-year relapse-free survival (RFS) was 18.7% in patients with CSF3R mutations, which was significantly lower than those with wide-type CSF3R (31.8%) (P = 0.015). The 5-year overall survival (OS) was also significantly different between patients with and without CSF3R mutations (17.5% versus 57.4%, P = 0.019). The prevalence of CSF3R mutations was high in AML patients with CEBPAdm, which indicated a poor prognosis, and CSF3R mutations may be a new potential candidate for prognostically re-stratifying AML patients with CEBPAdm.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acquired genetic abnormalities have an essential role in the pathogenesis of acute myeloid leukemia (AML). Systemic studies of the genomic landscape of AML, such as the date from the Cancer Genome Atlas (TCGA), revealed AML as a heterogeneous disease with nearly 2000 somatically mutated genes observed across 200 patients [1]. Cytogenetic and sequencing analyses of AML patients have revealed at least 11 genetic classes with distinct diagnostic features and clinical outcomes [2]. AML mutational status was reported to be associated with drug sensitivity in a recent study from the Beat AML program [3]. Deep sequencing of AML showed that the frequency of CCAAT/enhancer-binding protein α (CEBPA) gene mutations was less than 10% [1,2,3]. However, we found a relatively high incidence rate of CEBPA mutations (18.99%) by next-generation sequencing analysis of 553 consecutive AML patients [4]. The subgroup of CEBPAdm in AML patients has now been acknowledged in “The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia” as a definite entity, given its distinct biological and clinical features, as well as its prognostic significance [5]. AML with CEBPAdm was also identified as one of the 11 genomic subgroups proposed by Papaemmanuil and colleagues [2]. Although patients with CEBPAdm were associated with favorable outcomes [2, 6, 7], in recent years, the heterogeneity of patients with CEBPAdm was reported [4, 8]. However, how to re-stratify those patients was unclear.
Acquired mutations in granulocyte colony-stimulating factor receptor (CSF3R) gene are present in a majority of chronic neutrophilic leukemia and atypical chronic myeloid leukemia [9]. The frequency of CSF3R mutations was reported to be 1~3% in AML [10, 11]. However, in patients with CEBPAdm, the incidence rate of CSF3R mutations increased to approximately 16~30%, but the prognostic significance was not clear [4, 10, 11]. In this study, the clinical characteristics and prognostic value of CSF3R mutations in AML patients with CEBPAdm were explored.
Patients and methods
Patients and treatment
From January 1, 2012, to December 31, 2018, 101 AML patients with CEBPAdm from our hospital were retrospectively analyzed, which were categorized into FAB subtypes (M0–M7) based on morphological diagnoses. Patients were treated with the standard “3 + 7” regimen (darubicin/idarubicin + cytarabine) or CAG (aclarubicin + cytarabine + G-SCF) regimen for induction therapy. The response was assessed by bone marrow aspiration performed on days 14 and 28. The first consolidation therapy was the same as that generally used to achieve CR. Three to four courses of scheduled, high-dose cytarabine, at 1.5~3.0 g/m2, were administrated for consolidation therapy. All the patients gave informed consent prior to enrolment in the study. This study was approved by the ethics committee of Jilin University and conducted in accordance with the Declaration of Helsinki.
Cytogenetic and molecular mutation screening
Standard-culturing and chromosome-banding techniques were used to analyze the karyotypes. Their clonal abnormalities were defined and described according to the International System for Human Cytogenetic Nomenclature [12]. A sensitive next-generation sequencing was performed to screen molecular mutations in AML patients as described in our previously published study [4].
Statistics
Statistics Package for Social Sciences (SPSS) software (Version 17.0, SPSS Inc., Chicago, IL, USA) was used to calculate the statistical difference. For continuous variables, independent samples t test or Mann-Whitney U test was used to compare the differences between groups. The chi-square test or Fisher’s exact test was used to assess the statistical significance of differences between groups for categorical variables. The Kaplan-Meier method was employed for survival analysis, and the log-rank test was used to compare the differences between groups. P < 0.05 was considered significant.
