Abstract
We retrospectively compared the incidence of virus infections and outcome in the context of immune reconstitution in two different HLA-haploidentical transplantation (haplo-HSCT) settings. The first was a combined T-cell-replete and T-cell-deplete approach using antithymocyte globulin (ATG) prior to transplantation in patients with hematological diseases (cTCR/TCD group, 28 patients; median age 31 years). The second was a T-cell-replete (TCR) approach using high-dose posttransplantation cyclophosphamide (TCR/PTCY group, 27 patients; median age 43 years). The incidence of herpesvirus infection was markedly lower in the TCR/PTCY (22 %) than in the cTCR/TCD group (93 %). Recovery of CD4+ T cells on day +100 was faster in the TCR/PTCY group. CMV reactivation was 30 % in the TCR/PTCY compared to 57 % in the cTCR/TCD group, and control with antiviral treatment was superior after TCR/PTCY transplantation (100 vs 50 % cTCR/TCD). Twenty-five percent of the patients in the cTCR/TCD group but no patient in the TCR/PTCY group developed PTLD. While 1-year OS was not different (TCR/PTCY 59 % vs cTCR/TCD 39 %; p = 0.28), virus infection-related mortality (VIRM) was significantly lower after TCR/PTCY transplantation (1-year VIRM, 0 % TCR/PTCY vs 29 % cTCR/TCD; p = 0.009). On day +100, predictors of better OS were lymphocytes >300/μl, CD3+ T cells >200/μl, and CD4+ T cells >150/μl, whereas the application of steroids >1 mg/kg was correlated with worse outcome. Our results suggest that by presumably preserving antiviral immunity and allowing fast immune recovery of CD4+ T cells, the TCR approach using posttransplantation cyclophosphamide is well suited to handle the important issue of herpesvirus infection after haplo-HSCT.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
HLA-haploidentical hematopoietic stem cell transplantation (haplo-HSCT) is a valuable treatment option for patients with various hematological disorders who lack a suitable HLA-matched donor. In the past, the main risks limiting the benefit of haplo-HSCT were severe graft-versus-host disease (GvHD), graft rejection, and early death due to toxicity and infections [1, 2]. To allow for crossing of the HLA barrier, haplo-HSCT approaches were developed involving intensive preparative regimens and in vivo and in vitro T-cell depletion. Unwanted consequences of these approaches were reduction of cellular immunity and delayed immune reconstitution, resulting in high rates of mortality due to infections, toxicity, and relapse [3–7]. Whereas the use of reduced intensity conditioning (RIC) [8–10], infusion of mega doses of CD34+ cells [4, 8–11], and graft manipulations such as selective T-cell depletion [5] were helpful to achieve engraftment with lower rates of GvHD and toxicity, infectious complications remained an issue, in particular with a focus on viral infections in the early and intermediate posttransplantation phase, again primarily due to slow and impaired immune recovery [9, 12–15]. Thus, the employment of strategies that allow a faster and more robust immune reconstitution is an important task in haplo-HSCT.
Recently, the use of T-cell-replete (TCR) grafts and high-dose posttransplantation cyclophosphamide (PTCY) has shown promising results with low nonrelapse mortality (NRM) and improved immune reconstitution [16, 17]. At our center, another frequently used strategy of haplo-HSCT consisted of a conditioning regimen which contained absorbed antithymocyte globulin (ATG) and cyclophosphamide (CY) prior to transplantation, followed by a combined graft consisting of bone marrow and CD6-depleted peripheral blood stem cells (cTCR/TCD) which are sequentially applied. Successful induction of tolerance and full donor chimerism were reported in pediatric and adult patients with high-risk hematologic malignancies [18, 19].
In this study, we retrospectively evaluated the incidence of virus infections and their complications, immune recovery, and outcome in adults receiving a T-cell-replete haploidentical graft and PTCY (TCR/PTCY group) compared to those receiving a combination of a T-cell-replete and T-cell-deplete haploidentical graft (cTCR/TCD group) given sequentially.
Patients and methods
All patients receiving a HLA-haploidentical graft at our institution between April 2006 and April 2011 were enrolled in this retrospective study. Patient data were analyzed in particular with regard to microbiological data and absolute blood cell counts per microliter (leukocytes, neutrophils, platelets, lymphocytes, and their subsets). Reconstitution of T-cell subsets was assessed by flow cytometry on days +30, +100, and +180 after haplo-HSCT (range ±8 days). The occurrence of viral pathogens and virus infections and their clinical course were evaluated until day +180 and at 1 year after haplo-HSCT. All patients were treated according to institutional protocols and provided signed informed consent.
Infectious surveillance, prophylaxis, and supportive care
Before the start of conditioning, serology for herpesviruses (HSV, CMV, EBV, HHV-6, varicella zoster virus (VZV)), as well as respiratory viruses (influenzavirus A and B, parainfluenza and respiratory syncytial virus (RSV), adenovirus (ADV)), parvovirus B19, HIV, and hepatitis viruses, was assessed in all patients. Tests routinely performed during inpatient treatment included weekly monitoring of viral load in the blood (plasma), stool, urine, and oral lavage by quantitative real-time PCR specific for HSV, EBV, CMV, and HHV-6 and tests for RSV antigen (in oral lavage), ADV antigen (in oral lavage, urine, and stool), and polyomavirus JC and BK DNA (in urine). In the outpatient setting after haplo-HSCT (typically after day +30), PCR tests for CMV and EBV were routinely carried out every 7 or 14 days at least until day +100.
Routine prophylactic protective procedures for all patients included single unit isolation with air filters (HEPA), personnel wearing gloves and face masks, and prohibition of access of any persons presenting symptoms of upper or lower respiratory tract infection. All patients received standard antimicrobiological prophylaxis with acyclovir and trimethoprim-sulfamethoxazole. Acyclovir was administered at a daily dose of 4 × 400 mg orally (p.o.) or 3 × 500 mg intravenously (IV) over a period of at least 3–6 months after haplo-HSCT, or until discontinuation of immunosuppression and a CD4+ T-cell count >200/μl. CMV or EBV prophylaxis or high-dose intravenous immunoglobulins (IVIGs) were not administered on a routine basis. Patients were treated preemptively for CMV antigenemia or CMV DNA-emia (ganciclovir, foscarnet) and for EBV DNA-emia (cidofovir, rituximab).
