Abstract
Emerging epidemiological evidence suggests that patients with glucose-6-phosphate dehydrogenase (G6PD) deficiency may have a higher risk of developing diabetes. The aim of the review was to synthesise the evidence on the association between G6PD deficiency and diabetes. A systematic search on Medline, EMBASE, AMED and CENTRAL databases for studies published between January 1966 and September 2016 that assessed the association between G6PD deficiency and diabetes was conducted. This was supplemented by a review of the reference list of retrieved articles. We extracted data on study characteristics, outcomes and performed an assessment on the methodological quality of the studies. A random-effects model was used to compute the summary risk estimates. Fifteen relevant publications involving 949,260 participants were identified, from which seven studies contributed to the meta-analysis. G6PD deficiency was associated with a higher odd of diabetes (odds ratio 2.37, 95% confidence interval 1.50–3.73). The odds ratio of diabetes among men was higher (2.22, 1.31–3.75) compared to women (1.87, 1.12–3.12). This association was broadly consistent in the sensitivity analysis. Current evidence suggests that G6PD deficiency may be a risk factor for diabetes, with higher odds among men compared to women. Further research is needed to determine how G6PD deficiency moderates diabetes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glucose-6-phosphate dehydrogenase (G6PD) deficiency is one of the most common genetic disorders affecting approximately 400 million people worldwide [1]. Several recent studies have reported a relationship between G6PD deficiency and incidence of diabetes [2,3,4]. However, it remains unclear if G6PD deficiency itself may increase or decrease the risk of diabetes. In the current study, we systematically reviewed all available epidemiological evidence on the relationship between G6PD deficiency and prevalence of diabetes.
Methods
Data sources and searches
A literature search was performed up to September 2016 in Medline, EMBASE, CENTRAL and AMED for studies examining the association between patients with G6PD deficiency and diabetes. This was supplemented with a manual search of references cited by selected articles.
Study selection
We included cohort or cross-sectional studies that examined patients with either self-reported or diagnosed diabetes (including type 1, type 2, gestational, insulin-dependent and insulin-requiring diabetes) and diagnosed with G6PD deficiency. Two investigators (SWHL and NML) independently screened all studies and extracted data. Any discrepancies were resolved by discussion. To assess the quality of the included studies, we used the Cochrane risk of bias assessment tool for non-randomised studies of intervention (ROBINS-I) tool [5].
Data synthesis and statistical analysis
To summarise the relationship between G6PD deficiency and diabetes prevalence, we pooled the diabetes prevalence using the aggregate study-level data using a random-effects model. To evaluate the heterogeneity across studies, we used the Cochrane Q statistic and the I 2 statistic. We also explored the potential explanations by stratification of studies as well as using random-effects meta-regression analyses. All analyses were performed using Stata statistical software version 13.0 (StataCorp, College Station, TX, US).
Results
Description of studies and participants
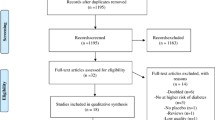
We identified 15 eligible studies representing a total of 949,260 participants (Supplementary Fig. S1), with sample sizes between 54 and 940,085. Participants aged 4 and 85 years were recruited over a period ranging from 1 month to 14 years. Most of the studies were cross-sectional, and seven were case-control studies with two being nested case-control studies (Table 1). Study population included six from Asia, five from Europe, three from Africa and one from South America. Six studies only included men in their study population while another one study included only women.
All studies had an unclear risk of bias for confounders, and the risk of bias from exposure measurement was unclear, given that most of the studies measured the presence of G6PD deficiency only once (Supplementary Table S1). The overall risk of bias for most studies was unclear except for one study which was judged to be at high risk as the controls were recruited at a different period [6].
Association between diabetes prevalence and G6PD deficiency
Several studies found a positive association between G6PD deficiency and diabetes [2, 3, 7,8,9,10]. For example, in the study by Heymann et al., the authors found that patients aged 45–64 years with G6PD deficiency had a 1.44 times higher prevalence of diabetes compared to those without G6PD deficiency at this age group. This was similarly noted in other studies which had found that G6PD enzyme activities were consistently reported to be lower among patients with diabetes compared to normal controls [11,12,13,14].
Two other studies examined the prevalence and severity of retinal complications in patients with G6PD deficiency and diabetes. Results of these studies were mixed, with one study reported an acceleration of microvascular complication [15] while another reported otherwise [16]. A similar trend was noted in studies examining the relationship between G6PD deficiency and glycaemic control. The study by Meloni and colleagues reported that HbA1c levels were lower in diabetes with G6PD deficiency compared to those with normal G6PD levels [17]. In contrast, Akter et al. reported that HbA1c levels were higher in G6PD-deficient individuals compared to those with normal G6PD [4].