Results
General characteristics of the patients
This cohort of AML patients was comprised of 53 male and 48 female patients with CEBPAdm. The median age was 43 years (9~79 years). Based on FAB classification, M2 had the highest frequency of 54.46% (55/101). The frequencies of M1, M4, M5, and M6 were 5.94% (6/101), 29.70% (30/101), 5.94% (6/101), and 3.96% (4/101), respectively. Seventy-eight patients successfully completed cytogenetic analysis; 72 cases (92.31%) presented with normal karyotype.
The characteristics of CEBPA mutations
Of the 101 patients with CEBPAdm, three patients presented with three mutation sites, two with two sites in the N-terminal, and the other one with two sites in the C-terminal. Totally, 205 genetic mutations, classifiable into 134 different kinds, were detected in the CEBPA gene. The most common mutated site was c.68dupC (7.32%, 15/205), followed by c.939_940insAAG (5.85%, 12/205) and c.936_937insCAG (4.88%, 10/205). The top ten mutation types were listed in Fig. 1.
The most common CEBPA mutation type was in-frame insertions (35.12%, 72/205), followed by frame-shift insertions (31.22%, 64/205). The frequencies of frame-shift deletions and in-frame deletions were 17.07% (35/205) and 9.27% (19/205), respectively. The occurrence rates of missense mutations and stop-gain mutations were 5.13% (11/205) and 3.85% (4/205), respectively. A majority of CEBPAdm patients (79.21%, 80/101) showed a combination of an N-terminal frame-shift and a C-terminal in-frame mutation.
The characteristics of CSF3R mutations
CSF3R mutations were detected in 20 patients, in which 15 patients (75.00%) carried T618I mutation. Four patients presented with two kinds of mutations (no. 4, no. 5, no. 9, and no. 19). The mutation information of CSF3R gene was summarized in Table 1.
Clinical characteristics of patients with CSF3R mutations
The clinical characteristics of patients with mutated and wide-type CSF3R gene were listed in Table 2. Compared with patients with wide-type CSF3R, those with mutated CSF3R presented with higher white blood cell counts (u = 3.178, P = 0.001) and lower platelet (u = 2.728, P = 0.006) in peripheral blood (Table 2).
Therapeutic response and long-term outcomes
Of the 101 AML patients with CEBPAdm, 22 refused chemotherapy. Totally, 79 patients received one course of induction therapy, 63 (79.75%) achieved complete remission (CR), 14 (17.72%) achieved a partial remission (PR), and the remaining two cases were evaluated as non-remission (NR). The CR rate was not significantly different between patients with and without CSF3R mutations (88.89% versus 77.05%; χ2 = 0.585, P = 0.445).
A total of 72 patients who received at least two courses of high-dose cytarabine consolidation entered long-term follow-up. The follow-up time ranged from 3 to 78 months (median, 18.5 months). Seven patients received allogeneic hematopoietic stem cell transplantation (one was core-blood transplantation). Five patients carried mutated CSF3R, and the remaining 2 cases were determined as wide-type CSF3R. Finally, 27 patients relapsed, and 20 patients died.
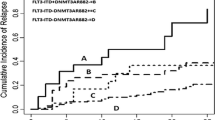
The 1-year, 3-year, and 5-year relapse-free survival (RFS) were 95.4%, 85.4%, 50.7%, respectively (Fig. 2a). The 1-year, 3-year, and 5-year overall survival (OS) were 97.1%, 93.6%, and 61.8%, respectively (Fig. 2b). The 5-year RFS was 18.7% in patients with CSF3R mutations (n = 18), which was significantly lower compared with those with wide-type CSF3R (31.8%) (n = 54) (median RFS, 11 months versus 44 months) (P = 0.015) (Fig. 3a). The 5-year OS was also significantly different between patients with and without CSF3R mutations (17.5% versus 57.4%, P = 0.019) (median RFS, 27 months versus not reaching) (Fig. 3b).
