Abstract
Primary central nervous system lymphoma (PCNSL) is a rare specific subtype of non-Hodgkin lymphoma limited to the brain, leptomeninges, spinal cord, or eyes without any systemic presentation and relapse which mostly takes place in CNS. In more than 95% of patients, it is of diffuse large B cell lymphoma (DLBCL) type. Categorizing PCNSL to germinal center cell like or activated B cell like, as we usually do for DLBCL NOS, may not be applicable for predicting outcome. Possible prognostic significance of MYC, BCL2, and/or BCL6 rearrangements may be important given what we know about their impact in systemic DLBCL, but we have limited knowledge about the status of double or triple hit molecular changes in PCNSL. Here, we have investigated prevalence of these molecular alterations in PCNSL. Two independent tissue microarrays constructed from 78 formalin-fixed paraffin-embedded blocks of confirmed PCNSL were tested for rearrangement of MYC, BCL2, and BCL6 by interphase fluorescent in situ hybridization (FISH) using break apart dual color probes. BCL6 translocation was detected in 15 (12%) cases. Translocation involving MYC and BCL2 was identified in 3 cases (3.8%) and 1 case (1.3%) respectively. One double hit lymphoma was discovered with both MYC/BCL2 translocation (1.3%). To the best of our knowledge, few organized studies have been conducted for MYC, BCL2, and/or BCL6 rearrangement in PCNSL. This study is evaluating large number of PCNSL. Double or triple hit events which are rarely seen in PCNSL.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Primary central nervous system lymphoma (PCNSL) is a rare specific type of non-Hodgkin lymphoma (NHL) limited to the brain, leptomeninges, spinal cord, or eyes without any systemic presentation, and relapse mostly takes place in CNS too [1]. It comprises 1–3% of all CNS malignancies and less than 5% of all NHL [2].
PCNSL accounts for diffuse large B cell lymphoma (DLBCL) in more than 95% of cases with rather distinct pathophysiology and prognostic features in comparison to systemic DLBCL [3]. DLBCLs are generally classified based on gene expression profile to germinal center B cell like (GCB) and activated B cell like (ABC). Most PCNSL show ABC-like phenotype and many studies have found that they originate from a late germinal center or early-post germinal center cell [1, 4].
However, a few studies discovered that classifying PCNSL into GCB and ABC-like subtypes have no significance in overall survival as it has in systemic DLBCL [5]. In 2016 revision of WHO classification of tumors, a new category, double or triple hit lymphoma (DHL or THL) designated as high-grade B cell lymphoma with gene arrangement of MYC, BCL2, and/or BCL6 having aggressive clinical course and poor response to conventional chemotherapy [6]. MYC is a transcription factor located on chromosome 8 (8q24) which regulates several genes and is involved in cell cycle, DNA damage repair, protein synthesis, and activation of P53. BCL2 naturally is an anti-apoptotic protein placed on 18q21; after rearrangement, it will be dysregulated resulting in survival advantages [7].
BCL6 serves a transcription repressor and as cell survival advantages in normal mature germinal center B cells and when overexpressed prevents apoptosis by transcriptional repression of P53 [8].
During the past 40 years, the incidence of PCNSL has been increasing even in immunocompetent patients. Genetic heterogeneity, difficulty in management and drug delivery, and inherent resistance to chemotherapy are challenges of PCNSL. To discover genetic alterations in these lymphomas and also to compare them with DLBCL NOS, here we report the in situ fluorescent findings of MYC, BCL2, and/or BCL6 rearrangement in these tumors.
Material and methods
Patient’s samples and tissue microarray (TMA)
Formalin-fixed paraffin-embedded blocks of 78 confirmed PCNSL from five medical hospitals in Tehran, Iran, were collected. All cases had been confirmed by immunohistochemistry and comprehensive clinical and radiological evaluation to exclude secondary involvement of the brain. Patient’s demographic data including age, sex, and tumor location was gathered. Two independent tissue microarrays made from duplicate 1-mm cores of tumor-formalin-fixed paraffin-embedded blocks.
