Abstract
Initial staging by positron emission tomography/computed tomography (PET/CT) scanning is recommended for patients with diffuse large B-cell lymphoma (DLBCL). Whether both PET/CT and bone marrow biopsy (BMB) are required remains unclear. This study examined whether staging by PET/CT is sufficient. Participants with untreated DLBCL assessed using both PET/CT and BMB were included. Patients received independent diagnostic assessments from a radiologist and a hematopathologist. Both hematoxylin–eosin staining and CD20 immunostaining were performed to determine the bone marrow involvement in BMB. A total of 84 patients were included. The number of patients with positive bone marrow involvement identified by PET/CT and BMB was 16 (19%) and 22 (26%), respectively. Eight (10%) patients showed positive results in both tests. When considering BMB as a reference, PET/CT showed 36% sensitivity and 87% specificity, with positive and negative predictive values of 50% and 79%, respectively. BMB-positive patients had shorter progression-free (PFS) and overall (OS) survival than their BMB-negative counterparts. Compared to PET/CT-negative patients, patients with positive results did not show any significant differences in PFS and OS. However, among 16 PET/CT-positive patients, poor PFS and OS were observed among patients who were also BMB positive. BMB remains a mandatory step in staging of untreated DLBCL patients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Diffuse large B-cell lymphoma (DLBCL), the most common subtype of non-Hodgkin lymphoma, can infiltrate all extramedullary sites. Bone marrow is one of the most commonly involved sites in patients with DLBCL, affected in 11–17% of cases examined with bone marrow biopsy (BMB) at initial staging [1,2,3,4]. Positron emission tomography/computed tomography (PET/CT) scanning is also recommended for staging of DLBCL. Although BMB is not “mandatory” according to the Lugano guidelines [5], both PET/CT scan and BMB are usually performed to assess bone marrow involvement (BMI) in patients with DLBCL; whether PET/CT scan alone is enough remains controversial.
Assessment of BMI is clinically relevant for several reasons. The Ann Arbor staging system includes BMI as a criterion for the highest clinical stage (stage IV) of DLBCL, while the International Prognostic Index (IPI) [6] uses it to stratify patients. In addition, the presence or absence of BMI might affect treatment strategy and can be used to monitor treatment effects. In PET/CT-based staging, DLBCL is listed as FDG-avid disease subtype alongside Hodgkin lymphoma [5], making PET/CT scanning useful in this patient group. This fact notwithstanding, prognostic indices such as IPI use BMB results as a measure of BMI.
The main advantage of BMB is the acquisition of histological specimen. Positive BMB is a definitive confirmation of BMI.4 In cases of positive BMB, discrimination of discordant or concordant involvement is also possible. Nevertheless, BMB is an invasive procedure associated with pain and stress.4 In addition, focal involvement detected by PET/CT scan can be missed by BMB. Due to required decalcification, BMB-based diagnosis is time-consuming. Although PET/CT allows to non-invasively surveil the entire bone marrow, it is associated with high risk of false-positive and/or false-negative results. This study examined whether PET/CT alone is enough in evaluating BMI in patients with DLBCL.
Materials and methods
This study was approved by the Medical Ethics Committee at St. Marianna University School of Medicine. While previous reports discussing the role of PET/CT and BMB as staging tools in patients with untreated DLBCL have used data based on medical records, here we performed a systematic review, for whom a radiologist (T.K.) and a hematopathologist (N.N.), both from external institutions, separately performed blind reviews. Both reviewers had been informed about the purpose of this study and blinded to each other’s and initial PET/CT and BMB findings. Pathological review was performed using specimens stained by hematoxylin–eosin and CD20 in all cases. Bone marrow invasion was reported if at least a cluster of large-sized lymphocytes with CD20 expression, excluding reactive lymph follicles, was present. We did not refer to the results of BM smear, flow cytometry, and cytogenetic analysis in this study.
