Abstract
Purpose
Hypomagnesemia, characterized by low magnesium levels, has been implicated in the pathophysiology of Type 2 Diabetes Mellitus (T2DM). This meta-analysis aims to provide a comprehensive assessment of hypomagnesemia prevalence in individuals with T2DM as well as its potential implications for diabetes management and complications.
Methods
We conducted a comprehensive systematic review and meta-analysis using databases like PubMed, Google Scholar, Science Direct, and Research Gate to identify relevant studies between January 2008 and August 2023. We focused on observational studies related to serum magnesium levels and Type 2 Diabetes in individuals aged 19 and older. Newcastle Ottawa tool was used for quality assessment. A random effect meta-analysis was performed to calculate the prevalence of hypomagnesemia in T2DM.
Results
We identified a total of 671 studies, and after screening 383 abstracts and full texts by two independent reviewers, we identified 19 eligible studies encompassing 4192 patients diagnosed with T2DM. The mean age was 55.4 (SD, 4.39) years with a mean HbA1C level of 8.01. The pooled prevalence of hypomagnesemia in T2DM was 32% (95% CI: 22–36%) out of 4192 cases. On subgroup analysis, the prevalence of hypomagnesemia in male and female were 19.8% and 20.1%, respectively. Geographically, Asia had the highest prevalence of hypomagnesemia with 31.9% (95% CI: 24–41.1%).
Conclusion
This meta-analysis highlights a significant prevalence of hypomagnesemia in individuals with T2DM, emphasizing the need for further investigation due to the intricate nature of the association between serum magnesium levels and T2DM.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes mellitus is a chronic condition characterized by hyperglycemia due to defective insulin secretion, insulin resistance, or both. Type 2 diabetes mellitus (T2DM) in particular is characterized by defective insulin receptors leading to insulin resistance in the body. Over 300 million individuals worldwide have type 2 diabetes mellitus (T2DM), and the prevalence is expected to climb to over 600 million in the coming decades with more than 75% of the adults residing in developing countries such as India, China and the USA [1].
Among the various complications of this global epidemic, its interplay with electrolyte imbalances is crucial for the understanding of this disease. One such electrolyte of importance is magnesium. Magnesium is a co-factor for various enzymes and is an integral part of protein, carbohydrate metabolism and DNA synthesis. Type 2 diabetes mellitus and the levels of magnesium are tightly regulated. Magnesium affects the insulin receptor sensitivity, insulin secretion in pancreatic beta cells and vascular tone. Insulin also affects the reabsorption of magnesium in the kidney [2].
Magnesium deficiency is strongly correlated with poorly controlled glycemic control, cardiovascular structural indices and metabolic syndrome,thus inducing or worsening existing diabetes [3].
Hypomagnesemia is also linked to microvascular complications (retinopathy, neuropathy and nephropathy) and secondary hypocalcemia, hypokalemia, and hypophosphatemia, further worsening cardiovascular and neuromuscular physiology [4].
Although certain metabolic studies show that Mg supplementation has a favorable effect on the action of insulin and in the metabolism of glucose, the mechanism involving DM and hypomagnesemia is yet unknown [5]. Due to its ability to increase insulin sensitivity and shield against diabetes and its consequences, magnesium has drawn a lot of interest. Nevertheless, the studies’ findings varied [6]. Although hypomagnesemia has been connected to additional risk factors for the development of diabetic retinopathy in Caucasian diabetics, black African diabetics have not shown this linkage [6].
The objective of this study is to systematically review and analyze the existing scientific literature to determine the prevalence of hypomagnesemia (low magnesium levels) in individuals with Type 2 Diabetes Mellitus T2DM and to explore the association between hypomagnesemia and T2DM, as well as its potential implications for diabetes management and its complications. This study aims to provide a comprehensive understanding of the relationship between magnesium status and T2DM, shedding light on the importance of magnesium in the context of this chronic metabolic disorder.