Preparative regimen
In the cTCR/TCD setting, conditioning consisted of total body irradiation (TBI) or busulfan, an infusion of irradiated donor peripheral blood leukocytes (buffy coat), antithymocyte globulin (rabbit ATG 20 mg/kg daily for 5 days), and cyclophosphamide (50 mg/kg daily for 4 days). The graft source was bone marrow given on day 0 followed on day +6 by CD6-depleted G-CSF-mobilized peripheral blood stem cells (PBSCs) [19]. GvHD prophylaxis consisted of short-course methotrexate (15 mg/m2 on day +1 and 10 mg/m2 on days +3 and +7) and cyclosporine A, which was discontinued on day +100 in the absence of GvHD.
In the TCR/PTCY setting, conditioning consisted of fludarabine and cyclophosphamide plus either TBI or melphalan, or treosulfan, alone or in combination with etoposide [20, 21]. The graft source was unstimulated bone marrow or unmanipulated G-CSF-mobilized PBSCs on day 0; GvHD prophylaxis consisted of high-dose PTCY (50 mg/kg on days +3 and +4), followed by tacrolimus and mycophenolate mofetil (MMF), each starting at day +5 after transplantation [17]. MMF was stopped on day +35, and tacrolimus was discontinued before day +180 after transplantation.
Definitions
Virus infection was defined as isolation of the virus or detection of viral proteins or nucleic acid in any body fluid or tissue specimen. Asymptomatic virus infection was defined as detection of any virus in the absence of related clinical symptoms or organ involvement, or both. Accordingly, symptomatic virus infection was defined by detection of virus and the presence of correlated symptoms. With the exception of CMV, virus disease was defined by virus detection in combination with corresponding symptoms and organ involvement shown by biopsy (culture, histopathologic testing, immune-histochemical analysis, and PCR-based techniques). CMV viremia, antigenemia, and end-organ disease were defined as published [22]. Otherwise, viremia was defined by detection of virus by PCR technique in peripheral blood samples.
Patients and/or donors who presented with a positive serology of CMV prior to transplantation were considered to be at risk for CMV reactivation. CMV seropositive patients who received a graft from a seropositive donor were defined as patients at intermediate risk for CMV reactivation and infection, and CMV seropositive patients with seronegative donors were considered to be at high risk [13, 23].
Death of patients with clinical findings attributable to infection and/or detection of an infectious pathogen at autopsy was defined as infection-related death. Other assessments were made utilizing standard definitions [24–26].
Statistical analysis
Analysis of estimated overall survival (OS), progression-free survival (PFS), infection-related mortality (IRM), and virus infection-related mortality (VIRM) was performed by means of the Kaplan-Meier method [27] using SAS 9.3 (SAS Institute Inc., Cary, NC). IRM was calculated from the day of transplantation until infection-related death, excluding death due to relapse, progressive disease, and/or toxicity. For calculation of VIRM, cases of infection-related death in the absence of virus infection were additionally excluded. Cumulative incidences (CI) of NRM and relapse were estimated by competing risk analysis using R 3.2.0. Associations between OS and patients’ and donors’ pretransplant characteristics and specific treatment modalities are presented as univariate hazard ratios.
Results
Transplantation and engraftment
Between 2006 and 2011, 55 patients underwent HSCT from a HLA-haploidentical donor at the Ludwig-Maximilians-University Hospital of Munich-Grosshadern, Germany. In 2006 and 2007, 28 patients received a haploidentical cTCR/TCD graft, while from 2009 to 2011, 27 patients received a HLA-haploidentical TCR graft using PTCY.
Acute myeloid leukemia (AML) was the most frequent underlying disease in both groups. The TCR/PTCY group contained fewer male patients (n = 14) at a higher median age (43 years) and a higher proportion of patients who had additional factors indicating high risk, such as transplantation in advanced phase including previous allogeneic HSCT. Bone marrow and CD6-depleted G-CSF-mobilized peripheral blood stem cells (PBSCs) were graft sources for all patients in the cTCR/TCD group. In the TCR/PTCY group, bone marrow was the most frequently used graft source (70 %). The characteristics of the two patient groups are shown in Table 1.
In the cTCR/TCD group, no patient died before day +30 and all patients achieved engraftment of neutrophils. In the TCR/PTCY group, three patients died early in aplasia; 24 patients showed neutrophil engraftment. Engraftment of platelets was achieved in 25 (89 %) patients in the cTCR/TCD group and in 22 (82 %) patients of the TCR/PTCY group. No primary graft rejection was observed in both groups.
Acute and chronic GvHD
Incidence of acute GvHD (aGvHD) grades II–IV was comparable in both groups. Skin was affected in all patients. Eleven (39 %) patients in the cTCR/TCD and eight (30 %) in the TCR/PTCY group required steroids as first-line treatment for aGvHD. Incidence of mild chronic GvHD was higher in the TCR/PTCY than in the cTCR/TCD group (Table 1).
Leukocyte/lymphocyte recovery and lymphocyte subsets after transplantation
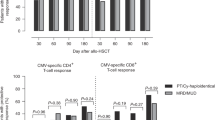
Median absolute numbers of total leukocytes and lymphocytes were generally similar in the two groups; a limited divergence was found only for leukocytes on day 100 ± 8, which were 1.5-fold higher in the TCR/PTCY group than in the cTCR/TCD group. The number of CD8+ T cells was higher in cTCR/TCD-transplanted patients at all three time points, with a nearly 5-fold higher median cell count at day 180 ± 8 for cTCR/TCD patients compared to the TCR/PTCY patients. In contrast, the recovery of CD4+ T cells was more pronounced in the TCR/PTCY group at all three time points; this divergence was most impressive on day 100 ± 1, when TCR/PTCY-transplanted patients had more than 2-fold larger numbers of CD4+ T cells than cTCR/TCD-transplanted patients. In addition, in the TCR/PTCY, NK-cell recovery on day 100 ± 8 was 2.3-fold higher, and B-cell recovery on day 100 ± 8 was 7.1-fold higher and 2.1-fold higher on day 180 ± 8 (Fig. 1).