Discussion
Genetic mutations can reveal the etiology of AML to some extent and offer some beneficial information for selecting therapeutic regimens and evaluating prognoses. Genomic landscapes of de novo AML showed that recurring coding mutations were discovered in 98.5% of patients, and 2585 validated somatic mutations in coding regions of the genome were discovered, with an average of 13 mutations per sample [1]. The Beat study also confirmed that a median of 13 somatic variants could be detected in patients with AML [3]. Papaemmanuil et al. reported that 96% of the samples had at least 1 driver mutation, and 86% of the samples carried at least 2 driver mutations [2]. The top 33 most common mutated genes across Beat AML and TCGA showed general similar frequencies, with higher occurrence of mutations in serine- and arginine-rich splicing factor 2 (SRSF2) observed in the Beat study [1, 3]. Our unpublished data demonstrated similar frequencies of these mutated genes, but there were some differences existed. Furthermore, the incidence rate of CEBPA mutations was higher in our cohort than those in Beat and TCGA AML studies (~ 6%) [1, 3]. The frequency of CEBPAdm was 4.29% in the German-Austrian AML study group [2], which was significantly lower than that in our cohort of patients. Patients with CEBPAdm are ascribed as a favorable group in both the NCCN guideline and European Leukemia Net. However, the heterogeneity of patients with CEBPAdm was also reported by some studies [4, 8]. As a result, increasing researchers have paid their attention to re-stratifying AML patients with CEBPAdm.
A combination of an N-terminal frame-shift and a C-terminal in-frame mutation was present in a high proportion of patients in this study, which was consistent with previous study [13]. Fasen et al. reported that the most frequent mutation site of CEBPA gene was p.Lys313del, followed by p.His24Alafs and p.Gln312del [13]. The most commonly mutated sites in this study were p.Pro23fs (7.32%, 15/205), p.Lys313_Val314insLys (5.85%, 12/205), and p.Gln312_Lys313insGln (4.88%, 10/205). This difference may be derived from ethnic backgrounds and geographic factors.
The frequency of CSF3R mutations was approximately 1~3% in the entire cohort of AML patients [10, 11]. We observed that the incidence rate of CSF3R mutations was significantly high (19.80%) in AML patients with CEBPAdm. Lavallée et al. analyzed 415 patients in their study and found 7 patients with CSF3R mutations (1.69%). Of 14 patients with CEBPAdm, four carried CSF3R mutations (28.57%, 4/14) [10]. In a cohort of 787 pediatric AML patients, 19 were detected with CSF3R mutations (2.41%). Of 53 patients with CEBPAdm, nine carried CSF3R mutations (16.98%, 9/53) [11]. Accordingly, both this and the previous studies indicated that the prevalence of CSF3R mutations was high in AML patients with CEBPAdm.
Of the 20 patients with CSF3R mutations, the majority of cases (n = 15, 75.00%) were determined as T618I mutation, which was also the most common mutation of CSF3R gene reported in the previous study [11]. Considering such fact and aberrant activation of the JAK-STAT signaling pathway, Lavallée et al. explored the sensitivity of these leukemia cells to JAK inhibitors. They found that leukemia cells with CSF3R mutations showed the most sensitivity to JAK inhibitor, ruxolitinib [10]. Hence, T618I was the major mutation type of CSF3R gene, and JAK inhibitors were the potential drugs to treat these patients.
CSF3R mutations occurred in higher rates in male than in female patients, and the frequency of M5 was lower in CSF3R-mutated patients than in those with wide-type CSF3R [11]. Patients with CSF3R mutations presented with lower bone marrow blasts compared with those without mutation [11]. In this study, we found that platelet was significantly lower in patients with CSF3R mutations compared with those without mutation. However, the white blood cell counts were significantly higher in CSF3R-mutated patients than in those without mutation. Further study is still needed to explore the clinical characteristics of patients with CSF3R mutations due to a relatively small number of participates included in both this and previous studies.
Prognostic re-stratification of AML patients with CEBPAdm has been reported in some studies [4, 14, 15]. GATA2 mutation was one of the most common mutations in patients with CEBPAdm, but its prognostic significance was controversial [13, 14]. In patients with CEBPAdm, the prognostic significance of CSF3R mutations was not clear. In our previous study, we found that CSF3R mutation was associated with an inferior RFS, but had no influence on OS [4]. After prolonging the follow-up time and increasing patient sample, in the present study, we found that CSF3R mutations were related to unfavorable outcomes for patients with CEBPAdm in terms of RFS and OS.