The subjects of this retrospective study were consecutive patients with DLBCL newly diagnosed at our institution (2008–2017) and staged with both PET/CT and BMB before initiation of treatment. Patients who underwent PET/CT at a single center (YUAI Clinic, Yokohama) were included. BMB was performed from a single side of the iliac crest. We excluded cases in which BMB specimens were qualitatively and/or quantitatively insufficient to determine the presence or absence of BMI. In addition, we excluded patients whose diagnostic specimens included a nodular growth pattern consistent with follicular lymphoma. In PET/CT-based assessment, cases with a score of 4 or 5 on the Deauville criteria for the bone marrow were classified as positive [5]. BMB-confirmed BMI was used as reference; involvement type was classified as either concordant or discordant. Concordant involvement was defined as marrow involvement with DLBCL, while discordant involvement was assigned when a diagnosis of small B-cell lymphoma would be made if the bone marrow was reviewed in isolation [3]. Most patients in our study were treated by both rituximab and Adriamycin-containing chemoimmunotherapy such as R-CHOP (Rituximab, Cyclophosphamide, Doxorubicin, Vincristine, and Prednisolone) regimen (intensive regimen) with curative intent.
In some cases, we were unable to extract from medical records data required to assess performance status using the International Prognostic Index (IPI) [6]. Instead, we used three variables of the SIL index (clinical stage, soluble interleukin-2 receptor concentration, and lactate dehydrogenase levels), associated with the outcomes of patients with DLBCL treated with R-CHOP regimen without data on performance status [7].
Statistical analysis
The Fisher’s exact probability test was used to determine statistically significant differences between the treatments of the groups. Overall survival (OS) was calculated from the date of diagnosis to the date of last follow-up or death, whichever occurred first. Progression-free survival (PFS) was calculated from the date of diagnosis to the date of disease progression, death from any cause, or last contact, whichever occurred first. Survival was estimated using the Kaplan–Meier curves and compared using the log-rank test. P values < 0.05 were considered to indicate a significant difference. Data were analyzed using GraphPad Prism version 7.
Results
This study included 84 patients with untreated DLBCL (82 cases of DLBCL, NOS and 2 of primary mediastinal large B-cell lymphoma), comprising 45 males and 39 females. Demographic and clinical characteristics of the participants are shown in Table 1. Median age at the time of diagnosis was 70 years (range, 19–86). The SIL index indicated standard-risk disease in 54 patients, high-risk in 29 patients, and unknown risk in 1 patient due to missing data on sIL-2R (data not shown). This finding was consistent with the original report on SIL index distribution,7 which showed 4-year PFS of 83% in standard-risk and of 52% in high-risk patients, respectively.
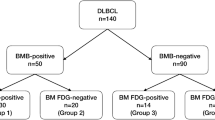
The number of PET/CT- and BMB-positive BMI cases was 16 (19%) and 22 (26%), respectively. Among 16 PET/CT-positive cases, 7 had focal and 9 had diffuse involvement patterns. Among 22 BMB-positive cases, 6 had discordant and 16 had concordant involvement patterns (Table 1). The overall distribution of positive and negative findings per testing method is presented in Table 2. Eight (10%) patients had positive BMI findings in both tests. Among 68 PET/CT-negative patients, 14 were BMB-positive and 9 of them were upstaged to clinical stage IV in Ann Arbor staging system. Using BMB results as a reference, PET/CT showed 36% sensitivity and 87% specificity with positive and negative predictive values of 50% and 79%, respectively. Five patients among those with diffuse involvement in PET/CT-based findings were also BMB-positive; concurrently, among patients with focal involvement on PET/CT scans, three were BMB-positive (Table 3). The tree diagram is shown in Fig. 1. All eight BMB-positive patients had concordant involvement. Distribution of patients between intensive/non-intensive regimens and BMB is shown in supplemental Table 1.