Methods
Eligibility criteria
Inclusion criteria
To be included in this systematic review, studies must meet several criteria. The studies focused on patients diagnosed with T2DM, studies that reported the prevalence of magnesium in patients with T2DM, manifestations, or complications associated with T2DM and individuals aged more than 19 years. The studies should be written in English and include human subjects only. Studies published only in the last 15 years are included.
Exclusion criteria
The systematic review excludes studies that do not pertain to T2DM or lack relevant information on the association with hypomagnesemia. Exclusion criteria were applied to certain study types, specifically, case reports and case series, owing to their limited generalizability. Furthermore, all forms of review articles, encompassing literature reviews, scoping reviews, and systematic reviews, were also excluded if published before 2008 or after 2023 and those not written in English. Studies involving pregnant patients, T1DM, patients less than 19 years of age and animal subjects are also excluded from this review.
Search Strategy
The following keywords are used to search PubMed, Google Scholar, Science Direct and Research Gate: (“Hyperglycemia,” “Diabetes Mellitus, Type 2,” “Insulin Resistance,” “Magnesium,” “Hypomagnesemia,” and “Magnesium Deficiency” to retrieve relevant studies focusing on the association of hypomagnesemia with T2DM. Booleans like AND and OR combine the keywords to search these databases. Supplementary Table 1 summarizes the search strategy used for this systematic review.
Study selection
Two reviewers scanned titles and abstracts independently and resolved conflicts through consensus. In the event of a continuing disagreement, a third reviewer intervened. All potentially relevant records are evaluated by the same reviewers. We kept track of the reasons why studies were excluded from this review. We utilized ChatGPT to improve the overall grammar, style, and coherence of the manuscript.
Assessment of the Methodological quality and risk of bias
The remaining studies underwent individual quality assessments conducted by two separate authors. These assessments employed the New Castle Ottawa scale [7]. Details regarding the quality assessment of the final [19] included reviews are presented in Supplementary Table 3.
Statistical analysis
In our meta-analysis, the primary goal was to aggregate the prevalence effect sizes across selected studies, employing the Comprehensive Meta-Analysis (CMA) software, version 4. Point prevalence and its 95% CI were extracted for each study. When studies presented varied prevalence data points, we selected the most pertinent for uniformity. Due to expected inter-study heterogeneity stemming from differences in populations, methodologies, and contexts, a random-effects model was preferred. We quantified this heterogeneity using the I2 statistic.
In our comprehensive sensitivity analysis, we iteratively omitted one study and recalculated the aggregated prevalence effect size, thereby ensuring the stability and robustness of our results. To investigate potential presence of publication bias, we crafted a funnel plot using Meta-Essentials [8]. Ideally, a symmetrical, inverted funnel indicates no evident publication bias. However, for a more comprehensive assessment of asymmetry, we further implemented Egger’s regression test and Begg & Mazumdar’s rank correlation test. These methodologies pinpoint potential biases suggested by deviations in the funnel plot.
Results
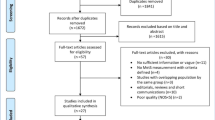
In this systematic review, a total of 671 records were initially identified through searches in various databases. There were no records identified from registers. After removing duplicate records (n = 288), 383 unique records remained for screening. During the screening phase, 350 records were excluded based on relevance and alignment with the research focus. Following this, 83 reports were sought for retrieval, and 267 reports were not retrieved. Out of the 24 reports assessed for eligibility, 5 were excluded from the systematic review: 3 due to inclusion of T1DM, one included patients less than 19 years of age and one included pregnant patients. Finally, 19 studies met the inclusion criteria and are included in the systematic review, forming the basis for the analysis and synthesis of the relevant data. Figure 1 illustrates the PRISMA flowchart summarizing details of the screening, identification and final inclusion of the studies. The data was extracted in a Microsoft Excel spreadsheet and was completed on Sept 17, 2023.