In conclusion, this study suggested that the prevalence of CSF3R mutations was high in AML patients with CEBPAdm. Patients with CSF3R mutations presented with a lower platelet and higher white blood cells in peripheral blood compared with those without mutation. Patients with mutated CSF3R had inferior RFS and OS compared with those with wide-type CSF3R. Accordingly, CSF3R mutations may be a new potential marker for prognostic re-stratification in AML patients with CEBPAdm.
References
Cancer Genome Atlas Research Network (2013) Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N Engl J Med 368:2059–2074. https://doi.org/10.1056/NEJMoa1301689
Papaemmanuil E, Gerstung M, Bullinger L, Gaidzik VI, Paschka P, Roberts ND, Potter NE, Heuser M, Thol F, Bolli N, Gundem G, van Loo P, Martincorena I, Ganly P, Mudie L, McLaren S, O’Meara S, Raine K, Jones DR, Teague JW, Butler AP, Greaves MF, Ganser A, Döhner K, Schlenk RF, Döhner H, Campbell PJ (2016) Genomic classification and prognosis in acute myeloid leukemia. N Engl J Med 374:2209–2221. https://doi.org/10.1056/NEJMoa1516192
Tyner JW, Tognon CE, Bottomly D, Wilmot B, Kurtz SE, Savage SL, Long N, Schultz AR, Traer E, Abel M, Agarwal A, Blucher A, Borate U, Bryant J, Burke R, Carlos A, Carpenter R, Carroll J, Chang BH, Coblentz C, d’Almeida A, Cook R, Danilov A, Dao KHT, Degnin M, Devine D, Dibb J, Edwards DK, Eide CA, English I, Glover J, Henson R, Ho H, Jemal A, Johnson K, Johnson R, Junio B, Kaempf A, Leonard J, Lin C, Liu SQ, Lo P, Loriaux MM, Luty S, Macey T, MacManiman J, Martinez J, Mori M, Nelson D, Nichols C, Peters J, Ramsdill J, Rofelty A, Schuff R, Searles R, Segerdell E, Smith RL, Spurgeon SE, Sweeney T, Thapa A, Visser C, Wagner J, Watanabe-Smith K, Werth K, Wolf J, White L, Yates A, Zhang H, Cogle CR, Collins RH, Connolly DC, Deininger MW, Drusbosky L, Hourigan CS, Jordan CT, Kropf P, Lin TL, Martinez ME, Medeiros BC, Pallapati RR, Pollyea DA, Swords RT, Watts JM, Weir SJ, Wiest DL, Winters RM, McWeeney SK, Druker BJ (2018) Functional genomic landscape of acute myeloid leukaemia. Nature 562:526–531. https://doi.org/10.1038/s41586-018-0623-z
Su L, Tan YH, Lin H, Liu XL, Yu L, Yang YP, Liu SS, Bai O, Yang Y, Jin FY, Sun JN, Liu CS, Liu QJ, Gao SJ, Li W (2018) Mutational spectrum of acute myeloid leukemia patients with double CEBPA mutations based on next-generation sequencing and its prognostic significance. Oncotarget 9:24970–24979. https://doi.org/10.18632/oncotarget.23873
Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, le Beau MM, Bloomfield CD, Cazzola M, Vardiman JW (2016) The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 127:2391–2405. https://doi.org/10.1182/blood-2016-03-643544
Fröhling S, Schlenk RF, Stolze I, Bihlmayr J, Benner A, Kreitmeier S, Tobis K, Döhner H, Döhner K (2004) CEBPA mutations in younger adults with acute myeloid leukemia and normal cytogenetics: prognostic relevance and analysis of cooperating mutations. J Clin Oncol 22:624–633
Dufour A, Schneider F, Metzeler KH, Hoster E, Schneider S, Zellmeier E, Benthaus T, Sauerland MC, Berdel WE, Büchner T, Wörmann B, Braess J, Hiddemann W, Bohlander SK, Spiekermann K (2010) Acute myeloid leukemia with biallelic CEBPA gene mutations and normal karyotype represents a distinct genetic entity associated with a favorable clinical outcome. J Clin Oncol 28:570–577. https://doi.org/10.1200/JCO.2008.21.6010