Occurrence of viral pathogens and virus infection
Cumulatively, 139 occurrences of virus infection were observed (Table 2), 71 of them asymptomatic, 68 symptomatic, and 20 associated with disease (Table 3). Virus infections affected 46 of 55 patients, whereas no virus infection was detected in nine patients only. In recipients of a cTCR/TCD graft, viral pathogens were detected more frequently than in recipients of a TCR graft using PTCY. Cumulatively, 87 occurrences of viral pathogens were identified in 27 patients (96 %) of the cTCR/TCD group. Within these occurrences, 51 patients were symptomatic or developed disease (n = 17). In the TCR/PTCY group, 52 occurrences were seen in 19 patients (70 %), 17 symptomatic or suffering from disease (n = 3) (Table 3).
The most frequently observed viral pathogens across both groups were HHV-6, polyomavirus JC/BK, EBV, CMV, HSV, and ADV (Table 2). In particular, symptomatic virus infection and disease were induced by the Herpesviridae HSV, VZV, CMV, EBV, and HHV-6 in 26 patients (93 %) of the cTCR/TCD group, but only in six patients (22 %) of the TCR/PTCY group. Besides the occurrence of HHV-6, for each of these Herpesviridae, the incidence of virus infection-related symptoms and disease was distinctly higher in the cTCD/TCR than in the TCR/PTCY group (Table 3).
Detection of individual viruses, associated symptoms, and disease
Detection of HSV (oral lavage, BAL) was presumably associated with prolonged stomatitis in four patients and pneumonitis in one patient of the cTCR/TCD and with prolonged stomatitis in two patients of the TCR/PTCY group.
No VZV infection was seen in both groups under acyclovir prophylaxis. After withdrawal of prophylaxis, herpes zoster was documented in two patients of the cTCR/TCD group.
The D−R+ constellation, predisposing to high risk of reactivation, was similarly frequent in both groups (n = 5 in each group) (Table 1). CMV DNA-emia was detected in the peripheral blood samples of 12 out of 21 patients at risk (57 %) in the cTCR/TCD and in 4 out of 13 patients at risk (31 %) in the TCR/PTCY group. Infection-associated symptoms were observed in four patients and end-organ disease in two patients (pneumonia, gastroenteritis) of the cTCR/TCD group (Table 3). In the TCR/PTCY group, two patients showed symptoms of gastroenteritis, but no one developed disease. Among the patients with high-risk D−R+ constellation, CMV reactivation was more frequent in the cTCR/TCD (5 of 5 patients) than in the TCR/PTCY group (1 of 5 patients). Preemptive treatment of CMV infection with antiviral drugs was superior and successful in the TCR/PTCY group (100 vs 50 % cTCR/TCD).
EBV infection associated with symptoms was observed in 12/28 patients in the cTCR/TCD (43 %) and in 2/27 patients of the TCR/PTCY group (7 %) (Table 3). Reactivation with a rapidly increasing virus load (PCR) in two consecutive peripheral blood samples, in combination with severe symptoms, prompted the histologically confirmed diagnosis of posttransplant lymphoproliferative disorder (PTLD) in 7/28 patients (25 %) in the cTCR/TCD group. No patient in the TCR/PTCY group was diagnosed with PTLD. Adoptive transfer of EBV-specific T cells was performed in five of our PTLD patients inducing a complete remission in patients who had early-stage PTLD [28].
HHV-6 infection was just as frequently detected in the cTCR/TCD (82 %) as in the TCR/PTCY group (78 %), while it was asymptomatic in more patients of the TCR/PTCY (18/21; 81 %) than of the cTCR/TCD group (12/23; 52 %) (Table 3). HHV-6-associated disease (gastroenteritis, cytopenia, pneumonitis) was assumed in three patients (11 %) of the cTCR/TCD and one patient (4 %; pneumonitis) of the TCR/PTCY group. Eleven patients in the cTCR/TCD and eight in the TCR/PTCY group showed concomitant CMV DNA-emia. All patients with HHV-6 infection had several other concomitant pathogens and suffered from grade II–IV aGvHD.
ADV-associated localized (n = 3) and disseminated disease (n = 1) was diagnosed in four patients in the cTCR/TCD group (Table 3). No patient in the TCR/PTCY group developed disseminated disease, and two patients in this group suffered from symptoms (e.g., hemorrhagic cystitis, conjunctivitis) in the absence of viremia (localized disease). In both groups, all symptomatic patients had grade III–IV aGvHD with gut and liver involvement and required steroids and other immunosuppressive agents.
A majority of patients with detection of BK virus in urine had no clinical symptoms (n = 11 in the cTCR/TCD group; n = 13 in the TCR/PTCY group; Table 3). Five patients of the cTCR/TCD (18 %) and four of the TCR/PTCY group (15 %) suffered from hemorrhagic cystitis (HC). Out of these, four patients in the cTCR/TCD group and two patients in the TCR/PTCY group required bladder irrigation and pain medication.
Outcome, virus infection-related mortality, and predictors of survival
After a median follow-up of 6.8 years (range 6.1–7.4) for the cTCR/TCD and 2.8 years (range 2.4–3.8) for the TCR/PTCY group, 1-year OS was 39 % (95 % confidence interval (95 % CI) 22–57) for the cTCR/TCD and 59 % (95 % CI 38–75) for the TCR/PTCY group (p = 0.28), respectively (Fig. 2). PFS after 1 year was 38 % (95 % CI 21–57) for the cTCR/TCD and 55 % (95 % CI 35–72) for the TCR/PTCY group (p = 0.47). CI of relapse after 1 year was 21 % (95 % CI 8–38) for the cTCR/TCD and 22 % (95 % CI 9–40) for the TCD/PTCY group (p = 0.56), and all relapsed patients died. Nineteen patients died without relapse (12 in the cTCR/TCD group; seven in the TCR/PTCY group). Causes of NRM were shown in Table 4. Taken together, the deaths of 11 patients in the cTCR/TCD and of six patients in the TCR/PTCY group were related to infections, and the deaths of eight patients in the cTCR/TCD and one patient in the TCR/PTCY group were related to virus infections. Virus infection causing PTLD was the cause of death in five patients of the cTCR/TCD group. The main cause of infection-related death in patients of the TCR/PTCY group was invasive fungal infection (n = 4). After TCR/PTCY transplantation, the CI of 1-year NRM was 22 % (95 % CI 9–39), while it was 39 % (95 % CI 21–57) after cTCR/TCD transplantation (p = 0.27).
