Abstract
Cytomegalovirus (CMV) DNAemia and CMV disease have been reported as more frequent in patients undergoing haploidentical allogeneic hematopoietic stem cell transplantation (Haplo-HSCT) than in those receiving HLA-matched allografts. This could be due to impaired CMV-specific T-cell reconstitution. Here, we conducted a multicenter observational study to assess CMV pp65 and IE-1-specific T cells kinetics in patients undergoing unmanipulated Haplo-HSCT with posttransplant cyclophosphamide (PT/Cy-haplo) and compared it with patients allografted with HLA-matched donors. Plasma CMV DNA load was monitored by real-time PCR and enumeration of CMV-specific IFN-γ-producing CD8+ and CD4+ T cells was performed by flow cytometry for intracellular cytokine staining at days +30, +60, +90, and +180 after transplantation. CMV DNAemia developed in 62 patients, occurring with comparable frequency in PT/Cy-haplo and MRD/MUD recipients (P = 0.14). There were no significant differences across groups in the number of patients either displaying detectable CMV-specific CD8+ and CD4+ T-cell responses or acquiring CMV-specific T-cell levels conferring protection against subsequent infection. CMV-specific T-cell counts were comparable between groups at most time points examined, irrespective of whether CMV DNAemia occurred or not prior to monitoring. Collectively the data suggest that PT/Cy-haplo recipients may reconstitute CMV-specific T-cell immunity to the same extent as patients undergoing HLA-matched allo-HSCT.
Similar content being viewed by others
Introduction
Haploidentical hematopoietic stem cell transplantation (Haplo-HSCT) is widely used to treat hematological malignancies, particularly in the absence of an HLA-matched donor, due to its availability for nearly all patients, safety, and favorable clinical outcome [1, 2]. Interestingly, Haplo-HSCT strategies employing T-cell-replete grafts and high-dose posttransplant cyclophosphamide (PT/Cy-haplo) [3] have demonstrated comparable overall and nonrelapse mortality to ones using either HLA-matched related (MRD) or unrelated (MUD) HSCT [1, 2].
Cytomegalovirus (CMV) causes significant morbidity and mortality following allogeneic hematopoietic stem cell transplantation (allo-HSCT) [4]. Discrepant data have been published regarding the incidence of CMV DNAemia and CMV disease in PT/Cy-haplo, with some studies reporting higher rates than those seen in other allo-HSCT platforms [5,6,7,8,9,10,11,12,13,14,15]. In a recent study, we observed a trend towards longer duration of initial episodes of CMV DNAemia, shorter CMV DNA doubling times and higher CMV DNA peak loads in PT/Cy-haplo recipients than in HLA-matched HSCT patients, despite similar incidence of CMV DNAemia [16]. Moreover, cases of CMV end-organ disease were only diagnosed in the PT/Cy-haplo population group [16]. To account for these findings, it was speculated that CMV-specific immune reconstitution could have been impaired or protracted in PT/Cy-haplo recipients [6,7,8,9, 16], although no data supporting this assumption were provided. To date, few studies have investigated the dynamics of CMV-specific T-cell reconstitution following HSCT-haplo [17,18,19,20,21,22]. Here, we conducted a multicenter study to assess the kinetics of CMV pp65 and IE-1-specific T cells in patients undergoing PT/Cy-haplo and compared them in patients allografted with HLA-matched donors. Previous studies showed that these CMV-specific T-cell subsets provide protection from and exert control over CMV DNAemia in the allo-HSCT setting [23,24,25].
Patients and methods
Study population
In this multicenter observational study, we prospectively recruited 97 consecutive adult patients who had undergone either PT/Cy-haplo (n = 71) or HLA-matched allo-HSCT (n = 26; MRD, n = 15; MUD, n = 11) at Hospital Regional Universitario of Málaga (HRUM), Spain (n = 41), Hospital Clínico Universitario of Salamanca (HCUS), Spain (n = 29), Hospital Clínico Universitario of Valencia (HCUV) (n = 18), or Hospital General Universitario Gregorio Marañón of Madrid (HGUGM), Spain (n = 9) between January 2016 and December 2018 (the length of the recruitment period varied across centers). Patients in this cohort were included in a previously published series [16]. The study period comprised the first 180 days after allo-HSCT. This study was approved by the research ethics committees at each institution. Informed consent was obtained from participants.