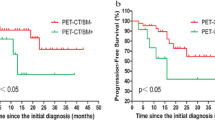
Overall, BMB-positive patients had shorter PFS (P = 0.006, Fig. 2a) and OS (P = 0.02, Fig. 2b) than did BMB-negative patients. Non-intensive regimen (not includes Adriamycin) was used as first-line therapy in 2 BMB-positive patients (9%) and 9 BMB-negative patients (15%), respectively. The proportion of patients who received non-intensive regimen was not significantly different. PET/CT-positive patients did not show any significant differences in PFS (Fig. 2c) or OS (Fig. 2d) compared to PET/CT-negative patients. Among 16 PET/CT-positive patients, significantly poorer PFS (P = 0.025, Fig. 3a) and OS (P = 0.04, Fig. 3b) were observed in BMB-positive patients. Among 68 PET/CT-negative patients, there were no significant differences in PFS (Fig. 3c) or OS (Fig. 3d) between BMB-positive and BMB-negative patients. There were no differences in outcomes between 6 discordant and 16 concordant involvement patients, classified based on BMB results (supplemental Fig. 1).
Progression-free (PFS) (a) and overall (OS) (b) survival among 84 patients with either positive or negative DLBCL bone marrow involvement confirmed by bone marrow biopsy. Significant differences were observed in both PFS (P = 0.006) and OS (P = 0.02). PFS (c) and OS (d) in positive and negative DLBCL bone marrow involvement cases confirmed by PET/CT scan among 84 patients. No significant difference was observed in either PFS (P = 0.20) or OS (P = 0.28)
Progression-free (PFS) (a) and overall (OS) (b) survival in positive and negative cases of DLBCL bone marrow involvement confirmed by bone marrow biopsy among 16 patients with PET/CT-positive findings. Significant differences were observed in both PFS (P = 0.03) and OS (P = 0.04). PFS (c) and OS (d) in positive and negative cases of DLBCL bone marrow involvement confirmed by bone marrow biopsy among 68 patients with PET/CT-negative findings. No significant difference was observed in either PFS (P = 0.13) or OS (P = 0.23)
Discussion
In assessing BMI in patients with DLBCL as part of a staging procedure, this study compared PET/CT and unilateral BMB. Bilateral BMB in DLBCL patients has been suggested as ineffective [8], while unilateral BMB is considered sufficient as an assessment method. Flow cytometry of the bone marrow aspirate is a useful method for assessing BMI, in particular, in cases involving a small number of lymphoma cells [9, 10]. However, both positive [11] and negative [12] impact of flow cytometry on prognosis has been associated with BMB-negative findings. The role of flow cytometry requires further research; however, differences in methodology such as antibody series and gating might limit uniform evaluation of associated findings, even in cases where flow cytometry is used to complement BMB-based diagnosis of BMI. In our study, all the 61 patients with BMB-negative findings showed flow cytometry-negative results, while 10 of 21 patients with BMB-positive findings showed flow cytometry positive results (supplemental Table 2). This might also show the indispensability of BMB in the staging procedure in DLBCL. Although previous studies have associated specific cytogenetic abnormalities and findings from interphase fluorescence in situ hybridization of bone marrow aspirates with worse prognosis [13], these parameters should be assessed at diagnostic sites such as lymph node. It might be difficult to diagnose lymphoma cell presence in bone marrow aspirates by microscopy from cases with very small numbers of lymphoma cells.
BMB was used to diagnose BMI in DLBCL patients before PET scans were introduced to routine clinical examination. However, the use of BMB for DLBCL staging is controversial in the PET era. In fact, several studies reported PET/CT scans to be able to replace BMB [14,15,16, 16], with some authors claiming PET scan superiority over BMB, ultimately arguing against the use of BMB altogether [17, 18, 18,19,20]. Some investigators have suggested that BMB should not constitute part of routine staging [21, 22], and that it is not required at all in PET/CT-negative patients [23]. Moreover, in a systematic review and meta-analysis, Adams et al. concluded that positive PET/CT findings obviate the need for BMB [24].