IRM was not significantly different between the two groups after 1 year (TCR/PTCY 15 % and cTCR/TCD 34 %; p = 0.20). In contrast, VIRM was significantly lower in the TCR/PTCY group being absent (0 %) at 1 year after haplo-HSCT, whereas it was 29 % (95 % CI 15–52) in the cTCR/TCD group (p = 0.009).
Risk factor analysis revealed a median absolute count of lymphocytes ≤300/μl (hazard ratio (HR) 0.161; p = 0.002), CD3+ cells ≤200/μl (HR 0.363; p = 0.04), and CD4+ cells ≤150/μl (HR 0.169; p = 0.05), all on day +100, and the application of steroids >1 mg/kg (HR 0.506; p = 0.05) as predictive of a negative outcome (OS) after haplo-HSCT (Table 5).
Discussion
This study reports the incidence of virus infection and related mortality in the context of immune reconstitution in two different HLA-haploidentical transplantation settings. We found that the incidence of infections caused by viruses of the herpesvirus family was markedly lower in the TCR group using PTCY than in the cTCR/TCD group using ATG prior to transplantation. The incidence of herpesvirus infection was 93 % in the cTCR/TCD, but only 22 % in the TCR/PTCY cohort. For other viruses, no clear differences between both groups could be observed. Improved control of herpesvirus in the TCR/PTCY cohort showed association with a fast recovery of total CD4+ T cells, a better response to antiviral treatment, and a decreased risk of death from viral infections.
The high overall incidence of detectable viral infections in our patient cohort (84 %) is consistent with reported data of transplantation from alternative donors, such as umbilical cord blood (UCB) or HLA-mismatched unrelated donor-derived transplantation [5, 9, 29–32]. However, when we separately consider our TCR/PTCY-transplanted cohort, we found an incidence of virus infection that is close to the reported incidence in HLA-matched transplantation [33–36].
The two regimens studied here represent different approaches to overcome the HLA barrier in haplo-HSCT. Our cTCR/TCD protocol involved ATG, which has been commonly used to deplete T cells in vivo, prevent rejection and GvHD [37, 38], and is known to cause prolonged lymphopenia through widespread depletion of lymphocytes, thus elevating the risk for infection [31]. In particular, ATG has a strong impact on the CD4+ T-cell compartment, leading to low CD4+ T-cell counts and low CD4+/CD8+ T-cell ratios [39]. Accordingly, CD4+ T-cell counts were decreased and recovery was slower in our cTCR/TCD group, while overall recovery of lymphocytes was similar in our two groups. It is of note that CD4+ T-cell reconstitution of our cTCR/TCD group is comparable to otherwise reported results of TCR haplo-HSCT involving ATG [33]; in both cases, CD4+ T-cell counts remained below 200/μl for 6 months after transplantation. Interestingly, a similarly impaired recovery of CD4+ T cells was only described in haplo-HSCT using CD34+ selected grafts [5]. In contrast, in our TCR/PTCY group, we observed CD4+ T-cell counts above 200/μl from 3 months onward, which is in a similar range as the CD4+ T-cell recovery previously observed after HLA-matched related transplantation [33, 40]. We assume that the possibility to omit ATG seems to be a major advantage of the TCR/PTCY haplo-HSCT approach by allowing a fast and improved immune recovery.
There is only one single-center study retrospectively comparing outcome and immune reconstitution in two different haplo-HSCT approaches. Improved immune reconstitution of T-cell subsets resulting in less infectious complications was reported by Ciurea and colleagues in 32 patients for the TCR approach using PTCY in comparison to a historical control of 33 patients who received TCD haplo-HSCT (CD34+ selected cells) and ATG [16]. CD4+ T cells were significantly lower in their TCD compared to the TCR/PTCY group resulting in a 1.5-fold increased risk to develop viral infections. Although our cTCR/TCD group is not completely equivalent to the TCD group reported in that study, our results from the TCR/PTCY cohort extend these earlier results by showing that this transplant procedure results in improved control of herpesvirus infection and decreased mortality from virus infections.
The frequency of CMV reactivation was higher in the cTCR/TCD (57 %) than in the TCR/PTCY group (31 %). In addition, preemptive treatment of CMV reactivation was successful in all patients of the TCR/PTCY group, whereas only 50 % in the cTCR/TCD group became negative for CMV DNA. The rate of CMV reactivation in the patients of the TCR/PTCY group was comparable to the incidence of 38 % CMV reactivation reported by Luznik and colleagues in the setting of T-cell-replete nonmyeloablative haplo-HSCT using PTCY [41] and to the incidence after HLA-matched transplantation [33, 36]. However, Raiola and colleagues observed a comparatively higher frequency of CMV reactivation (50 %) in their patients undergoing the TCR/PTCY haplo-approach; CMV was detected in 5/6 of their patients considered to be at high risk for reactivation [42], whereas in our TCR/PTCY group, only 1/5 of those patients reactivated. Median counts of CD4+ T cells at day +100 (140/μl) in their cohort were lower than in our TCR/PTCY group (200/μl). In contrast to our TCR/PTCY protocol, the Genoa group started postgrafting immunosuppression before PTCY was administered. While the impact of this alteration in immunosuppression regarding the induction of tolerance is so far unclear, one can speculate that this might lead to a mitigation of the preserving effect of PTCY on quiescent memory T cells responsible for antiviral immunity, as explained below. Compared to the Genoa TCR/PTCY group, a similar rate of CMV infection (57 %) and reactivation in patients at high risk (5/5) is observed in our cTCR/TCD group. In addition, these higher frequencies of CMV reactivation are reminiscent of the findings by other authors who reported on the outcome and immune reconstitution after a TCR haplo-HSCT approach using two grafts (G-CSF stimulated BM and PBSCs) and ATG for in vivo T-cell depletion, but no PTCY [33, 35].
Our results suggest that a better control of herpesvirus infection and successful antiviral treatment is promoted by the TCR/PTCY haplo-HSCT approach when no immunosuppression between allografting and the application of PTCY is administered and no ATG is used. This approach can induce tolerance to an allogeneic graft by aiming for depletion of proliferating mature T cells by inducing their apoptosis while sparing quiescent cells [16, 17, 41, 43]. Further, resistance to PTCY seems to be mediated by the presence of the enzyme ALDH (aldehyde-dehydrogenase), thus leading to a selective killing of naive T cells lacking ALDH, while memory T cells providing antiviral immunity can be spared [44, 45]. This is also clinically supported by an observation by Munchel and colleagues, who detected a donor-derived immunity against CMV returning by day +60 in 70 % of their patients after TCR/PTCY haplo-HSCT with an improved recovery of memory T cells resulting in decreased infection-related mortality [46].