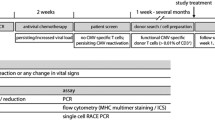
Management of CMV infection
The CMV RealTime CMV PCR (Abbott Molecular, Des Plaines, IL, USA), with limit of detection (LOD) of 31.4 IU/ml (95% CI) [26] was used to monitor plasma CMV DNA load at all centers except HGUGM where the Affigene CMV Trender kit (Cepheid AB, Bromma, Sweden) with LOD of 137 IU/ml was used according to the manufacturerʼs instructions. Monitoring began 1 or 2 weeks prior to allo-HSCT and was conducted on a weekly basis through day +100. From day +100 until day +180 patients at risk for recurrent episodes of CMV DNAemia were also monitored once a week [16]. The remaining patients were monitored at each planned visit and if testing positive were subsequently monitored once weekly. Preemptive antiviral therapy (PET) consisted of (val)ganciclovir or foscarnet administered at conventional doses. At HCUV PET was initiated when the plasma CMV DNA load reached ≥1500 IU/ml, or when the CMV DNA doubling time (dt) was ≤2.0 days, whichever occurred first [27]. Cut-off CMV DNA levels prompting initiation of PET were ≥600 IU/ml at HCUS and ≥156 IU/ml at HRUM.
Immunological monitoring
Enumeration of CMV-specific IFN-γ-producing CD8+ and CD4+ T cells was performed by flow cytometry for intracellular cytokine staining (BD Fastimmune, BD-Beckton Dickinson and Company-Biosciences, San Jose, CA, USA) as previously reported [23,24,25]. Two sets of 15-mer overlapping peptides encompassing the entire sequence of CMV pp65 and IE-1 proteins were used in combination for stimulation. A negative control (absence of peptide stimulation) was run in all experiments. The total number of CMV-specific CD8+ and CD4+ T cells was calculated by multiplying the corresponding percentage of CMV-specific T cells (after background subtraction) by the absolute number of CD8+ T and CD4+ T cells. Specific (detectable) responses were those >0.1% for each population [21,22,23]. Immunological monitoring was scheduled to be conducted by days +30, +60, +90, and +180 after allo-HSCT. Total CD3+/CD4+ and CD3+/CD8+ T cells were enumerated by flow cytometry.
CMV DNA doubling time
The CMV DNA dt was calculated as previously reported [28], and was given by dt = (t2 − t1) × [ln2/ln(q2/q1)], with q1 and t1 representing the CMV DNA (copies/ml) at the time of the first positive PCR (in days), and q2 and t2 representing CMV DNA at the time of the second positive PCR (in the absence of antiviral treatment).
Definitions
CMV DNAemia was defined as the detection of CMV DNA at any level in one or more plasma specimens. The overall duration of a given episode of viral DNAemia was the number of days elapsed between first detection of viral DNA in plasma and first negative (undetectable) PCR result. Recurrent episodes were those developing at least 15 days after clearance of the previous one. CMV disease was diagnosed as previously indicated [29]. Acute graft-versus-host disease (aGvHD) was diagnosed and graded as previously reported [30].
Statistical analysis
Frequency comparisons for categorical variables were carried out using the chi-square test (Fisher’s exact test). Differences between medians (for unpaired data) were compared using the Mann–Whitney U test (two independent variables). The Spearman’s rank test was used for analysis of correlation between continuous variables. Two-sided exact P values were reported. A P value < 0.05 was considered statistically significant. The analyses were performed using SPSS version 20.0 (SPSS, Chicago, IL, USA).
Results
Clinical characteristics of patients
Demographics, baseline and posttransplant characteristics of patients undergoing either PT/Cy-haplo or MRD/MUD are shown in Table 1. The two groups were comparable regarding age, sex, underlying hematological diseases, D/R CMV serostatus and incidence of aGvHD, but differed in the regimen used for aGvHD prophylaxis, with patients undergoing PT/Cy-haplo more frequently receiving sirolimus and mycophenolate mofetil. Also of interest are the following facts: (1) the number of patients receiving corticosteroids for aGvHD (21% in the PT/Cy-haplo group, 18% in the MUD group and 13%) in the MRD group) did not differ significantly (P = 0.42); (2) the frequency of patients and donors harboring HLA class I alleles presenting dominant CMV epitopes in pp65 (HLA-A0201) or in IE-1 (HLA-B0702) was comparable across groups (Supplementary Table 1); (3) No patient in this cohort received anti-thymocyte globulin or alemtuzumab.