These previous findings notwithstanding, in this study, we found that BMB-positive patients showed poorer prognosis compared with BMB-negative patients in the PET-positive group while the results of BMB did not affect outcomes in the PET-negative group. These findings suggest that BMB might be useful in the assessment of PET-positive patients and support management of PET-negative patients. Among 68 PET-negative patients, there were 14 BMB-positive cases (21%) whose outcomes were comparable to that of 54 patients who were both BMB and PET/CT negative. It is possible that PET-negative patients have smaller amount of tumor cells in the bone marrow than PET-positive patients. However, the relatively small number of patients included in this study limits the interpretation.
The proportion of BMB-positive and PET-negative patients was higher in the present study than in the previous reports [14, 15, 17, 18, 18,19,20,21,22,23], which might be accounted for by the routine usage of CD20 immunostaining in this study, which was not used consistently in the previous reports. It is unknown whether PET/CT-based staging alone could be used with the existing prognostic indexes such as the IPI, which was established in pre-PET era. BMI would affect the assessment of 2 out of 5 factors such as clinical stage and number of extranodal involvement sites. Overall, we recommend that at present, patients with DLBCL undergo disease staging using PET/CT scans and BMB. During restaging, BMB might no longer be required to document CR if the PET/CT assessment is negative in patients with initial BMI [25]. However, recent prospective studies have shown that BMB at restaging can affect response [26].
Routine CD20 immunostaining in this study contributed to the accuracy of BMI assessment and discrimination of discordant or concordant involvement. Discordant involvement suggests an indolent B-cell lymphoma that involves the bone marrow and that might be clonally related to nodal DLBCL [27]. Unlike previous studies, the present study did not observe prognostic differences between involvement types [28], both of which were associated with poor outcomes; previously, several reports demonstrated that concordant involvement might have a negative impact on prognosis [2, 3, 27]. The risk of central nervous system involvement was elevated in patients with concordant involvement [27]. Use of BMB to confirm involvement types might be clinically relevant.
In the evaluation of BMI by PET/CT scans, focal FDG accumulation generally used to be treated as positive; however, BMB from the applicable part would be needed to confirm BMI histologically. Although most diffuse FDG accumulation was also revealed to be BMI in aggressive lymphoma by BMB [29], it would help differentiate reactive inflammatory uptake from active lymphoma involvement [30]. In the present study, as many as 44% of patients with diffuse FDG accumulation detected by PET/CT scan had negative BMB results. These patients’ PET/CT findings were likely false-positive results due to reactions such as acceleration of hematopoiesis or inflammation.
Conclusion
This study is characterized by the review method that a hematopathologist and a radiologist separately reviewed the results without being informed about the initial medical records. PET/CT showed only 36% sensitivity for BMI. Among PET/CT-positive patients, poor outcome was observed in patients who were also BMB-positive. We recommend that patients with positive PET/CT findings undergo mandatory BMB, as it might affect outcomes. Some patients with negative PET/CT scan findings might have positive BMB results. Overall, both PET/CT and BMB should be performed in detecting BMI in previously untreated patients with DLBCL.
References
The Non-Hodgkin’s Lymphoma Classification Project. A clinical evaluation of the international lymphoma study group classification of non-Hodgkin’s lymphoma. Blood. 1997;89:3909–18.
Chung R, Lai R, Wei P, Lee J, Hanson J, Belch AR, et al. Concordant but not discordant bone marrow involvement in diffuse large B-cell lymphoma predicts a poor clinical outcome independent of the international prognostic index. Blood. 2007;110:1278–82.
Sehn LH, Scott DW, Chhanabhai M, Berry B, Ruskova A, Berkahn L, et al. Impact of concordant and discordant bone marrow involvement on outcome in diffuse large B-cell lymphoma treated with R-CHOP. J Clin Oncol. 2011;29:1452–7.
Adams HJ, Nievelstein RA, Kwee TC. Opportunities and limitations of bone marrow biopsy and bone marrow FDG-PET in lymphoma. Blood Rev. 2015;29:417–25.
Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32:3059–68.
International Non-Hodgkin’s Lymphoma Prognostic Factors Project. A predictive model for aggressive non-Hodgkin’s lymphoma. N Engl J Med. 1993;329:987–94.