We found 19 studies that included 4192 patients published between 2012 and 2023 (Supplementary Table 2). Among these, 1146 individuals with diabetes mellitus were discovered to be suffering from hypomagnesemia. Table 1 contains information regarding the studies, such as research design types, prevalence of hypomagnesemia, mean serum magnesium levels, sex ratio, mean age, and total participants. Notably, 17 of the publications were cross-sectional studies [9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25], with the remaining two being cohort studies [26] and case control studies [27], respectively.
Meta-analysis
Based on the cumulative data from 19 studies, the pooled prevalence effect size for hypomagnesemia in individuals with type 2 diabetes mellitus was estimated to be 0.30, with a 95% confidence interval ranging from 0.23 to 0.39 (Fig. 2). This suggests that approximately 30% of individuals with type 2 diabetes mellitus, as reported in these studies, might have hypomagnesemia. The confidence interval further indicates that, with 95% certainty, this prevalence rate lies between 23% and 39%. Regarding study heterogeneity, the Tau2 value of 0.71 indicates significant between-study variation. The Chi2 test yielded a value of 506.4 with 18 degrees of freedom and a p < 0.0001, emphasizing pronounced heterogeneity. The I2 statistic, standing at 96.4%, underscores an extremely high variation across studies, suggesting that the majority of the observed variability can be attributed to genuine differences in outcomes rather than mere random fluctuations.
Subgroup analysis
Gender-based prevalence
In our analysis of hypomagnesemia prevalence among T2DM patients, 6 studies were identified for both genders, each encompassing 1461 observations. Males demonstrated a prevalence of 19.8% (95% CI: 15.6–24.7%), slightly lower than females with a prevalence of 20.1% (95% CI: 8.4–40.7%) (Table 2).
Geographical prevalence
In Asia, the pooled prevalence from various countries revealed diverse figures: India at 28.2% (95% CI: 18.4–40.6%), Pakistan at 41.2% (95% CI: 25.7–58.6%), Indonesia at 17.1% (95% CI: 11.1–25.2%), Palestine at 10.9% (95% CI: 8.2–14.5%), Nepal with the highest of 49.7% (95% CI: 42.3–57.1%), Saudi Arabia at 28.4% (95% CI: 23.4–33.9%), and Turkey at 52.5% (95% CI: 41.6–63.1%). The pooled estimate for Asia was 31.9% (95% CI: 24–41.1%).As for Europe, the data from the Netherlands showed a prevalence of 9.5% (95% CI: 7.8–11.6%), while Spain reported a significantly higher figure at 48% (95% CI: 41.1–54.9%). Europe’s combined prevalence was 23.7% (95% CI: 3.6–72.2%). From Africa, Nigeria was the sole representative, with a reported prevalence of 27.3% (95% CI: 20.8–34.8%).
Sensitivity analysis
A sensitivity analysis was executed by iteratively omitting one study at a time and recalculating the aggregated prevalence effect size (Fig. 3). Remarkably, the removal of individual studies did not produce any notable shifts in heterogeneity, confirming the consistent nature of the included studies. The prevalence estimates, during this analysis, displayed a tight range from a lower bound of 29% (with a 95% confidence interval of 22–36%) to an upper bound of 32% (with a 95% confidence interval of 25–40%). Such marginal fluctuations on individual study exclusion underscore the stability of the aggregated findings. In essence, this sensitivity analysis affirms the reliability of our results, suggesting that they are not overly influenced by any single included study.
Publication Bias
To assess potential publication bias among the 19 included studies, multiple diagnostic methods were utilized. The Egger Regression test was employed first. The intercept estimate was −0.6 with a standard error (SE) of 0.87, and its 95% confidence interval (CI) ranged from −2.43 to 1.23. Notably, the t-test for the intercept rendered a p-value of 0.5, suggesting no significant evidence of funnel plot asymmetry. Subsequently, Begg & Mazumdar’s rank correlation test was performed. With Kendall’s Tau a at −0.18 and a resulting p-value of 0.139, this test also indicated no significant publication bias.