Along that line and in consistence with other observations [47, 42, 48], we did not observe EBV-associated PTLD in our TCR/PTCY group. In contrast, PTLD occurred in 25 % of our patients of the cTCR/TCD group. A similar high incidence of PTLD has so far only been reported after UCB transplantation using ATG [49], while it is not commonly reported after TCD haplo-HSCT using serotherapy [5, 6]. Highly effective and sustained in vitro and in vivo T-cell depletion using a very high dosage of ATG might explain this finding in our cohort.
There is some evidence that improved immune recovery is associated with better transplant outcome [50, 51]. Accordingly, early recovery of absolute lymphocyte counts has been reported to serve as a simple surrogate marker for better outcome after haplo-HSCT [52, 53], and a CD4+ T-cell count >200/μl 3 months after transplantation predicted better OS and lower NRM [51]. We identified a count of lymphocytes >300/μl and of CD4+ T cells >150/μl, both on day +100, as a predictor of better OS. As reported by others [54], the application of steroids significantly influenced the mortality related to virus infection in our cohort.
The main limitations of our retrospective study were low patient numbers and two different observational periods for the two groups, which might imply less statistical power and some older results of the cTCR/TCD group. However, by allowing a comparison between a previous, difficult and a newer, easier to perform strategy, this might serve as an example for the progress which we were able to deliver in haplo-HSCT in the past. Although risk distribution was not well balanced in our two groups (44 % second transplants in the TCR/PTCY vs 0 % in the cTCR/TCD group), we could not reveal significant differences in outcome regarding PFS and relapse incidence but observed a trend to a higher NRM in the cTCR/TCD group. While VIRM mortality was significantly lower after the TCR/PTCY approach, IRM was not different. This may be due to the following reasons: First, as reported by our group previously for the TCR/PTCY haplo-HSCT approach [20, 21], invasive pulmonary aspergillosis caused severe morbidity and mortality in our patients undergoing a second TCR/PTCY haplo-HSCT, while virus infection played a minor role, and immune reconstitution proved to be favorable using PTCY. Second, probably a high dosage of ATG promoted the development of PTLD in the cTCR/TCD group, but consequent preemptive management of EBV reactivation and adoptive immunotherapy with EBV-specific T cells were available for patients of the cTCR/TCD group.
Taken together, we suggest that TCR/PTCY haplo-HSCT is associated with a fast, improved immune recovery of CD4+ T cells and a lower incidence of herpesvirus infection, resulting in significantly lower virus infection-related mortality when compared to a combined TCR/TCD HLA-haploidentical transplantation approach using ATG. Presumably, by using PTCY, antiviral immunity responsible for the control of latent viruses, such as herpesviruses, might be preserved. The incidence of virus infection and immune recovery in the TCR/PTCY group was similar to that after a HLA-matched transplantation. Thus, the TCR/PTCY approach is well suited to handle the important problem of impaired immune recovery and herpesvirus infection after haplo-HSCT.
References
Anasetti C, Beatty PG, Storb R, Martin PJ, Mori M, Sanders JE, Thomas ED, Hansen JA (1990) Effect of HLA incompatibility on graft-versus-host disease, relapse, and survival after marrow transplantation for patients with leukemia or lymphoma. Hum Immunol 29(2):79–91
Powles RL, Morgenstern GR, Kay HE, McElwain TJ, Clink HM, Dady PJ, Barrett A, Jameson B, Depledge MH, Watson JG, Sloane J, Leigh M, Lumley H, Hedley D, Lawler SD, Filshie J, Robinson B (1983) Mismatched family donors for bone-marrow transplantation as treatment for acute leukaemia. Lancet 1(8325):612–615
Ash RC, Horowitz MM, Gale RP, van Bekkum DW, Casper JT, Gordon-Smith EC, Henslee PJ, Kolb HJ, Lowenberg B, Masaoka T et al (1991) Bone marrow transplantation from related donors other than HLA-identical siblings: effect of T cell depletion. Bone Marrow Transplant 7(6):443–452
Aversa F, Tabilio A, Velardi A, Cunningham I, Terenzi A, Falzetti F, Ruggeri L, Barbabietola G, Aristei C, Latini P, Reisner Y, Martelli MF (1998) Treatment of high-risk acute leukemia with T-cell-depleted stem cells from related donors with one fully mismatched HLA haplotype. N Engl J Med 339(17):1186–1193. doi:10.1056/NEJM199810223391702
Aversa F, Terenzi A, Tabilio A, Falzetti F, Carotti A, Ballanti S, Felicini R, Falcinelli F, Velardi A, Ruggeri L, Aloisi T, Saab JP, Santucci A, Perruccio K, Martelli MP, Mecucci C, Reisner Y, Martelli MF (2005) Full haplotype-mismatched hematopoietic stem-cell transplantation: a phase II study in patients with acute leukemia at high risk of relapse. J Clin Oncol 23(15):3447–3454. doi:10.1200/JCO.2005.09.117
Ciceri F, Labopin M, Aversa F, Rowe JM, Bunjes D, Lewalle P, Nagler A, Di Bartolomeo P, Lacerda JF, Lupo Stanghellini MT, Polge E, Frassoni F, Martelli MF, Rocha V (2008) A survey of fully haploidentical hematopoietic stem cell transplantation in adults with high-risk acute leukemia: a risk factor analysis of outcomes for patients in remission at transplantation. Blood 112(9):3574–3581. doi:10.1182/blood-2008-02-140095