Incidence of CMV DNAemia and CMV disease
A total of 62 patients (63.9%) developed one (n = 47) or more (n = 15) episodes of CMV DNAemia at a median of 33 (−7 to 123) days after transplantation. CMV DNAemia developed at a comparable frequency (P = 0.14) in both PT/Cy-haplo (n = 43; 59.7%) and MRD/MUD recipients (n = 19; 76%). First episodes of CMV DNAemia were documented prior to day +30 in 22 out of 62 patients (PT/Cy-haplo, n = 15; MRD/MUD, n = 7), between days +30 and +60 in 36 patients (PT/Cy-haplo, n = 24; MRD/MUD, n = 12), and after day +60 in the remaining four patients (all PT/Cy-haplo). The number of patients experiencing recurrent CMV DNAemia was similar in the two groups (P = 0.92). Likewise, PET was used comparably across groups (P = 0.54). Three cases of CMV disease (gastrointestinal) were diagnosed, in all cases in PT/Cy-haplo recipients.
No significant differences were found across groups regarding virological features of CMV DNAemia episodes, although a trend towards shorter CMV dts and higher CMV DNA peak loads was observed in the PT/Cy-haplo setting (Table 2).
Immune reconstitution following transplantation
Data from a median of three blood specimens per patient (range, 1–4) were available for analyses. In detail, samples for immunological monitoring were available for 78, 65, 58, and 41 patients by days +30, +60, +90, and +180, respectively. As shown in Table 3, total lymphocyte, CD3+/CD8+ and CD3+/CD4+ T-cell counts were not significantly different across groups by days +30, +60, and +180. However, a noticeably higher number of CD3+/CD4+T cells was observed by day +90 in patients undergoing PT/Cy-haplo.
Kinetics of CMV-specific T-cell reconstitution across transplantation groups
Detectable CMV-specific CD8+ and CD4+ T-cell responses were found to increase in frequency over time, in both PT/Cy-haplo and MRD/MUD recipients, reaching 85% of patients in both groups at the end of the follow-up period (Fig. 1). The number of patients displaying detectable responses was not significantly different across groups at most time points, but more patients had detectable CMV-specific CD4+ T-cell responses by day +30 in the MRD/MUD group (P = 0.03). Separate subanalyses comparing PT/Cy-haplo versus MRD recipients (Supplementary Table 2) and PT/Cy-haplo versus MUD patients (Supplementary Table 3) were performed. The data suggested that reconstitution of CMV-specific CD8+ T cells (but not CMV-specific CD4+ T cells) occurred at a faster rate in PT/Cy-haplo than in MUD patients (but not than in MRD recipients.
Frequency of detectable and protective (against CMV DNAemia) CMV-specific IFN-γ-CD8+ and CD4+ T cells at different time points following unmanipulated haploidentical hematopoietic stem cell transplantation and high-dose posttransplant cyclophosphamide (PT/Cy-haplo) or HLA-matched related (MRD) or unrelated (MUD) allogeneic hematopoietic stem cell transplantation P values for comparisons across groups are shown.
From a quantitative standpoint, there were no significant differences in CMV-specific CD8+ and CD4+ T-cell counts across comparison groups at most time points, irrespective of whether CMV DNAemia did (Table 4) or did not (Table 5) occur prior to immunological monitoring. In fact, CMV-specific CD8+ T-cell counts measured by day +60 were only significantly higher in PT/Cy-haplo than in MRD/MUD recipients in the subset of patients with preceding CMV DNAemia. Overall, correlation between CMV-specific CD8+ and CD4+ T cells was similar in PT/Cy-haplo and MRD/MUD patients (Table 6).
Occurrence of CMV DNAemia had no significant impact on level of CMV-specific CD8+ and CD4+ T-cell reconstitution at the end of the follow-up period (day +180) in either allo-HSCT modality (Table 7).
In PT/Cy-haplo CMV-seropositive recipients (R), donor (D) CMV serostatus had no apparent effect on the magnitude of CMV-specific CD8+ and CD4+ T-cell immune reconstitution at the end of follow-up, although recovery appeared faster in D+/R+ than in D−/R+ recipients (Supplementary Table 4).