Visually, the funnel plot was symmetrical, offering qualitative evidence supporting the absence of notable bias (Fig. 4). In summary, both statistical evaluations and the funnel plot suggest minimal publication bias among the studies, underscoring the reliability of our meta-analysis.
Discussion
In this systematic review and meta-analysis, we attempted to combine the prevalent impact sizes of hypomagnesemia in people with type 2 diabetes mellitus by combining information from 19 chosen research. Our data showed a pooled prevalence effect size of 0.30, indicating that about 30% of people with type 2 diabetes mellitus may experience hypomagnesemia. A reliable estimate of this prevalence rate was given by the 95% confidence interval, which covered the range of 0.23 to 0.39. This prevalence rate suggests that a substantial proportion of individuals with type 2 diabetes may be at risk of hypomagnesemia. The observed high heterogeneity across the studies, with an I2 statistic of 96.4%, reflects genuine variations in outcomes among different populations and methodologies rather than random fluctuations. Importantly, sensitivity analysis demonstrated the robustness of these findings, with minimal shifts in prevalence estimates upon excluding individual studies, reaffirming the consistency of the included research. Furthermore, the absence of significant publication bias, as indicated by both statistical tests and a symmetrical funnel plot, enhances the credibility of our meta-analysis results. In sum, this study highlights the pressing need for clinical attention to magnesium status in T2DM management and underscores the reliability and generalizability of the prevalence estimates obtained.
Our analysis also revealed significant heterogeneity among the included studies, emphasizing the necessity for a careful interpretation of these results. Despite the observed heterogeneity, our meta-analysis discuss the clinical significance of assessing magnesium status in individuals with type 2 diabetes mellitus. Hypomagnesemia, as identified in approximately 30% of our study population, may have implications for diabetes management and overall health. Magnesium is involved in numerous physiological processes, including glucose metabolism and insulin sensitivity. Therefore, identifying and addressing magnesium deficiency in individuals with type 2 diabetes mellitus is imperative for optimizing their health outcomes. Moreover, the average duration of diabetes varied among the studies, with some investigating relatively recent diagnoses and others focusing on individuals with longstanding diabetes exceeding a decade.
In our meta-analysis, we encountered variations in cut-off values for defining hypomagnesemia across the included studies, ranging from 0.6 mMol/L to <1.6 mg/dL. Normal serum magnesium levels typically fall within the range of 1.7 to 2.2 mg/dL or 0.7 to 0.9 mmol/L, although slight variations may exist based on laboratory standards [6]. Furthermore, the choice of assay method, such as the calmagite dye method, photometric method, or spectrophotometry, introduced variability in magnesium measurements among studies. Acknowledging these variations is crucial for understanding the observed heterogeneity in prevalence rates and ensuring the reliability of the reported findings.
Not only has hypomagnesemia been associated with to type 2 diabetes, but a number of studies have found an unfavorable association between glycemic management and blood Mg levels [28,29,30]. Although several authors have claimed that diabetes itself may cause hypomagnesemia, others have found that consuming more magnesium may reduce the chance of developing type 2 diabetes [5, 31,32,33].
Gender differences in the prevalence of hypomagnesemia are evident across the studies. Siddiqui et al. and Kocyigit et al. observe a higher prevalence among males [17, 27], while Lamsal et al., Hamarshih et al., and Odusan et al. find a higher prevalence among females [13, 21, 23]. These gender disparities may be caused by hormonal changes, dietary preferences, or other aspects of magnesium metabolism. Additionally, the mean age and average duration of diabetes among the study populations warrant consideration in the context of these findings. The included studies reflected a wide age range, with mean ages spanning from the early 40 s to late 50 s, indicating that hypomagnesemia is a concern across different age groups of individuals with type 2 diabetes.