Fluorescence in situ hybridization
Interphase FISH was performed on two TMAs using probes specific for translocation detection. The Zyto Light SPECT dual color, orange and green, breaks apart rearrangement probe against MYC, BCL2, and BCL6 used following the manufacture instructions briefly as follows: 4-μm sections were prepared and mounted onto positively charged glass slides. Slides were placed in a 70 °C hot plate for 10 min, de-paraffinized with xylene (2 times, 10 min each), dehydrated in 100%, 100%, 90%, and 70% ethanol each for 5 min, washed 2 × 2 min in distilled water (DW), and then incubated in pre-warmed Heat Pretreatment Solution Citric (PT1) at 98 °C for 15 min. Slides were transferred immediately to DW for 2 × 2 min. After blotting off, Pepsin Solution (ES1) applied and incubated for 10 min at 37 °C in humidity chamber. Washed for 5 min in Wash Buffer SSC (WB1) and 1 min in DW. Dehydrated in 70%, 90%, and 100% ethanol each for 1 min. Ten-micrometer ZytoLight FISH probe applied. The slides were denatured at 75 °C for 10 min on a hot plate and transferred to a humidity chamber and hybridized overnight at 37 °C in oven then covered by coverslip and sealed by rubber cement. After removing the cement and coverslip (by submerging in Wash Buffer for 2 × 5 min at 37 °C), the slides incubated in 70%, 90%, and 100% ethanol each for 1 min and air-dried in the dark. Pipetted 30 μm DAPI/DuraTect-Solution (MT7) onto slides and covered by the coverslip in the dark for 15 min. Signals were analyzed by fluorescent microscope (Nikon E600) with proper filters and images taken by digital camera and Genesis software. Break apart signals in more than 10% of nuclei were considered positive for translocation [9].
Result
We studied 78 confirmed cases of PCNSL. They were composed of 51 men and 27 women between 7 and 84 years old (mean 53.8). Most of them located in supratentorial site.
Among 18 positive FISH cases, including 14 male and 4 female patients, the age range was between 13 and 81 years.
None of these cases were immunodeficient. Sixteen tumors were located in the brain (including 2 in cerebellum) and 2 were found in the spinal cord. Most of the tumors were single mass except one which was located in the brain. Regarding immunohistochemistry, 17 tumors were CD20 and BCL2 positive with no CD3 reactivity. BCL6 and MUM1 were expressed (> 30%) in 6 and 12 tumors respectively and CD10 was positive in 4 tumors. In addition, Ki67 expression was between 30 and 98%. All were CD3 negative.
Translocation involving MYC was identified in 3 cases (3.8%) (Fig. 1a, b) and BCL6 translocation was detected in 15 (12%) and BCL2 translocation in just one case (1.3%) (Fig. 2a, b). For all positive cases, the translocation partner was unknown.
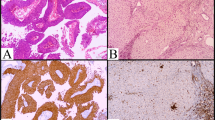
Only one case of double hit lymphoma with MYC/BCL2 rearrangement (1.3%) identified who was a 81-year-old man with single spinal cord mass and IHC profile as CD20+, BCL2+, BCL6+, CD10+, MUM1-, and Ki67 30% (Fig. 3a–d). No triple rearrangement was seen.