Lacerda JF, Martins C, Carmo JA, Lourenco F, Juncal C, Rodrigues A, Vilalobos I, Moura MC, Ligeiro D, Martinho A, Lacerda JM (2003) Haploidentical stem cell transplantation with purified CD34 cells after a chemotherapy-alone conditioning regimen. Biol Blood Marrow Transplant 9(10):633–642. doi:10.1016/S1083
Ogawa H, Ikegame K, Yoshihara S, Kawakami M, Fujioka T, Masuda T, Taniguchi Y, Hasei H, Kaida K, Inoue T, Kim EH, Kawase I (2006) Unmanipulated HLA 2-3 antigen-mismatched (haploidentical) stem cell transplantation using nonmyeloablative conditioning. Biol Blood Marrow Transplant 12(10):1073–1084. doi:10.1016/j.bbmt.2006.06.007
Rizzieri DA, Koh LP, Long GD, Gasparetto C, Sullivan KM, Horwitz M, Chute J, Smith C, Gong JZ, Lagoo A, Niedzwiecki D, Dowell JM, Waters-Pick B, Liu C, Marshall D, Vredenburgh JJ, Gockerman J, Decastro C, Moore J, Chao NJ (2007) Partially matched, nonmyeloablative allogeneic transplantation: clinical outcomes and immune reconstitution. J Clin Oncol 25(6):690–697. doi:10.1200/JCO.2006.07.0953
Sykes M, Preffer F, McAfee S, Saidman SL, Weymouth D, Andrews DM, Colby C, Sackstein R, Sachs DH, Spitzer TR (1999) Mixed lymphohaemopoietic chimerism and graft-versus-lymphoma effects after non-myeloablative therapy and HLA-mismatched bone-marrow transplantation. Lancet 353(9166):1755–1759. doi:10.1016/S0140-6736(98)11135-2
Handgretinger R, Klingebiel T, Lang P, Schumm M, Neu S, Geiselhart A, Bader P, Schlegel PG, Greil J, Stachel D, Herzog RJ, Niethammer D (2001) Megadose transplantation of purified peripheral blood CD34(+) progenitor cells from HLA-mismatched parental donors in children. Bone Marrow Transplant 27(8):777–783. doi:10.1038/sj.bmt.1702996
Bethge WA, Haegele M, Faul C, Lang P, Schumm M, Bornhauser M, Handgretinger R, Kanz L (2006) Haploidentical allogeneic hematopoietic cell transplantation in adults with reduced-intensity conditioning and CD3/CD19 depletion: fast engraftment and low toxicity. Exp Hematol 34(12):1746–1752. doi:10.1016/j.exphem.2006.08.009
Boeckh M, Erard V, Zerr D, Englund J (2005) Emerging viral infections after hematopoietic cell transplantation. Pediatr Transplant 9(Suppl 7):48–54. doi:10.1111/j.1399-3046.2005.00442.x
Dykewicz CA (2001) Summary of the guidelines for preventing opportunistic infections among hematopoietic stem cell transplant recipients. Clin Infect Dis 33(2):139–144. doi:10.1086/321805
Einsele H, Bertz H, Beyer J, Kiehl MG, Runde V, Kolb HJ, Holler E, Beck R, Schwerdfeger R, Schumacher U, Hebart H, Martin H, Kienast J, Ullmann AJ, Maschmeyer G, Kruger W, Niederwieser D, Link H, Schmidt CA, Oettle H, Klingebiel T (2003) Infectious complications after allogeneic stem cell transplantation: epidemiology and interventional therapy strategies—guidelines of the Infectious Diseases Working Party (AGIHO) of the German Society of Hematology and Oncology (DGHO). Ann Hematol 82(Suppl 2):S175–185. doi:10.1007/s00277-003-0772-4
Ciurea SO, Mulanovich V, Saliba RM, Bayraktar UD, Jiang Y, Bassett R, Wang SA, Konopleva M, Fernandez-Vina M, Montes N, Bosque D, Chen J, Rondon G, Alatrash G, Alousi A, Bashir Q, Korbling M, Qazilbash M, Parmar S, Shpall E, Nieto Y, Hosing C, Kebriaei P, Khouri I, Popat U, de Lima M, Champlin RE (2012) Improved early outcomes using a T cell replete graft compared with T cell depleted haploidentical hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 18(12):1835–1844. doi:10.1016/j.bbmt.2012.07.003
Luznik L, O’Donnell PV, Symons HJ, Chen AR, Leffell MS, Zahurak M, Gooley TA, Piantadosi S, Kaup M, Ambinder RF, Huff CA, Matsui W, Bolanos-Meade J, Borrello I, Powell JD, Harrington E, Warnock S, Flowers M, Brodsky RA, Sandmaier BM, Storb RF, Jones RJ, Fuchs EJ (2008) HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant 14(6):641–650. doi:10.1016/j.bbmt.2008.03.005
Schuster FR, Meisel R, Fuhrer M, Reuther S, Hauer J, Tischer J, Feuchtinger T, Laws HJ, Kolb HJ, Borkhardt A (2013) Anti-leukaemic activity of a novel haploidentical-transplantation approach employing unmanipulated bone marrow followed by CD6-depleted peripheral blood stem cells in children with refractory/relapsed acute leukaemia. Br J Haematol 162(6):802–807. doi:10.1111/bjh.12455
Kolb H-J, Bigalke I, Simoes B, Falk C, Tischer J, Hill W, Ledderose G (2004) CD6-depleted mobilized stem cells for modification of HVG and GVH reactions after HLA-haploidentical marrow transplantation. ASH Annu Meet Abstr 104(11):978
Tischer J, Engel N, Fritsch S, Prevalsek D, Hubmann M, Schulz C, Zoellner AK, Bucklein V, Lippl S, Reibke R, Rieger CT, Ledderose G, Stemmler HJ, Hiddemann W, Schmid C, Hausmann A (2014) Second haematopoietic SCT using HLA-haploidentical donors in patients with relapse of acute leukaemia after a first allogeneic transplantation. Bone Marrow Transplant 49(7):895–901. doi:10.1038/bmt.2014.83
Tischer J, Stemmler HJ, Engel N, Hubmann M, Fritsch S, Prevalsek D, Schulz C, Zoellner AK, Bucklein V, Hill W, Ledderose G, Hausmann A (2013) Feasibility of clofarabine cytoreduction followed by haploidentical hematopoietic stem cell transplantation in patients with relapsed or refractory advanced acute leukemia. Ann Hematol 92(10):1379–1388. doi:10.1007/s00277-013-1862-6
Ljungman P, Griffiths P, Paya C (2002) Definitions of cytomegalovirus infection and disease in transplant recipients. Clin Infect Dis 34(8):1094–1097. doi:10.1086/339329