Numerous studies show a correlation between the prevalence of hypomagnesemia and the duration of diabetes. Both Noor et al. and Siddiqui et al. found that hypomagnesemia increases with ongoing diabetes, which raises the possibility that long-term glycemic dysregulation may be a factor in magnesium imbalance [16, 17]. Wanders et al. indicate a decreased prevalence of hypomagnesemia in people with type 2 diabetes mellitus who are treated in primary care settings, which counteracts this tendency [26]. This discrepancy highlights the complexity of the magnesium-diabetes interactions as well as the possible impact of healthcare environments and management techniques on magnesium status.
Studies by Dasgupta et al., Lamsal et al., Nayyar et al., Hamarshih et al., and Rao et al. draw attention to the connection between hypomagnesemia and numerous microvascular problems in type 2 diabetes [9, 10, 13, 20, 23]. Diabetic retinopathy and nephropathy, as well as inadequate glycemic control, are among the consequences. This regular pattern highlights the potential clinical use of determining the magnesium status in people with type 2 diabetes, since hypomagnesemia may be a marker for an increased risk of microvascular problems. Previous studies conducted by various researchers have provided valid substantiation for the adverse outcomes linked to reduced magnesium levels [5, 28,29,30, 34, 35].
Azeez et al. and Alswat et al. explore the connection between hypomagnesemia and cardiometabolic risk factors in type 2 diabetes [14, 19]. Their findings demonstrate the potential value of plasma magnesium as a cardiovascular risk measure in resource-constrained environments. The association between magnesium levels and cardiovascular health is still being studied because cardiovascular problems are a major issue in diabetes care.
Dasgupta et al., Nayyar et al., and Rao et al. provide insights into the association between hypomagnesemia and glycemic control [9, 10, 20]. Hypomagnesemia is linked to poorer glycemic control, suggesting that magnesium supplementation could potentially aid in glycemic management. This finding is of clinical relevance, as it raises the possibility of magnesium interventions to improve diabetes outcomes.
Adebayo et al. and Kumar et al. emphasized the importance of including electrolyte assessments, including magnesium levels, as part of routine screening in diabetic patients [11, 15]. Early detection and management of electrolyte imbalances, including hypo and hypermagnesemia, are crucial for optimizing diabetes care. Routine monitoring of magnesium levels in diabetes patients may help identify individuals at risk of complications.
According to Pham et al., Mg concentration between 2.0 and 2.5 mg/dl may be favorable. in patients with diabetes [6]. Although the correction of low serum Mg levels has never been proved to be protective against chronic diabetic complications, intervention is justified because hypomagnesemia has been associate to many adverse clinical outcomes [6].
Strengths
The sensitivity analysis performed in this study strengthens the reliability of our findings. The consistent nature of the aggregated prevalence estimates, with minimal fluctuations upon the exclusion of individual studies, suggests that our results are not unduly influenced by any single study. This stability enhances the credibility of our conclusions regarding the prevalence of hypomagnesemia in individuals with type 2 diabetes mellitus. Our assessment of publication bias, employing both the Egger Regression test and Begg & Mazumdar’s rank correlation test, revealed no significant evidence of bias among the included studies. The symmetrical funnel plot provided additional qualitative support for the absence of notable bias. These findings suggest that our meta-analysis is not unduly influenced by publication bias, further enhancing the trustworthiness of our results.
Limitations
Although our meta-analysis sheds light on the frequency of hypomagnesemia in people with type 2 diabetes, there are a number of limitations that need to be acknowledged. The generalizability of our findings may be constrained by the heterogeneity of the included research. Because most research is cross-sectional in nature, causality or temporal connections cannot be established. Additionally, the absence of information on dietary intake and magnesium supplementation in the included studies restricts our capacity to evaluate potential confounding variables.
Conclusion
The purpose of this meta-analysis is to look into the prevalence of hypomagnesemia in people with type 2 diabetes mellitus, in order to shed light on the complicated link between magnesium status and diabetes-related comorbidities. Our goal is to combine the information already available from numerous studies that each provided a distinctive perspective on this association.