Schlenk RF, Taskesen E, van Norden Y, Krauter J, Ganser A, Bullinger L, Gaidzik VI, Paschka P, Corbacioglu A, Gohring G, Kundgen A, Held G, Gotze K, Vellenga E, Kuball J, Schanz U, Passweg J, Pabst T, Maertens J, Ossenkoppele GJ, Delwel R, Dohner H, Cornelissen JJ, Dohner K, Lowenberg B (2013) The value of allogeneic and autologous hematopoietic stem cell transplantation in prognostically favorable acute myeloid leukemia with double mutant CEBPA. Blood 122:1576–1582. https://doi.org/10.1182/blood-2013-05-503847
Maxson JE, Gotlib J, Pollyea DA, Fleischman AG, Agarwal A, Eide CA, Bottomly D, Wilmot B, McWeeney SK, Tognon CE, Pond JB, Collins RH, Goueli B, Oh ST, Deininger MW, Chang BH, Loriaux MM, Druker BJ, Tyner JW (2013) Oncogenic CSF3R mutations in chronic neutrophilic leukemia and atypical CML. N Engl J Med 368:1781–1790. https://doi.org/10.1056/NEJMoa1214514
Lavallée VP, Krosl J, Lemieux S et al (2016) Chemo-genomic interrogation of CEBPA mutated AML reveals recurrent CSF3R mutations and subgroup sensitivity to JAK inhibitors. Blood 127:3054–3061. https://doi.org/10.1182/blood-2016-03-705053
Maxson JE, Ries RE, Wang YC, Gerbing RB, Kolb EA, Thompson SL, Guidry Auvil JM, Marra MA, Ma Y, Zong Z, Mungall AJ, Moore R, Long W, Gesuwan P, Davidsen TM, Hermida LC, Hughes SB, Farrar JE, Radich JP, Smith MA, Gerhard DS, Gamis AS, Alonzo TA, Meshinchi S (2016) CSF3R mutations have a high degree of overlap with CEBPA mutations in pediatric AML. Blood 127:3094–3098. https://doi.org/10.1182/blood-2016-04-709899
Shaffer LG, Slovak ML, Campbell LJ (eds) (2009) ISCN 2009: an international system for human cytogenetic nomenclature (2009). Karger, Basel
Fasan A, Haferlach C, Alpermann T, Jeromin S, Grossmann V, Eder C, Weissmann S, Dicker F, Kohlmann A, Schindela S, Kern W, Haferlach T, Schnittger S (2014) The role of different genetic subtypes of CEBPA mutated AML. Leukemia 28:794–803. https://doi.org/10.1038/leu.2013.273
Fasan A, Eder C, Haferlach C, Grossmann V, Kohlmann A, Dicker F, Kern W, Haferlach T, Schnittger S (2013) GATA2 mutations are frequent in intermediate-risk karyotype AML with biallelic CEBPA mutations and are associated with favorable prognosis. Leukemia 27:482–485. https://doi.org/10.1038/leu.2012.174
Theis F, Corbacioglu A, Gaidzik VI, Paschka P, Weber D, Bullinger L, Heuser M, Ganser A, Thol F, Schlegelberger B, Göhring G, Köhne CH, Germing U, Brossart P, Horst HA, Haase D, Götze K, Ringhoffer M, Fiedler W, Nachbaur D, Kindler T, Held G, Lübbert M, Wattad M, Salih HR, Krauter J, Döhner H, Schlenk RF, Döhner K (2016) Clinical impact of GATA2 mutations in acute myeloid leukemia patients harboring CEBPA mutations: a study of the AML study group. Leukemia 30:2248–2250. https://doi.org/10.1038/leu.2016.185
Acknowledgments
We thank the Department of Hematology of the First Hospital, Bethune Medical College of Jilin University, for their assistance in this work.
Funding
This work was supported by the Clinical Research Foundation of First Hospital of Jilin University (No. LCFYJJ2017005).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All the patients gave informed consent prior to enrolment in the study. This study was approved by the ethics committee of Jilin University and conducted in accordance with the Declaration of Helsinki.
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Su, L., Gao, S., Tan, Y. et al. CSF3R mutations were associated with an unfavorable prognosis in patients with acute myeloid leukemia with CEBPA double mutations. Ann Hematol 98, 1641–1646 (2019). https://doi.org/10.1007/s00277-019-03699-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-019-03699-7