Discussion
Primary CNS lymphomas is a rare extranodal lymphoma showing aggressive clinical course with low response to current treatment regimens. Little is known about it from the point of molecular pathogenesis, so identification of new prognostic markers and treatment goals will have high clinical priority [10]. Biologic studies of PCNSL are challenging because of rarity of disease and sparse available tissue material. The gene expression signature of PCNSL includes genes involved in B cell differentiation, proliferation, apoptosis, and cytokine signaling [11]. In this study, we have found that the prevalence of BCL6, MYC, and BCL2 rearrangements is 12%, 3.8%, and 1.3% respectively. The prevalence of the above-mentioned specific translocations in our series is different from systemic DLBCL to some extent; however, there are some similarities too. Regarding PCNSL in other studies, they have some likeness in common that we will discuss in the following sentences. In systemic DLBCL, BCL6 rearrangement is around 15.3% [12] in the work of Oliveira et al. and lower than the result of Iqbal et al. (19–36%) [13]. Regarding BCL6 rearrangement studies of PCNSL, Cady’s et al. found (17%) [10], but it was lower than the other studies 23–37% [14, 15], and 38% [1]. The prevalence of MYC translocation in our study was nearly similar to other previous reports of systemic DLBCL 4–14% [16,17,18] and also PCNSL 3% [10], but in comparison with other studies of PCNSL like Brunn et al. [19] and Son et al. [20] which were 8% and 7% respectively, our result was slightly lower too. In the other study, proto-oncogene MYC was also found to be highly expressed in PCNSL, and there were evidence of somatic mutations in this genes [11]. The logical reason for the latter issue could be that FISH may not detect other gene deregulation other than translocation. The prevalence of BCL2 translocation in systemic DLBCL has been reported as 12.5% [12] to 20–30% [8]. This is incompatible with our data in PCNSL. However, it was compatible with other studies of PCNSL which showed rare BCL2 rearrangement [1, 19]. It might be partly due to the outnumbered activated B cell type (ABC) in PCNSL than systemic DLBCL [21]. Furthermore, we found out a case of DHL with both MYC and BCL2 rearrangement. It stands to reason that DHL is rare and comprised 7–10% of all B cell lymphoma [8, 12] and besides, few handful studies were carried out on PCNSL in this regard. In Cady’s series [10], just one DHL in PCNSL was reported but it was MYC/BCL6 DHL. In addition, Yin et al. worked on 33 cases of PCNSL and reported 2 cases of MYC/BCL2 DHL. They also described that DHL had poor impact on outcome [22]. Although PCNSL and systemic DLBCL have morphologic and immunologic similarity, a few discrepancies (such as different incidence of the above mentioned translocations) can suggest possible effect of brain environment and even more different pathogenesis between them somehow. Therefore, other molecular profiling and prognostic biomarkers should be investigated [11, 23].
Conclusion
PCNSL is a rare form of extranodal NHL, typically described as DLBCL that is confined to the nervous system. Our knowledge of PCNSL biology due to rarity of the disease and the paucity of available research specimens is inadequate and should be improved. Our study was one of the first few organized FISH studies for MYC, BCL2, and BCL6 simultaneously for assessing the presence and also the prevalence of their rearrangements to investigate the possible underlying molecular cause. So by understanding their roles, new modalities of treatment and rather prognostic factors will be introduced. We also suggest correlating the mentioned rearrangements prospectively in more cases with survival to assess their effect on prognosis as systemic DLBCL in future studies.
References