Meta-analysis
Pooled results of the seven studies that examined the prevalence of diabetes in G6PD deficiency showed that overall, patients with G6PD deficiency were associated with an increased risk of diabetes. The summary odds ratio (OR) was 2.37 (95% CI 1.50–3.73), with higher odds among men compared to women (OR 2.22 (95% CI 1.31–3.75) vs. 1.87 (1.12–3.12); Fig. 1). However, there was evidence of heterogeneity in the estimates (I 2 = 88.2%, p < 0.001). We subsequently examined the potential sources of heterogeneity which could have influenced the results. We found that when stratified according to study design, these associations are consistent in cross-sectional studies but not case-control studies. Other factors such as gender, types of diabetes examined as well as diabetes definitions were not associated with overall effect size (Supplementary Table S2). In meta-regression analyses, we found that study design accounted for 24% of the total heterogeneity. The data was, however, insufficient to analyse the role of different G6PD variants or degree of G6PD enzyme activity on the prevalence of diabetes. Sensitivity analyses suggest the results were broadly consistent with some form of asymmetry noted suggesting small study effects (Supplementary Fig. S2).
Discussion
To our knowledge, this is the first systematic review and meta-analysis to-date that examines the relationship between G6PD deficiency and diabetes. Overall, the pooled results from 7 studies with 893,408 participants suggest that patients with G6PD deficiency have a 2.37 times increased odds of developing diabetes compared to unaffected individuals, with men appearing more likely to be affected compared to women.
This finding raises several potential ramifications from a public health perspective which merits some discussion. As G6PD deficiency is an x-linked hereditary disease, the defect commonly affects males as opposed to females [18]. Furthermore, findings from several studies [19, 20] have also suggested that males and Asians also have a higher risk of developing diabetes. Taken together, this suggests that there may be a further increase in the prevalence of diabetes among future generations since these are genetically transmitted. In addition, because diabetes compounds cardiovascular risk factors, we are likely to expect an increase in prevalence of cardiovascular disease in the future. As such, we recommend that all G6PD-deficient individuals should be screened for diabetes regularly and at a younger age compared to the unaffected population. These findings also suggest that it may be beneficial to include this information on association especially in patient information leaflets given to all parents with G6PD-deficient children.
Strengths and limitations
This analysis offers several strengths. Firstly, as a systematic review and meta-analysis, the study has greater power to detect any differences than any of the individual studies identified. Secondly, the study used a broad range of keywords as well as inclusion of a citation tract to identify for recently published studies, without any language restrictions. Thirdly, this study was done in accordance with the PRISMA [21] and MOOSE [22] guidelines. Finally, this is the largest confirmatory work to date examining the association between G6PD deficiency and diabetes.
This study has several limitations. Firstly, many of the studies reported in the review did not systematically examine the class of G6PD enzyme variant of their patients. This information would have provided researchers useful insights into the possible implications of this deficiency, as different variants have been shown to correspond with various levels of enzyme activities [18]. Another limitation of the review is the wide variation in the methodologies of the included studies, with fewer than half of the eligible studies having contributed suitable data for our meta-analysis. Furthermore, in our meta-analyses, substantial heterogeneity existed despite grouping studies according to patient and study characteristics as well as outcome definition. This suggests that much of the heterogeneity was unexplained and precluded the presentation of summary estimates. Finally, studies were included from over three decades, in which the definition and criteria for diabetes have been revised regularly. For example, in 1997, the threshold of diabetes diagnosis using fasting plasma glucose was lowered [23]. As such, inclusion of older studies may have led to an underestimation of diabetes prevalence.
In summary, patients with G6PD deficiency appear to have an increased odd of developing diabetes compared to unaffected controls. As the current unprecedented growth of diabetes is becoming a national and worldwide public health problem, further studies into how G6PD deficiency increases risk of diabetes is required. A large longitudinal study that measures glycosylated haemoglobin, fasting plasma glucose at baseline and over time should be established to fill in the gaps in our understanding of mechanisms by which G6PD deficiency moderates diabetes especially by sex. In addition, clinicians also need to consider having more routine screening of diabetes especially among G6PD-deficient individuals, in view of the increased risk especially among men.