Tomita N, Sakai R, Fujisawa S, Fujimaki K, Taguchi J, Hashimoto C, et al. SIL index, comprising stage, soluble interleukin-2 receptor, and lactate dehydrogenase, is a useful prognostic predictor in diffuse large B-cell lymphoma. Cancer Sci. 2012;103:1518–23.
Wang J, Weiss LM, Chang KL, Slovak ML, Gaal K, Forman SJ, et al. Diagnostic utility of bilateral bone marrow examination: significance of morphologic and ancillary technique study in malignancy. Cancer. 2002;94:1522–31.
Kim B, Lee ST, Kim HJ, Kim SH. Bone marrow flow cytometry in staging of patients with B-cell non-Hodgkin lymphoma. Ann Lab Med. 2015;35:187–93.
Cabezas-Quintario MA, Gomez P, Yuste-Del Pozo V, Valencia-Mesa AL, Sosa G, Ricard P, et al. Bone marrow trephine biopsy involvement by lymphoma: pattern of involvement and concordance with flow cytometry, in 10 years from a single institution. Clin Transl Oncol. 2016;18:537–40.
Martín-Moro F, Piris-Villaespesa M, Marquet-Palomanes J, Garcia-Cosio M, Villarrubia J, Lario A, et al. Bone marrow infiltration by flow cytometry at diffuse large B-cell lymphoma NOS diagnosis implies worse prognosis without considering bone marrow histology. Cytometry B Clin Cytom. 2019. https://doi.org/10.1002/cyto.b.21863.
Wolach O, Fraser A, Luchiansky M, Shaporo C, Radnay J, Shpilberg O, et al. Can flow cytometry of bone marrow aspirate predict outcome of patients with diffuse large B cell lymphoma? A retrospective single centre study. Hematol Oncol. 2015;33:42–7.
Kim S, Kim H, Kang H, Kim J, Eom H, Kim T, et al. Clinical significance of cytogenetic aberrations in bone marrow of patients with diffuse large B-cell lymphoma: prognostic significance and relevance to histologic involvement. J Hematol Oncol. 2013;6:76.
Hong J, Lee Y, Park Y, Kim SG, Hwang KH, Park SH, et al. Role of FDG-PET/CT in detecting lymphomatous bone marrow involvement in patients with newly diagnosed diffuse large B-cell lymphoma. Ann Hematol. 2012;91:687–95.
Adams HJ, Kwee TC, Fijnheer R, Dubois SV, Nievelstein RA, de Klerk JM. Bone marrow 18F-fluoro-2-deoxy-d-glucose positron emission tomography/computed tomography cannot replace bone marrow biopsy in diffuse large B-cell lymphoma. Am J Hematol. 2014;89:726–31.
Adams HJ, Kwee TC, Fijnheer R, Dubois SV, Nievelstein RA, de Klerk JM. Direct comparison of visual and quantitative bone marrow FDG-PET/CT findings with bone marrow biopsy results in diffuse large B-cell lymphoma: does bone marrow FDG-PET/CT live up to its promise? Acta Radiol. 2015;56:1230–5.
Chen-Liang TH, Martín-Santos T, Jerez A, Rodriguez-Garcia G, Senent L, Martinez-Millan C, et al. Bone marrow biopsy superiority over PET/CT in predicting progression-free survival in a homogeneously-treated cohort of diffuse large B-cell lymphoma. Cancer Med. 2017;6:2507–14.
Khan AB, Barrington SF, Mikhaeel NG, Hunt AA, Cameron L, Morris T, et al. PET-CT staging of DLBCL accurately identifies and provides new insight into the clinical significance of bone marrow involvement. Blood. 2013;122:61–7.
Berthet L, Cochet A, Kanoun S, Berriolo-Riedinger A, Humbert O, Toubeau M, et al. In newly diagnosed diffuse large B-cell lymphoma, determination of bone marrow involvement with 18F-FDG PET/CT provides better diagnostic performance and prognostic stratification than does biopsy. J Nucl Med. 2013;54:1244–50.