Yang X-L, Liu Y-B (2017) Advances in pathobiology of primary central nervous system lymphoma. Chin Med J 130(16):1973–1979. https://doi.org/10.4103/0366-6999.211879
Provencher S, Ferlay C, Alaoui-Slimani K, Devidas A, Lepretre S, de Prijck B, Sebban C, de la Fouchardiere A, Chassagne-Clement C, Ketterer N (2011) Clinical characteristics and outcome of isolated extracerebral relapses of primary central nervous system lymphoma: a case series. Hematol Oncol 29(1):10–16. https://doi.org/10.1002/hon.944
Campo E, Swerdlow SH, Harris NL, Pileri S, Stein H, Jaffe ES (2011) The 2008 WHO classification of lymphoid neoplasms and beyond: evolving concepts and practical applications. Blood 117(19):5019–5032. https://doi.org/10.1182/blood-2011-01-293050
Montesinos-Rongen M, Purschke F, Küppers R, Deckert M (2014) Immunoglobulin repertoire of primary lymphomas of the central nervous system. J Neuropathol Exp Neurol 73(12):1116–1125. https://doi.org/10.1097/NEN.0000000000000133
Raoux D, Duband S, Forest F, Trombert B, Chambonnière ML, Dumollard JM, Khaddage A, Gentil-Perret A, Péoc'h M (2010) Primary central nervous system lymphoma: immunohistochemical profile and prognostic significance. Neuropathology 30(3):232–240. https://doi.org/10.1111/j.1440-1789.2009.01074.x
Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, Advani R, Ghielmini M, Salles GA, Zelenetz AD (2016) The 2016 revision of the World Health Organization (WHO) classification of lymphoid neoplasms. Blood 127(20):2375–2390. https://doi.org/10.1182/blood-2016-01-643569
Li S, Seegmiller AC, Lin P, Wang XJ, Miranda RN, Bhagavathi S, Medeiros LJ (2015) B-cell lymphomas with concurrent MYC and BCL2 abnormalities other than translocations behave similarly to MYC/BCL2 double-hit lymphomas. Mod Pathol 28(2):208–217. https://doi.org/10.1038/modpathol.2014.95
Rosenthal A, Younes A (2017) High grade B-cell lymphoma with rearrangements of MYC and BCL2 and/or BCL6: double hit and triple hit lymphomas and double expressing lymphoma. Blood Rev 31(2):37–42. https://doi.org/10.1016/j.blre.2016.09.004
Yan L-X, Liu Y-H, Luo D-L, Zhang F, Cheng Y, Luo X-L, Xu J, Cheng J, Zhuang H-G (2014) MYC expression in concert with BCL2 and BCL6 expression predicts outcome in Chinese patients with diffuse large B-cell lymphoma, not otherwise specified. PLoS One 9(8):e104068. https://doi.org/10.1371/journal.pone.0104068
Cady FM, O’Neill BP, Law ME, Decker PA, Kurtz DM, Giannini C, Porter AB, Kurtin PJ, Johnston PB, Dogan A (2008) Del (6)(q22) and BCL6 rearrangements in primary CNS lymphoma are indicators of an aggressive clinical course. J Clin Oncol 26(29):4814–4819. https://doi.org/10.1200/JCO.2008.16.1455
Mrugala MM, Rubenstein JL, Ponzoni M, Batchelor TT (2009) Insights into the biology of primary central nervous system lymphoma. Curr Oncol Rep 11(1):73–80. https://doi.org/10.1007/s11912-009-0012-8
Oliveira CC, Maciel-Guerra H, Kucko L, Hirama EJ, Brilhante AD, Quevedo FC, da Cunha IW, Soares FA, Niero-Melo L, dos Reis PP (2017) Double-hit lymphomas: clinical, morphological, immunohistochemical and cytogenetic study in a series of Brazilian patients with high-grade non-Hodgkin lymphoma. Diagn Pathol 12(1):3. https://doi.org/10.1186/s13000-016-0593-0
Iqbal J, Greiner T, Patel K, Dave B, Smith L, Ji J, Wright G, Sanger W, Pickering D, Jain S (2007) Distinctive patterns of BCL6 molecular alterations and their functional consequences in different subgroups of diffuse large B-cell lymphoma. Leukemia 21(11):2332–2343. https://doi.org/10.1038/sj.leu.2404856
Montesinos-Rongen M, Zühlke-Jenisch R, Gesk S, Martín-Subero JI, Schaller C, Van Roost D, Wiestler OD, Deckert M, Siebert R (2002) Interphase cytogenetic analysis of lymphoma-associated chromosomal breakpoints in primary diffuse large B-cell lymphomas of the central nervous system. J Neuropathol Exp Neurol 61(10):926–933. https://doi.org/10.1093/jnen/61.10.926