Based on the results of our meta-analysis, it is clear that hypomagnesemia is a prevalent concern among individuals with type 2 diabetes mellitus. The prevalence estimates differed between research, underlining the influence of elements including diabetes duration, glycemic management, and gender. Notably, we highlighted the persistent link between hypomagnesemia and microvascular problems, emphasizing the importance of this relationship in the management of diabetes. Furthermore, the possible impact of magnesium on cardiovascular health and glycemic management is identified as significant areas of interest that require additional exploration.
Longitudinal studies to establish causal links between magnesium levels and diabetes-related outcomes are recommended for future research. Understanding how magnesium supplementation affects glycemic control and prevents complications may provide insightful information. Standardization of cut-off values and assay methodologies for magnesium measurements is also necessary to improve cross-study comparability. It is also important to continue researching how gender and healthcare interventions affect magnesium levels in diabetes. Overall, treating hypomagnesemia in patients with type 2 diabetes mellitus is critical for enhancing diabetic treatment and enhancing long-term health outcomes.
References
H. King, R.E. Aubert, W.H. Herman, Global burden of diabetes, 1995-2025: prevalence, numerical estimates, and projections. Diabetes Care 21(9), 1414–1431 (1998).
L.M.M. Gommers, J.G.J. Hoenderop, R.J.M. Bindels, J.H.F. De Baaij, Hypomagnesemia in type 2 diabetes: A vicious circle? Diabetes [Internet]. 65(1), 3–13 (2023). https://diabetesjournals.org/diabetes/article/65/1/3/34908/Hypomagnesemia-in-Type-2-Diabetes-A-Vicious-Circle.
M. Barbagallo, L.J. Dominguez, Magnesium metabolism in type 2 diabetes mellitus, metabolic syndrome and insulin resistance. Arch. Biochem. Biophys. 458(1), 40–47 (2007).
G. Liamis, E. Liberopoulos, F. Barkas, M. Elisaf, Diabetes mellitus and electrolyte disorders. World J. Clin. Cases 2(10), 488–496 (2014).
W.H.L. Kao, A.R. Folsom, F.J. Nieto, J.P. Mo, R.L. Watson, F.L. Brancati, Serum and dietary magnesium and the risk for type 2 diabetes mellitus: The atherosclerosis risk in communities study. Arch. Intern. Med. [Internet] 159(18), 2151 (2023). https://doi.org/10.1001/archinte.159.18.2151.
P.C.T. Pham, P.M.T. Pham, S.V. Pham, J.M. Miller, P.T.T. Pham, Hypomagnesemia in patients with type 2 diabetes. Clin. J. Am. Soc. Nephrol. [Internet] 2(2), 366–373 (2023). https://journals.lww.com/01277230-200703000-00033.
G.A. Wells, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. Department of Epidemiology and Community Medicine, University of Ottawa, Canada. University of Ottawa, Room A 3227 (2011): 451.
R. Suurmond, H. Van Rhee, T. Hak, Introduction, comparison, and validation of Meta‐Essentials: A free and simple tool for meta‐analysis. Res. Synth. Methods [Internet] 8(4), 537–553 (2023). https://doi.org/10.1002/jrsm.1260. Available from https://onlinelibrary.wiley.com/doi/.
A. Dasgupta, D. Sarma, U.K. Saikia, Hypomagnesemia in type 2 diabetes mellitus. Indian J. Endocrinol. Metab. [Internet] 16(6), 1000 (2023). https://journals.lww.com/indjem/fulltext/2012/16060/hypomagnesemia_in_type_2_diabetes_mellitus.24.aspx.
S.B. Nayyar, H.S. Brar, S. Kukreja, K. Kaur, Study of serum magnesium level in type 2 diabetes mellitus with nephropathy. Int J Adv Med [Internet]. 2019 Jul 24 [cited 6(4), 1145–1150 (2023). https://www.ijmedicine.com/index.php/ijam/article/view/1466.