Acquisition of protective CMV-specific IFN-γ-producing CD8+ and CD4+ T-cell responses
We previously reported that no patients displaying pp65/IE-1 CMV-specific CD8+ and CD4+ T-cell counts above 1.0 and 1.2 cells/µL, respectively, within the first year after allo-HSCT, went on to develop CMV DNAemia [25]. In the current series, one patient in the PT/Cy-haplo group and one patient undergoing MUD remained unprotected despite exhibiting CMV-specific CD8+ T-cell counts by day +30 above the mentioned threshold, while three patients (two in the PT/Cy-haplo group and one MUD) displaying CMV-specific CD4+ T-cell responses above the corresponding cut-off by day +30 were subsequently diagnosed with CMV DNAemia. All five patients received corticosteroids for Grade II aGvHD.
The number of patients acquiring CMV-specific CD8+ and CD4+ T-cell responses conferring protection against subsequent CMV DNAemia rose over time, with no significant differences between PT/Cy-haplo and MRD/MUD patients at any time point examined (Fig. 1). Nevertheless, more patients in the PT/Cy-haplo group than in the MUD subgroup (but not in the MRD subgroup) exhibited protective CMV-specific CD8+ T-cell responses by days +60 and +90 (Supplementary Tables 2 and 3).
Discussion
To our knowledge, this is the first report on the kinetics of CMV-specific T-cell reconstitution in PT/Cy-haplo recipients. Previous studies addressing this topic included patients undergoing other Haplo-HSCT modalities, as is discussed later [17,18,19,20].
We focused on enumerating pp65/IE-1 CMV pp65/IE-1-specific IFN-γ-producing CD8+ and CD4+ cells, since these functional T-cell subset specificities were shown to afford protection against CMV DNAemia in allo-HSCT recipients over the first year following transplantation [23,24,25].
Consistent with previous reports [1, 31], total CD3+/CD8+ and CD3+/CD4+ T-cell recovery appeared to follow similar kinetics in PT/Cy-haplo and MRD/MUD recipients, with no significant differences in the respective cell counts at most time points examined. Likewise, we found no substantial variations between PT/Cy-haplo recipients and MRD/MUD patients in CMV-specific T-cell reconstitution dynamics within the first 6 months after transplantation, either from a qualitative standpoint (frequency of detectable and protective responses at any given time point) or from a quantitative perspective (cell counts of the respective CMV-specific T-cell populations at the times of immunological monitoring). Nevertheless, acquisition of detectable and protective CMV-specific CD8+ T-cell responses, but not of CMV-specific CD4+ T-cell responses) appeared to occur more rapidly in PT/Cy-haplo recipients than in MUD patients, whereas no differences were seen between PT/Cy-haplo and MRD recipients. In this sense, it should be stressed that PT/Cy-haplo, MRD, and MUD recipients did not differ in certain parameters known to have an impact on the degree of reconstitution of CMV-specific T cells [1], namely, receipt of corticosteroids for treatment of aGvHD or harboring HLA class I specificities presenting highly immunogenic epitopes mapping on pp65 and IE-1 (HLA-A0207 for pp65 and HLA-B0702 for IE-1). The scarce number of MUD and MRD patients in the current cohort precludes drawing robust conclusions from these subanalyses, though.
Reconstitution of CMV-specific CD8+ and CD4+ T-cell immunity in the PT/Cy-haplo setting appeared to be slower in CMV D−/R+ than in CMV D+/R+ patients, as occurs in patients undergoing other allo-HSCT modalities [1], yet degree of recovery at end of follow-up was quite similar. Furthermore, overall, CMV-specific CD8+ and CD4+ T-cell counts were similarly correlated regardless of the allo-HSCT platform used. Thus, the subtle dissimilarities across groups in the virological features of CMV DNAemia episodes reported herein in line with previous observations [16], namely shorter in CMV DNA dts and higher CMV DNA peak levels in PT/Cy-haplo, could not be explained by differences in the kinetics of CMV-specific T-cell reconstitution. Nevertheless, we cannot rule out that the magnitude and timing of expansion in these functional CMV-specific T-cell subsets following CMV DNAemia, which we showed to inversely correlate with CMV DNA peak loads reached within episodes [32], could have been different across groups.
Direct comparison of the data presented here with those published by our group a few years ago [25] comprising a much larger cohort of HLA-matched allo-HSCT recipients, supports the notion that CMV-specific T-cell reconstitution in PT/Cy-haplo recipients proceeds comparably with that in MRD/MUD patients. For example, around 20% of patients who underwent MRD/MUD were found to have acquired protective CMV-specific CD8+ and CD4+ T-cell responses by day +30 after transplantation in our previous study [25]; by day +90, 36% of patients were reported to display protective CMV-specific CD4+ T-cell responses [25]. These figures are remarkably similar to those found in PT/Cy-haplo recipients in the current study.