Ganepola S, Gentilini C, Hilbers U, Lange T, Rieger K, Hofmann J, Maier M, Liebert UG, Niederwieser D, Engelmann E, Heilbronn R, Thiel E, Uharek L (2007) Patients at high risk for CMV infection and disease show delayed CD8+ T-cell immune recovery after allogeneic stem cell transplantation. Bone Marrow Transplant 39(5):293–299. doi:10.1038/sj.bmt.1705585
Bacigalupo A, Ballen K, Rizzo D, Giralt S, Lazarus H, Ho V, Apperley J, Slavin S, Pasquini M, Sandmaier BM, Barrett J, Blaise D, Lowski R, Horowitz M (2009) Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant 15(12):1628–1633. doi:10.1016/j.bbmt.2009.07.004
Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, Martin P, Chien J, Przepiorka D, Couriel D, Cowen EW, Dinndorf P, Farrell A, Hartzman R, Henslee-Downey J, Jacobsohn D, McDonald G, Mittleman B, Rizzo JD, Robinson M, Schubert M, Schultz K, Shulman H, Turner M, Vogelsang G, Flowers ME (2005) National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant 11(12):945–956. doi:10.1016/j.bbmt.2005.09.004
Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, Thomas ED (1995) 1994 Consensus conference on acute GVHD grading. Bone Marrow Transplant 15(6):825–828
Kaplan E, Meier P (1958) Nonparametric estimation from incomplete observations. J Am Stat Assoc 53:457–481
Moosmann A, Bigalke I, Tischer J, Schirrmann L, Kasten J, Tippmer S, Leeping M, Prevalsek D, Jaeger G, Ledderose G, Mautner J, Hammerschmidt W, Schendel DJ, Kolb HJ (2010) Effective and long-term control of EBV PTLD after transfer of peptide-selected T cells. Blood 115(14):2960–2970. doi:10.1182/blood-2009-08-236356
Mulanovich VE, Jiang Y, de Lima M, Shpall EJ, Champlin RE, Ciurea SO (2011) Infectious complications in cord blood and T-cell depleted haploidentical stem cell transplantation. Am J Blood Res 1(1):98–105
Parody R, Martino R, Rovira M, Vazquez L, Vazquez MJ, de la Camara R, Blazquez C, Fernandez-Aviles F, Carreras E, Salavert M, Jarque I, Martin C, Martinez F, Lopez J, Torres A, Sierra J, Sanz GF (2006) Severe infections after unrelated donor allogeneic hematopoietic stem cell transplantation in adults: comparison of cord blood transplantation with peripheral blood and bone marrow transplantation. Biol Blood Marrow Transplant 12(7):734–748. doi:10.1016/j.bbmt.2006.03.007
Ruggeri A, Peffault de Latour R, Carmagnat M, Clave E, Douay C, Larghero J, Cayuela JM, Traineau R, Robin M, Madureira A, Ribaud P, Ferry C, Devergie A, Purtill D, Rabian C, Gluckman E, Toubert A, Socie G, Rocha V (2011) Outcomes, infections, and immune reconstitution after double cord blood transplantation in patients with high-risk hematological diseases. Transpl Infect Dis 13(5):456–465. doi:10.1111/j.1399-3062.2011.00632.x
Verdeguer A, de Heredia CD, Gonzalez M, Martinez AM, Fernandez-Navarro JM, Perez-Hurtado JM, Badell I, Gomez P, Gonzalez ME, Munoz A, Diaz MA (2011) Observational prospective study of viral infections in children undergoing allogeneic hematopoietic cell transplantation: a 3-year GETMON experience. Bone Marrow Transplant 46(1):119–124. doi:10.1038/bmt.2010.52
Chang YJ, Zhao XY, Huo MR, Xu LP, Liu DH, Liu KY, Huang XJ (2012) Immune reconstitution following unmanipulated HLA-mismatched/haploidentical transplantation compared with HLA-identical sibling transplantation. J Clin Immunol 32(2):268–280. doi:10.1007/s10875-011-9630-7
Dominietto A, Raiola AM, Bruno B, van Lint MT, Frassoni F, Grazia CD, Gualandi F, Bregante S, Varaldo R, Ghiso A, Bacigalupo A (2011) Rapid immune reconstitution following unmanipulated haploidentical BMT with post-transplant high dose cyclophosphamide. ASH Annu Meet Abstr 118((21):3050
Lu DP, Dong L, Wu T, Huang XJ, Zhang MJ, Han W, Chen H, Liu DH, Gao ZY, Chen YH, Xu LP, Zhang YC, Ren HY, Li D, Liu KY (2006) Conditioning including antithymocyte globulin followed by unmanipulated HLA-mismatched/haploidentical blood and marrow transplantation can achieve comparable outcomes with HLA-identical sibling transplantation. Blood 107(8):3065–3073. doi:10.1182/blood-2005-05-2146
Rieger CT, Rieger H, Kolb HJ, Peterson L, Huppmann S, Fiegl M, Ostermann H (2009) Infectious complications after allogeneic stem cell transplantation: incidence in matched-related and matched-unrelated transplant settings. Transpl Infect Dis 11(3):220–226. doi:10.1111/j.1399-3062.2009.00379.x
Finke J, Bethge WA, Schmoor C, Ottinger HD, Stelljes M, Zander AR, Volin L, Ruutu T, Heim DA, Schwerdtfeger R, Kolbe K, Mayer J, Maertens JA, Linkesch W, Holler E, Koza V, Bornhauser M, Einsele H, Kolb HJ, Bertz H, Egger M, Grishina O, Socie G, Group AT-FT (2009) Standard graft-versus-host disease prophylaxis with or without anti-T-cell globulin in haematopoietic cell transplantation from matched unrelated donors: a randomised, open-label, multicentre phase 3 trial. Lancet Oncol 10(9):855–864
Pidala J, Tomblyn M, Nishihori T, Ayala E, Field T, Fernandez H, Perez L, Locke F, Alsina M, Ochoa JL, Perkins J, Tate C, Shapiro J, Conwell M, Bookout R, Anasetti C (2011) ATG prevents severe acute graft-versus-host disease in mismatched unrelated donor hematopoietic cell transplantation. Biol Blood Marrow Transplant 17(8):1237–1244. doi:10.1016/j.bbmt.2010.12.705