P. Kumar, S. Bhargava, P.K. Agarwal, A. Garg, A. Khosla. Association of serum magnesium with type 2 diabetes mellitus and diabetic retinopathy. J. Fam. Med. Prim. Care [Internet]. 2019 May [cited 8(5), 1671–1677 (2023). https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6559114/.
P. Dwipayana, M.R. Saraswati, W. Gotera, A.A.G. Budhiarta, K. Suastika, S. Malik, et al. Hypomagnesemia is a risk factor for metabolic syndrome and type 2 diabetes mellitus in native Balinese. Fam. Med. Community Health [Internet]. 2013 Mar 1 [cited 2023 Sep 18];1(1). Available from: https://fmch.bmj.com/content/1/1/14.
R. Sangroula, Fasting serum magnesium level and its association with type 2 diabetes mellitus and its chronic complications. J. Inst. Med. Nepal 43, 97–101 (2022).
T. Azeez, O. Sonuga, Hypomagnesemia as a cardiometabolic risk marker in type 2 diabetes mellitus: Implications for Sub-Saharan Africa. Ann. Clin. Cardiol. [Internet]. 2021 [cited 3(1), 29 (2023). http://www.onlineacc.org/text.asp?2021/3/1/29/317104.
T. Adebayo, A. Ajayi, S. Shoyinka, F. Oyetunji, A. Ajetunmobi, O. Olaniyan, Assessment of magnesium n type II diabetes mellitus and its correlation to glycated haemoglobin. Life Sci. ume 2, 30–36 (2022).
M.M. Noor, Q. Nazir, T.M. Khan, S. Gillani, M.A. Abbasi, A. Rauf et al. Association between low serum magnesium level and type 2 diabetes mellitus in abbottabad. J. Ayub. Med. Coll. Abbottabad. 31(2), 226–229 (2019).
M.U. Siddiqui, I. Ali, M. Zakariya, S.P. Asghar, M.R. Ahmed, G.H. Ibrahim. FREQUENCY OF HYPOMAGNESEMIA IN PATIENTS WITH UNCONTROLLED TYPE II DIABETES MELLITUS: Hypomagnesemia in Type 2 Diabetes Mellitus. Pak. Armed Forces Med. J. [Internet]. 2016 Dec 31 [cited 66(6), 845–850 (2023). https://pafmj.org/index.php/PAFMJ/article/view/1025.
M. Hasan, S. Sultan, S. Ashfaq, S. Irfan, Hypomagnesemia in type II diabetes mellitus patients; an experience from a tertiary care center. Ann. PIMS 13, 146–150 (2017).
K. Alswat, Type 2 diabetes control and complications and their relation to serum magnesium level. Arch Med Sci AMS [Internet]. 2021 Mar 18 [cited 18(2), 307–313 (2023). https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8924817/.
Y. Rao, D.R. Vanamali. Serum magnesium levels in type 2 diabetes. Int J Res Med Sci. 2016 Jan 1;
O. Odusan, O. Familoni, A. Odewabi, A. Idowu, A. Adekolade, Patterns and correlates of serum magnesium levels in subsets of type 2 diabetes mellitus patients in Nigeria. Indian J. Endocrinol. Metab. 21, 439 (2017).
R. Paladiya, A. Pitliya, A. Choudhry, D. Kumar, S. Ismail, A. Mohammed, et al. Association of low magnesium level with duration and severity of type 2 diabetes. Cureus. 2021 May 27;13.
Hamarshih M, Hamshari S, Nazzal Z, Snobar F, Mletat R, Abu-Mazen O, et al. Hypomagnesemia and Poor Glycemic Control among Type 2 Diabetic Patients: A Cross-Sectional Study. Indian Journal of Endocrinology and Metabolism [Internet]. 2022;26(6). Available from: https://journals.lww.com/indjem/fulltext/2022/11000/hypomagnesemia_and_poor_glycemic_control_among.12.aspx.