Relevant to the above, sirolimus was used more frequently in the PT/Cy-haplo group. Given recent evidence that this m-TOR inhibitor drug significantly improves CMV-specific effector memory T-cell function via modulation of the environmental milieu [33], we postulated whether the differential use of sirolimus across groups might have introduced bias. Unfortunately, the limited number of patients undergoing sirolimus treatment without CMV DNAemia prior to the first immunological monitoring time point precluded meaningful statistical analysis.
In a previous study [25] peripheral levels of CMV pp65/IE-1-specific IFN-γ-producing CD8+ and CD4+ cells through day +365 after transplantation in a cohort of allo-HSCT patients devoid of PT/Cy-haplo recipients were found to be comparable regardless of whether or not CMV DNAemia occurred prior to immunological monitoring, which supports the idea that subclinical CMV reactivation operates as a potent stimulator of CMV-specific T-cell expansion [34]. Here, we extend this observation to PT/Cy-haplo recipients, in whom reconstitution of CMV-specific functional T-cell responses appeared to develop comparably with MRD/MUD recipients, irrespective of presence or absence of a preceding CMV DNAemia episode.
In a rather heterogeneous allo-HSCT population, protective cut-off levels for both CMV-specific CD8+ and CD4+ T-cell subsets were defined as ≥1 CMV-specific CD8+ T cells/µL and ≥1.2 CMV-specific CD4+ T cells/µL [23, 25]. The clinical validity of these threshold levels appeared to largely stand for PT/Cy-haplo recipients. In effect, only three patients developed CMV DNAemia despite having CMV-specific T-cell counts above established cut-offs (one CD8+ and two CD4+). Nonetheless, all three patients were receiving corticosteroids for Grade II aGvHD,and it is known that ex vivo frequencies of CMV-specific T cells do not correlate with in vivo protection against CMV DNAemia in this setting [35].
As previously mentioned, several studies have examined CMV-specific T-cell responses in the setting of Haplo-HSCT platforms other than PT/Cy-haplo. Noviello et al. included patients undergoing Haplo-HSCT with either CD34+ selection or posttransplant sirolimus as GvHD prophylaxis [20]. Enumeration of CMV-specific T cells was done by IFN-γ Enzyme-Linked ImmunoSpot (ELISpot), which cannot discriminate between CMV-specific CD8+ and CD4+ T cells, following stimulation with CMV-infected fibroblasts lysates. Despite notable differences between Noviello’s study and ours regarding not only method used for immunological evaluation and Haplo-HSCT platform, but also their use of CMV prophylaxis during conditioning and up to day 20 after transplantation, protective levels of CMV-specific T cells against viremia (>one spot forming unit/µL) were observed in 21.7, 29, and 52.7% of patients by days 30, 90 and 180 after transplantation. These numbers are quite close to those found in the present study for CMV-specific CD8+and CD4+ T cells.
Kato et al. [19] enumerated CMV-specific CD8+ T cells targeting the HLA-A2-restricted NLVPMVATV peptide in a small cohort of T-cell replete Haplo-HSCT recipients not receiving PT/Cy, and found that patients with resolving CMV antigenemia had significantly higher median CMV T-cell counts than those displaying persistent CMV antigenemia. The kinetics of recovery of these CMV-specific T cells was not investigated. Luo et al. [17] conducted a prospective study on T cell-depleted (ATG) Haplo-HSCT recipients and used a combination of pentamer quantification of pp65-specific CD8+ T cells with functional ELISpot to characterize the pattern of CMV immune restoration after transplantation. CMV-specific T-cell reconstitution was found to be delayed in comparison with unmanipulated HLA-matched HSCTs. Similar findings were reported by other authors in patients receiving partially HLA-matched allografts with either ex vivo or in vivo (alemtuzumab) T-cell depletion [21, 22]. Collectively, data from the above studies and those presented here support the assumption that CMV-specific T-cell reconstitution in Haplo-HSCT develops comparably with that in HLA-matched allo-HSCT, as long as T-cell depletion is avoided.