Cortés-Romera M, Sabaté-Llobera A, Mercadal-Vilchez S, Climent-Esteller F, Serrano-Maestro A, Gamez-Cenzano C, et al. Bone marrow evaluation in initial staging of lymphoma: 18F-FDG PET/CT versus bone marrow biopsy. Clin Nucl Med. 2014;39:e46-52.
Ujjani CS, Hill EM, Wang H, Nassif S, Esposito G, Ozdemirli, et al. 18F-FDG PET-CT and trephine biopsy assessment of bone marrow involvement in lymphoma. Br J Haematol. 2016;174:410–6.
El Karak F, Bou-Orm IR, Ghosn M, Kattan J, Farhat F, Ibrahim T, et al. PET/CT Scanner and bone marrow biopsy in detection of bone marrow involvement in diffuse large B-cell lymphoma. PLoS ONE. 2017;12:e0170299.
Alzahrani M, El-Galaly TC, Hutchings M, Hansen JW, Loft A, Johnsen HE, et al. The value of routine bone marrow biopsy in patients with diffuse large B-cell lymphoma staged with PET/CT: a Danish-Canadian study. Ann Oncol. 2016;27:1095–9.
Vishnu P, Wingerson A, Lee M, Mandelson MT, Aboulafia DM. Utility of bone marrow biopsy and aspirate for staging of diffuse large B-cell lymphoma in the era of positron emission tomography with 2-deoxy-2-[fluorine-18]fluoro-deoxyglucose integrated with computed tomography. Clin Lymphoma Myeloma Leuk. 2017;17:631–6.
Cerci JJ, Györke T, Fanti S, Paez D, Meneghetti JC, Redondo F, et al. Combined PET and biopsy evidence of marrow involvement improves prognostic prediction in diffuse large B-cell lymphoma. J Nucl Med. 2014;55:1591–7.
Adams HJ, Kwee TC, de Keizer B, Fijnheer R, de Klerk JM, Nievelstein RA. FDG PET/CT for the detection of bone marrow involvement in diffuse large B-cell lymphoma: systematic review and meta-analysis. Eur J Nucl Med Mol Imaging. 2014;41:565–74.
Jackson AE, Smeltzer JP, Habermann TM, Jones JM, Burnette B, Ristow K, et al. The utility of restaging bone marrow biopsy in PET-negative patients with diffuse large B-cell lymphoma and baseline bone marrow involvement. Am J Hematol. 2014;89:865–7.
Rutherford SC, Herold M, Hiddemann W, Kostakoglu L, Marcus R, Martelli M, et al. Impact of bone marrow biopsy on response assessment in immunochemotherapy-treated lymphoma patients in GALLIUM and GOYA. Blood Adv. 2020;4:1589–93.
Brudno J, Tadmor T, Pittaluga S, Nicolae A, Polliack A, Dunleavy K. Discordant bone marrow involvement in non-Hodgkin lymphoma. Blood. 2016;127:965–70.
Park MJ, Park SH, Park PW, Seo YH, Kim KH, Seo JY, et al. Prognostic impact of concordant and discordant bone marrow involvement and cell-of-origin in Korean patients with diffuse large B-cell lymphoma treated with R-CHOP. J Clin Pathol. 2015;68:733–8.
Adams HJ, Kwee TC, Fijnheer R, Dubois SV, Nievelstein RA, de Klerk JM. Diffusely increased bone marrow FDG uptake in recently untreated lymphoma: incidence and relevance. Eur J Haematol. 2015;95:83–9.
Abramson JS. Bone marrow biopsies for staging of diffuse large B-cell lymphoma: are we looking too closely? Leuk Lymphoma. 2017;58:4–5.
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
About this article
Cite this article
Saiki, Y., Tomita, N., Uchida, A. et al. Biopsy remains indispensable for evaluating bone marrow involvement in DLBCL patients despite the use of positron emission tomography. Int J Hematol 113, 675–681 (2021). https://doi.org/10.1007/s12185-021-03080-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-021-03080-3