Schwindt H, Akasaka T, Zühlke-Jenisch R, Hans V, Schaller C, Klapper W, Dyer MJ, Siebert R, Deckert M (2006) Chromosomal translocations fusing the BCL6 gene to different partner loci are recurrent in primary central nervous system lymphoma and may be associated with aberrant somatic hypermutation or defective class switch recombination. J Neuropathol Exp Neurol 65(8):776–782. https://doi.org/10.1097/01.jnen.0000229988.48042.ae
Copie-Bergman C, Cuillière-Dartigues P, Baia M, Briere J, Delarue R, Canioni D, Salles G, Parrens M, Belhadj K, Fabiani B (2015) MYC-IG rearrangements are negative predictors of survival in DLBCL patients treated with immunochemotherapy: a GELA/LYSA study. Blood 126(22):2466–2474. https://doi.org/10.1182/blood-2015-05-647602
Tzankov A, Xu-Monette ZY, Gerhard M, Visco C, Dirnhofer S, Gisin N, Dybkaer K, Orazi A, Bhagat G, Richards KL (2014) Rearrangements of MYC gene facilitate risk stratification in diffuse large B-cell lymphoma patients treated with rituximab-CHOP. Mod Pathol 27(7):958–971. https://doi.org/10.1038/modpathol.2013.214
Kojima M, Nishikii H, Takizawa J, Aoki S, Noguchi M, Chiba S, Ando K, Nakamura N (2013) MYC rearrangements are useful for predicting outcomes following rituximab and chemotherapy: multicenter analysis of Japanese patients with diffuse large B-cell lymphoma. Leuk lymphoma 54(10):2149–2154. https://doi.org/10.3109/10428194.2013.771398
Brunn A, Nagel I, Montesinos-Rongen M, Klapper W, Vater I, Paulus W, Hans V, Blümcke I, Weis J, Siebert R (2013) Frequent triple-hit expression of MYC, BCL2, and BCL6 in primary lymphoma of the central nervous system and absence of a favorable MYC low BCL2 low subgroup may underlie the inferior prognosis as compared to systemic diffuse large B cell lymphomas. Acta Neuropathol 126(4):603–605. https://doi.org/10.1007/s00401-013-1169-7
Son S-M, Ha S-Y, Yoo H-Y, Oh D, Kim S-J, Kim W-S, Ko Y-H (2017) Prognostic impact of MYC protein expression in central nervous system diffuse large B-cell lymphoma: comparison with MYC rearrangement and MYC mRNA expression. Mod Pathol 30(1):4–782. https://doi.org/10.1097/01.jnen.0000229988.48042.ae
Izumi K, Fujita K, Fukutsuka K, Hayashida M, Akasaka T, Ohno H (2016) MYC/BCL2/BCL6 triple-hit lymphoma that presented with a suprasellar tumor and meningeal dissemination: a case report. Tenri Med Bull 19(1):24–33. https://doi.org/10.12936/tenrikiyo.19-007
Yin W, Zhu X, Yang H, Sun W, Wu M (2018) Survival of patients with primary central nervous system diffuse large B-cell lymphoma: impact of gene aberrations and protein overexpression of bcl-2 and C-MYC, and selection of chemotherapy regimens. Zhonghua Bing Li Xue Za Zhi 47(1):32–38. https://doi.org/10.3760/cma.j.issn.0529-5807.2018.01.007
Montesinos-Rongen M, Brunn A, Bentink S, Basso K, Lim W, Klapper W, Schaller C, Reifenberger G, Rubenstein J, Wiestler O (2008) Gene expression profiling suggests primary central nervous system lymphomas to be derived from a late germinal center B cell. Leukemia 22(2):400–405. https://doi.org/10.1038/sj.leu.2405019
Author information
Authors and Affiliations
Contributions
Anahita Nosrati participated in assessing FISH cases, drafting, proofreading, and finally writing of the manuscript. Ahmad Monabati and Akbar Safaei both participated in final assessing of all FISH cases and are essentially responsible for writing of manuscript and submission. Alireza Sadeghipour participated in introducing and preparing the clinical data of patients. Fatemeh Radmanesh and Sajjadeh Movahedinia, both participated in introducing clinical data of patients. All authors critically revised and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Nosrati, A., Monabati, A., Sadeghipour, A. et al. MYC, BCL2, and BCL6 rearrangements in primary central nervous system lymphoma of large B cell type. Ann Hematol 98, 169–173 (2019). https://doi.org/10.1007/s00277-018-3498-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-018-3498-z