References
Nkhoma ET, Poole C, Vannappagari V, Hall SA, Beutler E (2009) The global prevalence of glucose-6-phosphate dehydrogenase deficiency: a systematic review and meta-analysis. Blood Cells Mol Dis 42(3):267–278. doi:10.1016/j.bcmd.2008.12.005
Heymann AD, Cohen Y, Chodick G (2012) Glucose-6-phosphate dehydrogenase deficiency and type 2 diabetes. Diabetes Care 35(8):e58. doi:10.2337/dc11-2527
Adinortey MB, Owusu RK, Galyuon IKA, Ekloh W, Owusu I, Larbi DA (2011) G6PD deficiency—a potential risk factor for development of diabetes mellitus. Journal of Medicine and Medical Science 2(8):1017–1021
Akter N, Begum N, Ferdousi S (2011) Glucose-6-phosphate dehydrogenase (G6PD) status in female type 2 diabetes mellitus and its relationship with HbA 1 C. Journal of Bangladesh Society of Physiologist 5(2):6. doi:10.3329/jbsp.v5i2.6778
Sterne J, Higgins J, Reeves B (2016) ROBINS-I: a tool for assessing risk of bias in non-randomized studies of interventions. http://www.riskofbias.info. Accessed 15 Mar 2016
Meloni T, Pacifico A, Forteleoni G, Meloni GF (1992) G6PD deficiency and diabetes mellitus in northern Sardinian subjects. Haematologica 77(1):94–95
Saha N (1979) Association of glucose-6-phosphate dehydrogenase deficiency with diabetes mellitus in ethnic groups of Singapore. J Med Genet 16(6):431–434
Saeed TK, Hamamy HA, Alwan AAS (1985) Association of glucose-6-phosphate dehydrogenase deficiency with diabetes mellitus. Diabet Med 2(2):110–112
Niazi GA (1991) Glucose-6-phosphate dehydrogenase deficiency and diabetes mellitus. Int J Hematol 54(4):295–298
Santana MS, Monteiro WM, Costa MRF, Sampaio VS, Brito MAM, Lacerda MVG, Alecrim MGC (2014) High frequency of diabetes and impaired fasting glucose in patients with glucose-6-phosphate dehydrogenase deficiency in the western Brazilian Amazon. Am J Trop Med Hyg 91(1):74–76
Mahmoud AA, Nor El-Din AK (2013) Glucose-6-phosphate dehydrogenase activity and protein oxidative modification in patients with type 2 diabetes mellitus. Journal of biomarkers 2013:430813. doi:10.1155/2013/430813
Festus O, Dada F, Iweka F, Eyaufe A, Osagie R, Akiyang E (2012) Assessment of the activity of glucose-6-phosphate dehydrogenase in patients with type 2 diabetes mellitus in Ekpoma, South-South Nigeria. International Journal of Community Research 1(2):45–48
Rashidi H, Shafiei M, Hamidian R (2009) Erythrocytic glucose-6-phosphate dehydrogenase activity in diabetic patients. Pakistan Journal of Medical Sciences 25(4):665–668
Wan GH, Tsai SC, Chiu DT (2002) Decreased blood activity of glucose-6-phosphate dehydrogenase associates with increased risk for diabetes mellitus. Endocrine 19(2):191–195. doi:10.1385/endo:19:2:191
Cappai G, Songini M, Doria A, Cavallerano JD, Lorenzi M (2011) Increased prevalence of proliferative retinopathy in patients with type 1 diabetes who are deficient in glucose-6-phosphate dehydrogenase. Diabetologia 54(6):1539–1542. doi:10.1007/s00125-011-2099-3
Pinna A, Contini EL, Carru C, Solinas G (2013) Glucose-6-phosphate dehydrogenase deficiency and diabetes mellitus with severe retinal complications in a Sardinian population, Italy. Int J Med Sci 10(13):1907–1913. doi:10.7150/ijms.6776
Meloni T, Pacifico A, Forteleoni G, Meloni GF (1994) HbA1c levels in diabetic Sardinian patients with or without G6PD deficiency. Diabetes Res Clin Pract 23(1):59–61
Beutler E (1996) G6PD: population genetics and clinical manifestations. Blood Rev 10(1):45–52
Logue J, Walker JJ, Colhoun H, Leese GP, Lindsay RS, McKnight JA, Morris AD, Pearson D, Petrie JR, Philip S, Wild S, Sattar N (2011) Do men develop type 2 diabetes at lower body mass indices than women? Diabetologia 54(12):3003–3006. doi:10.1007/s00125-011-2313-3
Choi YJ, Kim HC, Kim HM, Park SW, Kim J, Kim DJ (2009) prevalence and management of diabetes in Korean adults: Korea National Health and Nutrition Examination Surveys 1998–2005. Diabetes Care 32(11):2016–2020. doi:10.2337/dc08-2228
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ (Clin Res Ed) 339. doi:10.1136/bmj.b2535
Stroup DF, Berlin JA, Morton SC et al (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA 283(15):2008–2012. doi:10.1001/jama.283.15.2008
World Health Organization (2006) Definition and diagnosis of diabetes mellitus and intermediate hyperglycaemia: report of a WHO/IDF consultation
Acknowledgements
The authors wish to thank Anthony Heymann of the University of Tel Aviv for sharing the information required from his studies.
Author contributions
SWHL, NML and YKL undertook the literature search and reviewed the abstracts and full articles. SWHL conceived the idea, drafted the initial manuscript and performed the statistical analysis. All authors designed the study, contributed to the discussion and critically reviewed the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Source of funding
None.
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Supplementary Figure S1
(DOCX 36 kb)
Supplementary Figure S2
(DOCX 15 kb)
Supplementary Table S1
(DOCX 157 kb)
Supplementary Table S2
(DOCX 14 kb)
Rights and permissions
About this article
Cite this article
Lai, Y.K., Lai, N.M. & Lee, S.W.H. Glucose-6-phosphate dehydrogenase deficiency and risk of diabetes: a systematic review and meta-analysis. Ann Hematol 96, 839–845 (2017). https://doi.org/10.1007/s00277-017-2945-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-017-2945-6