Muller TF, Grebe SO, Neumann MC, Heymanns J, Radsak K, Sprenger H, Lange H (1997) Persistent long-term changes in lymphocyte subsets induced by polyclonal antibodies. Transplantation 64(10):1432–1437
Shenoy S, Mohanakumar T, Todd G, Westhoff W, Dunnigan K, Adkins DR, Brown RA, DiPersio JF (1999) Immune reconstitution following allogeneic peripheral blood stem cell transplants. Bone Marrow Transplant 23(4):335–346. doi:10.1038/sj.bmt.1701581
Luznik L, Jalla S, Engstrom LW, Iannone R, Fuchs EJ (2001) Durable engraftment of major histocompatibility complex-incompatible cells after nonmyeloablative conditioning with fludarabine, low-dose total body irradiation, and posttransplantation cyclophosphamide. Blood 98(12):3456–3464
Raiola AM, Dominietto A, Ghiso A, Di Grazia C, Lamparelli T, Gualandi F, Bregante S, Van Lint MT, Geroldi S, Luchetti S, Ballerini F, Miglino M, Varaldo R, Bacigalupo A (2013) Unmanipulated haploidentical bone marrow transplantation and posttransplantation cyclophosphamide for hematologic malignancies after myeloablative conditioning. Biol Blood Marrow Transplant 19(1):117–122. doi:10.1016/j.bbmt.2012.08.014
Mayumi H, Himeno K, Tokuda N, Nomoto K (1986) Drug-induced tolerance to allografts in mice. VII. Optimal protocol and mechanism of cyclophosphamide-induced tolerance in an H-2 haplotype-identical strain combination. Transplant Proc 18(2):363–369
Kanakry CG, Ganguly S, Zahurak M, Bolanos-Meade J, Thoburn C, Perkins B, Fuchs EJ, Jones RJ, Hess AD, Luznik L (2013) Aldehyde dehydrogenase expression drives human regulatory T cell resistance to posttransplantation cyclophosphamide. Sci Transl Med 5(211):211ra–157. doi:10.1126/scitranslmed.3006960
Kastan MB, Schlaffer E, Russo JE, Colvin OM, Civin CI, Hilton J (1990) Direct demonstration of elevated aldehyde dehydrogenase in human hematopoietic progenitor cells. Blood 75(10):1947–1950
Munchel A, Kesserwan C, Symons HJ, Luznik L, Kasamon YL, Jones RJ, Fuchs EJ (2011) Nonmyeloablative, HLA-haploidentical bone marrow transplantation with high dose, post-transplantation cyclophosphamide. Pediatr Rep 3(Suppl 2):e15. doi:10.4081/pr.2011.s2.e15
Kanakry JA, Kasamon YL, Gocke CD, Tsai HL, Davis-Sproul J, Ghosh N, Symons H, Bolanos-Meade J, Gladstone DE, Swinnen LJ, Luznik L, Fuchs EJ, Jones RJ, Ambinder RF (2013) Outcomes of related donor HLA-identical or HLA-haploidentical allogeneic blood or marrow transplantation for peripheral T cell lymphoma. Biol Blood Marrow Transplant 19(4):602–606. doi:10.1016/j.bbmt.2013.01.006
Solomon SR, Sizemore CA, Sanacore M, Zhang X, Brown S, Holland HK, Morris LE, Bashey A (2012) Haploidentical transplantation using T cell replete peripheral blood stem cells and myeloablative conditioning in patients with high-risk hematologic malignancies who lack conventional donors is well tolerated and produces excellent relapse-free survival: results of a prospective phase II trial. Biol Blood Marrow Transplant 18(12):1859–1866. doi:10.1016/j.bbmt.2012.06.019
Brunstein CG, Weisdorf DJ, DeFor T, Barker JN, Tolar J, van Burik JA, Wagner JE (2006) Marked increased risk of Epstein-Barr virus-related complications with the addition of antithymocyte globulin to a nonmyeloablative conditioning prior to unrelated umbilical cord blood transplantation. Blood 108(8):2874–2880. doi:10.1182/blood-2006-03-011791
Berger M, Figari O, Bruno B, Raiola A, Dominietto A, Fiorone M, Podesta M, Tedone E, Pozzi S, Fagioli F, Madon E, Bacigalupo A (2008) Lymphocyte subsets recovery following allogeneic bone marrow transplantation (BMT): CD4+ cell count and transplant-related mortality. Bone Marrow Transplant 41(1):55–62. doi:10.1038/sj.bmt.1705870
Kim DH, Sohn SK, Won DI, Lee NY, Suh JS, Lee KB (2006) Rapid helper T-cell recovery above 200 × 106/l at 3 months correlates to successful transplant outcomes after allogeneic stem cell transplantation. Bone Marrow Transplant 37(12):1119–1128. doi:10.1038/sj.bmt.1705381
Chang YJ, Zhao XY, Xu LP, Liu DH, Liu KY, Chen YH, Wang Y, Zhang XH, Zhao XS, Han W, Chen H, Wang FR, Lv M, Huang XJ (2013) Early lymphocyte recovery predicts superior overall survival after unmanipulated haploidentical blood and marrow transplant for myelodysplastic syndrome and acute myeloid leukemia evolving from myelodysplastic syndrome. Leuk Lymphoma 54(12):2671–2677. doi:10.3109/10428194.2013.783912
Ciurea SO, Mulanovich V, Jiang Y, Bassett R, Rondon G, McMannis J, de Lima M, Shpall EJ, Champlin RE (2011) Lymphocyte recovery predicts outcomes in cord blood and T cell-depleted haploidentical stem cell transplantation. Biol Blood Marrow Transplant 17(8):1169–1175. doi:10.1016/j.bbmt.2010.11.020
Wolfromm A, Porcher R, Legoff J, Peffault de Latour R, Xhaard A, de Fontbrune FS, Ribaud P, Bergeron A, Socie G, Robin M (2014) Viral respiratory infections diagnosed by multiplex PCR after allogeneic hematopoietic stem cell transplantation: long-term incidence and outcome. Biol Blood Marrow Transplant 20(8):1238–1241. doi:10.1016/j.bbmt.2014.04.004
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tischer, J., Engel, N., Fritsch, S. et al. Virus infection in HLA-haploidentical hematopoietic stem cell transplantation: incidence in the context of immune recovery in two different transplantation settings. Ann Hematol 94, 1677–1688 (2015). https://doi.org/10.1007/s00277-015-2423-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-015-2423-y