The current study has several limitations: First, the small size of the MRD/MUD cohort; second, the unavailability of a number of blood specimens initially planned to be collected for immunological analyses; third, polyfunctionality of CMV-specific T cells (i.e., secretion of other cytokines or chemokines, such as tumor necrosis factor-alpha, macrophage inflammatory protein-1b, or interleukin-2, or exhibiting degranulation markers (CD107a)) was not explored. In this sense, there is some evidence indicating that polyfunctional T cells may be more effective than monofunctional T cells in controlling CMV replication in this clinical setting [36, 37]. Nevertheless, in a previous study we showed that the predictive value of total CMV-specific IFN-γ-producing CD8+ T cells for the occurrence of CMV DNAemia was comparable with that of the detection of polyfunctional CD8+ T cells (IFN-γ/TNF-α/CD107a+) [38]. Fourth, two distinct PCR assays were used across centers. Nevertheless, this latter drawback is unlikely to undermine our conclusions, as CMV DNA monitoring was performed using the Abbott real-time PCR assay in all but nine patients. Needless to say, we cannot rule out that CMV-specific T-cell antigen specificities potentially contributing to control of CMV DNAemia in allo-HSCTs other than those investigated herein may have differed across groups.
In summary, our data suggested that PT/Cy-haplo recipients are capable of CMV-specific T-cell immune reconstitution to the same extent as patients undergoing HLA-matched allo-HSCT. Nevertheless, further studies are warranted to validate our findings.
References
McCurdy SR, Luznik L. Immune reconstitution after T-cell replete HLA-haploidentical transplantation. Semin Hematol. 2019;56:221–26.
Gagelmann N, Bacigalupo A, Rambaldi A, Hoelzer D, Halter J, Sanz J, et al. Haploidentical stem cell transplantation with posttransplant cyclophosphamide therapy vs other donor transplantations in adults with hematologic cancers: a systematic review and meta-analysis. JAMA Oncol. 2019;5:1739–48.
Luznik L, O’Donnell PV, Symons HJ, Chen AR, Leffell MS, Zahurak M, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transpl. 2008;14:641–50.
Pérez-Romero P, Blanco P, Giménez E, Solano C, Navarro D. An update on the management and prevention of cytomegalovirus infection following allogeneic hematopoietic stem cell transplantation. Future Virol. 2015;10:113–34.
Slade M, Goldsmith S, Romee R, DiPersio JF, Dubberke ER, Westervelt P, et al. Epidemiology of infections following haploidentical peripheral blood hematopoietic cell transplantation. Transpl Infect Dis. 2017;19. https://doi.org/10.1111/tid.12629.
Goldsmith SR, Slade M, DiPersio JF, Westervelt P, Lawrence SJ, Uy GL, et al. Cytomegalovirus viremia, disease, and impact on relapse in T-cell replete peripheral blood haploidentical hematopoietic cell transplantation with post-transplant cyclophosphamide. Haematologica. 2016;101:e465–8.
Shmueli E, Or R, Shapira MY, Resnick IB, Caplan O, Bdolah-Abram T, et al. High rate of cytomegalovirus drug resistance among patients receiving preemptive antiviral treatment after haploidentical stem cell transplantation. J Infect Dis. 2014;209:557–61.
Lin CH, Su YJ, Hsu CY, Wang PN, Teng CJ. Haploidentical allogeneic hematopoietic stem cell transplantation increases the risk of cytomegalovirus infection in adult patients with acute leukemia. Transpl Infect Dis. 2019;20:e13096.
Chen Y, Xu LP, Liu KY, Chen H, Chen YH, Zhang XH, et al. Risk factors for cytomegalovirus DNAemia following haploidentical stem cell transplantation and its association with host hepatitis B virus serostatus. J Clin Virol. 2016;75:10–15.
Hammerstrom AE, Lombardi LR, Pingali SR, Rondon G, Chen J, Milton DR, et al. Prevention of cytomegalovirus reactivation in haploidentical stem cell transplantation. Biol Blood Marrow Transpl. 2018;24:353–8.
Baker M, Wang H, Rowley SD, Cai L, Pecora AL, Skarbnik A, et al. Comparative outcomes after haploidentical or unrelated donor bone marrow or blood stem cell transplantation in adult patients with hematological malignancies. Biol Blood Marrow Transpl. 2016;22:2047–55.
Raj RV, Hari P, Pasquini M, Epperla N, D’Souza A, Fenske T, et al. Impact of haploidentical hematopoietic cell transplantation conditioning intensity on the incidence and severity of post-transplantation viral infections. Bone Marrow Transpl. 2016;51:1602–4.