A. Lecube, J.A. Baena-Fustegueras, J.M. Fort, D. Pelegrí, C. Hernández, R. Simó. Diabetes is the main factor accounting for hypomagnesemia in obese subjects. PLOS ONE [Internet]. 2012 Jan 24 [cited 7(1), e30599 (2023). https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0030599.
D.S.S. Antin, D.M. Kashinkunti, D.A.V. Kataria, D. Dhananjaya, D.S. Alevoor. A cross sectional study of fasting serum magnesium levels in the patients with type 2 diabetes mellitus and its relation to diabetic complications. In 2014. Available from: https://www.semanticscholar.org/paper/A-Cross-Sectional-Study-of-Fasting-Serum-Magnesium-Antin-Kashinkunti/20bac9323008517456c34af67fc9a1bf08003bba.
F. Waanders, R.P.F. Dullaart, M.J. Vos, S.H. Hendriks, H. van Goor, H.J.G. Bilo et al. Hypomagnesaemia and its determinants in a contemporary primary care cohort of persons with type 2 diabetes. Endocrine 67(1), 80–86 (2020).
E. Kocyigit, M. Akturk, E. Koksal, Relationships between serum and dietary magnesium, calcium, and metabolic parameters in women with type 2 diabetes mellitus. Clin. Nutr. ESPEN 54, 304–310 (2023).
L.M. Resnick, R.K. Gupta, H. Gruenspan, J.H. Laragh, Intracellular free magnesium in hypertension: relation to peripheral insulin resistance. J. Hypertens. Suppl. J. Int Soc. Hypertens. 6(4), S199–S201 (1988).
F. Liao, A.R. Folsom, F.L. Brancati, Is low magnesium concentration a risk factor for coronary heart disease? The Atherosclerosis Risk in Communities (ARIC) Study. Am. Heart J. 136(3), 480–490 (1998).
P. McNair, C. Christiansen, S. Madsbad, E. Lauritzen, O. Faber, C. Binder et al. Hypomagnesemia, a risk factor in diabetic retinopathy. Diabetes 27(11), 1075–1077 (1978).
R. Lopez-Ridaura, W.C. Willett, E.B. Rimm, S. Liu, M.J. Stampfer, J.E. Manson et al. Magnesium intake and risk of type 2 diabetes in men and women. Diabetes Care 27(1), 134–140 (2004).
Y. Song, J.E. Manson, J.E. Buring, S. Liu, Dietary magnesium intake in relation to plasma insulin levels and risk of type 2 diabetes in women. Diabetes Care 27(1), 59–65 (2004).
R.M. van Dam, F.B. Hu, L. Rosenberg, S. Krishnan, J.R. Palmer, Dietary calcium and magnesium, major food sources, and risk of type 2 diabetes in U.S. black women. Diabetes Care 29(10), 2238–2243 (2006).
A. Hatwal, A.S. Gujral, R.P. Bhatia, J.K. Agrawal, H.S. Bajpai, Association of hypomagnesemia with diabetic retinopathy. Acta Ophthalmol. (Copenh) 67(6), 714–716 (1989).
F.J. Rodriguez, A.L. Folpe, C. Giannini, A. Perry, athology of peripheral nerve sheath tumors: diagnostic overview and update on selected diagnostic problems. Acta Neuropathol (Berl) [Internet]. 2012 Mar [cited 123(3), 295–319 (2023). http://springerlink.bibliotecabuap.elogim.com/10.1007/s00401-012-0954-z.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by A.P., V.B., M.B.P., Suraj, A.D., A.P. and S.N. The first draft of the manuscript was written by A.P. and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethics approval
This systematic review is based on published data from literature and therefore does not involve active patient participation in the design, conduct, report or dissemination plans of the research. Therefore, it does not need ethical approval or consent for participation.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pitliya, A., Vasudevan, S.S., Batra, V. et al. Global prevalence of hypomagnesemia in type 2 diabetes mellitus – a comprehensive systematic review and meta-analysis of observational studies. Endocrine 84, 842–851 (2024). https://doi.org/10.1007/s12020-023-03670-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-023-03670-7