Tischer J, Engel N, Fritsch S, Prevalsek D, Hubmann M, Schulz C, et al. Virus infection in HLA-haploidentical hematopoietic stem cell transplantation: incidence in the context of immune recovery in two different transplantation settings. Ann Hematol. 2015;94:1677–88.
Chang YJ, Zhao XY, Huo MR, Xu LP, Liu DH, Liu KY, et al. Immune reconstitution following unmanipulated HLA-mismatched/haploidentical transplantation compared with HLA-identical sibling transplantation. J Clin Immunol. 2012;32:268–80.
Crocchiolo R, Bramanti S, Vai A, Sarina B, Mineri R, Casari E, et al. Infections after T-replete haploidentical transplantation and high-dose cyclophosphamide as graft-versus-host disease prophylaxis. Transpl Infect Dis. 2015;17:242–9.
Huntley D, Giménez E, Pascual MJ, Hernández-Boluda JC, Gago B, Vázquez L, et al. Incidence, features, and outcomes of cytomegalovirus DNAemia in unmanipulated haploidentical allogeneic hematopoietic stem cell transplantation with post-transplantation cyclophosphamide. Transpl Infect Dis. 2019;22:e13206.
Luo XH, Huang XJ, Li D, Liu KY, Xu LP, Liu DH. Immune reconstitution to cytomegalovirus following partially matched-related donor transplantation: impact of in vivo T-cell depletion and granulocyte colony-stimulating factor-primed peripheral blood/bone marrow mixed grafts. Transpl Infect Dis. 2013;15:22–33.
Luo XH, Chang YJ, Huang XJ. Improving cytomegalovirus-specific T cell reconstitution after haploidentical stem cell transplantation. J Immunol Res. 2014;63:1951.
Kato R, Tamaki H, Ikegame K, Yoshihara S, Kaida K, Taniguchi K, et al. Early detection of cytomegalovirus-specific cytotoxic T lymphocytes against cytomegalovirus antigenemia in human leukocyte antigen haploidentical hematopoietic stem cell transplantation. Ann Hematol. 2015;94:1707–15.
Noviello M, Forcina A, Veronica V, Crocchiolo R, Stanghellini MT, Carrabba M, et al. Early recovery of CMV immunity after HLA-haploidentical hematopoietic stem cell transplantation as a surrogate biomarker for a reduced risk of severe infections overall. Bone Marrow Transpl. 2015;50:1262–4.
Kanda Y, Oshima K, Asano-Mori Y, Kandabashi K, Nakagawa M, Sakata-Yanagimoto M, et al. In vivo alemtuzumab enables haploidentical human leukocyte antigen-mismatched hematopoietic stem-cell transplantation without ex vivo graft manipulation. Transplantation. 2005;79:1351–7.
Lilleri D, Gerna G, Fornara C, Chiesa A, Comolli G, Zecca M, et al. Human cytomegalovirus-specific T cell reconstitution in young patients receiving T cell-depleted, allogeneic hematopoietic stem cell transplantation. J Infect Dis. 2009;15:829–36.
Solano C, Benet I, Clari MA, Nieto J, de la Cámara R, López J, et al. Enumeration of cytomegalovirus-specific interferongamma CD8+ and CD4+ T cells early after allogeneic stem cell transplantation may identify patients at risk of active cytomegalovirus infection. Haematologica. 2008;93:1434–6.
Tormo N, Solano C, Benet I, Clari MA, Nieto J, de la Cámara R, et al. Lack of prompt expansion of cytomegalovirus pp65 and IE-1-specific IFNgamma CD8+ and CD4+ T cells is associated with rising levels of pp65 antigenemia and DNAemia during pre-emptive therapy in allogeneic hematopoietic stem cell transplant recipients. Bone Marrow Transpl. 2010;45:543–9.
Tormo N, Solano C, Benet I, Nieto J, de la Cámara R, López J, et al. Reconstitution of CMV pp65 and IE-1-specific IFN-γ CD8(+) and CD4(+) T-cell responses affording protection from CMV DNAemia following allogeneic hematopoietic SCT. Bone Marrow Transpl. 2011;46:1437–43.
Clari MÁ, Bravo D, Costa E, Muñoz-Cobo B, Solano C, Remigia MJ, et al. Comparison of the new Abbott Real Time CMV assay and the Abbott CMV PCR Kit for the quantitation of plasma cytomegalovirus DNAemia. Diagn Microbiol Infect Dis. 2013;75:207–9.
Solano C, Giménez E, Piñana JL, Vinuesa V, Poujois S, Zaragoza S, et al. Preemptive antiviral therapy for CMV infection in allogeneic stem cell transplant recipients guided by the viral doubling time in the blood. Bone Marrow Transpl. 2016;51:718–21.
Muñoz-Cobo B, Solano C, Costa E, Bravo D, Clari MÁ, Benet I, et al. Dynamics of cytomegalovirus (CMV) plasma DNAemia in initial and recurrent episodes of active CMV infection in the allogeneic stem cell transplantation setting: implications for designing preemptive antiviral therapy strategies. Biol Blood Marrow Transpl. 2011;17:1602–11.
Ljungman P, Boeckh M, Hirsch HH, Josephson F, Lundgren J, Nichols G, et al. Definitions of cytomegalovirus infection and disease in transplant patients for use in clinical trials. Clin Infect Dis. 2017;64:87–89.
Glucksberg H, Storb R, Fefer A, Buckner CD, Neiman PE, Clift RA, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974;18:295–304.
McCurdy SR, Vulic A, Symons HJ. Comparable and robust immune reconstitution after HLA-haploidentical or HLA-matched allogeneic transplantation (BMT) utilizing post-transplantation cyclophosphamide. Biol Blood Marrow Transpl. 2015;21:S71–71.
Tormo N, Solano C, Benet I, Nieto J, de la Cámara R, Garcia-Noblejas A, et al. Kinetics of cytomegalovirus (CMV) pp65 and IE-1-specific IFNgamma CD8+ and CD4+ T cells during episodes of viral DNAemia in allogeneic stem cell transplant recipients: potential implications for the management of active CMV infection. J Med Virol. 2010;82:1208–15.
Bak S, Tischer S, Dragon A, Ravens S, Pape L, Koenecke C, et al. Selective effects of mTOR inhibitor sirolimus on naïve and CMV-specific T cells extending its applicable range beyond immunosuppression. Front Immunol. 2018;9:2953.
Hakki M, Riddell SR, Storek J, Carter RA, Stevens-Ayers T, Sudour P, et al. Immune reconstitution to cytomegalovirus after allogeneic hematopoietic stem cell transplantation: impact of host factors, drug therapy, and subclinical reactivation. Blood. 2003;102:3060–7.
Navarro D, Amat P, de la Cámara R, López J, Vázquez L, Serrano D, et al. Efficacy and safety of a preemptive antiviral therapy strategy based on combined virological and immunological monitoring for active cytomegalovirus infection in allogeneic stem cell transplant recipients. Open Forum Infect Dis. 2016;3:ofw107.
Lacey S, La Rosa C, Zhou W, Sharma MC, Martinez J, Krishnan A, et al. Functional comparison of T cells recognizing cytomegalovirus pp65 and immediate-early antigen polypeptides in hematopoietic stem-cell transplant and solid organ transplant recipients. J Infect Dis. 2006;194:1410–21.
Zhou W, Longmate J, Lacey SF, Palmer JM, Gallez-Hawkins G, Thao L, et al. Impact of donor CMV status on viral infection and reconstitution of multifunction CMV specific T cells in CMV-positive transplant recipients. Blood. 2009;113:6465–76.
Giménez E, Muñoz-Cobo B, Solano C, Amat P, de la Cámara R, Nieto J, et al. Functional patterns of cytomegalovirus (CMV) pp65 and immediate early-1-specific CD8(+) T cells that are associated with protection from and control of CMV DNAemia after allogeneic stem cell transplantation. Transpl Infect Dis. 2015;17:361–70.
Acknowledgements
EG holds a Juan Rodés research contract from the Carlos III Health Institute (Ref. JR18/00053). Eliseo Albert holds a Río Hortega research contract from the Carlos III Health Institute (Ref. CM18/00221).
Funding
This research was supported by grant 15/0090 from FIS (Fondo de Investigaciones Sanitarias), Ministerio de Sanidad y Consumo, Spain.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Huntley, D., Giménez, E., Pascual, M.J. et al. Reconstitution of cytomegalovirus-specific T-cell immunity following unmanipulated haploidentical allogeneic hematopoietic stem cell transplantation with posttransplant cyclophosphamide. Bone Marrow Transplant 55, 1347–1356 (2020). https://doi.org/10.1038/s41409-020-0865-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-020-0865-x
- Springer Nature Limited