Abstract
The prognosis of patients with stage III/IV NK/T-cell lymphoma (NTCL) is extremely poor. Although l-asparaginase (l-asp) is effective for NTCL, its significance has not been clearly demonstrated. In addition, there are few studies comparing treatment outcomes in stage III/IV NTCL. This study evaluated the efficacy of l-asp-based chemotherapy and prognostic factors in stage III/IV NTCL. Seventy patients with newly diagnosed stage III/IV NTCL were enrolled between January 2000 and February 2013. Patients received ifosfamide, etoposide, methotrexate, and prednisolone (IMEP) plus l-asp (N = 22) or combination chemotherapy without l-asp (N = 48) as a first-line treatment. Clinical prognostic factors, treatment outcomes, and prognostic scores were compared between the groups. After a median follow-up period of 12.8 months (range, 1.1–186.6 months), median overall survival (OS) and progression-free survival (PFS) were 11.3 and 5.6 months, respectively. Treatment outcomes were superior in patients treated with IMEP plus l-asp compared to those treated with chemotherapy without l-asp (overall response rate, 90.0 vs. 34.8 %, P < 0.001; complete remission rate, 65.0 vs. 21.7 %, P = 0.001). The OS and PFS were significantly higher for the IMEP plus l-asp group compared with the chemotherapy without l-asp group. In a multivariate analysis, the use of chemotherapy without l-asp was an independent predictor of reduced OS (hazards ratio (HR) = 2.18, 95 % confidence interval (CI) 1.08–4.40; P = 0.030) and PFS (HR = 2.29, 95 % CI 1.22–4.29; P = 0.010). IMEP plus l-asp is active against stage III/IV NTCL, and it is an independent predictor of improved survival.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
NK/T-cell lymphoma (NTCL) is defined as a distinct clinicopathologic disease in the World Health Organization (WHO) classification [1]. It is most common in Asia and rare in Western populations. In Korea, it accounts for 6.3 % of all non-Hodgkin’s lymphoma with a male preponderance.
Patients with stage III/IV NTCL follow a very aggressive clinical course [2], and long-term outcomes with conventional combination chemotherapy are unsatisfactory. Because NTCL cells frequently express p-glycoprotein [3], which leads to multidrug resistance (MDR), anthracycline-based combination chemotherapy has limited activity in the treatment of NTCL. Therefore, non-anthracycline-based chemotherapy regimens including ifosfamide, etoposide, methotrexate, and prednisolone (IMEP) have been used in NTCL [4]. In addition, l-asparaginase (l-asp), which is not affected by MDR, demonstrated anti-tumor activity against NK cell tumors in vitro [5]. Recent phase II studies demonstrated the efficacy of l-asp-based combination chemotherapy in newly diagnosed stage IV or refractory NTCL [6–8]. Nearly 80 % of patients with stage IV or refractory/relapsed NTCL responded to an l-asp-based regimen with a complete response (CR) rate of 45–66 %. Although combination chemotherapy is the standard treatment for advanced NTCL, there have been no studies to compare different chemotherapy regimens in stage III/IV NTCL. In addition, the prognostic significance of l-asp-based regimens has not been clearly elucidated in a relatively homogenous NTCL subset. Therefore, this study was conducted to evaluate the efficacy of l-asp-based combination chemotherapy and prognostic factors in advanced stage III/IV NTCL.
Patients and methods
Patients
A total of 70 patients who were newly diagnosed with NTCL between January 2000 and February 2013 were retrospectively identified from Seoul National University Hospital, Seoul National University Bundang Hospital, and Seoul National University Boramae Medical Center using the following inclusion criteria: (1) pathologically confirmed NTCL according to the WHO criteria [1, 9]; (2) previously untreated, stage III/IV NTCL; and (3) received chemotherapy with curative intent. Patients who received radiotherapy or surgery alone (N = 7) or a less intensive chemotherapy regimen (N = 6) were excluded. The staging work-up data were collected including complete blood count; blood chemistry including lactate dehydrogenase (LDH); computed tomography (CT) of the neck, chest, and abdomen; bone marrow examination; and otolaryngologic examination of upper aerodigestive tract (UAT). UAT-NTCL was defined as a primary tumor involving the nasal cavity, nasopharynx, oral cavity, oropharynx, and hypopharynx, whereas non-UAT (NUAT)-NTCL referred to a primary tumor outside the UAT [10]. Clinical demographics and prognostic factors were retrieved including age, sex, B symptoms, Eastern Cooperative Oncology Group performance status (ECOG PS), serum LDH level, Ann Arbor stage, number of extranodal sites, and international prognostic index (IPI) score [11]. This outcome research using human subjects was reviewed and approved by the institutional review board of each participant center and was conducted in accordance with the precepts established by the Declaration of Helsinki.
Treatment and response evaluation
All patients received first-line chemotherapy consisting of cyclophosphamide, doxorubicin, vincristine, and prednisolone (CHOP; N = 15); cyclophosphamide, vincristine, doxorubicin, bleomycin, procarbazine, and prednisolone (COPBLAM-V; N = 4); cyclophosphamide, vincristine, doxorubicin, and dexamethasone (Hyper-CVAD; N = 1); IMEP (N = 28); or IMEP plus l-asp (N = 22). CHOP, COPBLAM-V, and Hyper-CVAD were anthracycline-based or CHOP-like regimens. Although selection of chemotherapy regimens was at the discretion of the physicians, IMEP plus l-asp was mainly used since 2007. Treatment was planned up to six to eight cycles and given until disease progression, unacceptable toxicities, or patient’s refusal. Eight and four patients received sequential radiotherapy and stem cell transplantation at relapse, respectively.
The conventional IMEP regimen consists of ifosfamide at a dose of 1.5 g/m2 intravenously on days 1 to 3 with adequate hydration of 2 l of half-saline per day and mesna to prevent hemorrhagic cystitis, methotrexate at a dose of 30 mg/m2 intravenously on days on 3 and 10, etoposide at a dose of 100 mg/m2 intravenously on days 1 to 3, and prednisolone at a dose of 60 mg/m2 orally on days 1 to 5, every 3 weeks. IMEP plus l-asp derived from Escherichia coli was administered as follows: IMEP plus l-asp at a dose of 6,000 IU/m2 on days 4, 6, 8, 11, 13, and 15 before January 2010 (N = 7) and modified IMEP plus l-asp (methotrexate 30 mg/m2 on day 4 and l-asp 6,000 IU/m2 on days 1, 3, 5, 7, 9, and 11 after January 2010; N = 15).
Clinical responses were assessed by physical and otolaryngologic examinations and CT scans using the response criteria for lymphoma [12]. Adverse events could be assessed in patients treated with IMEP plus l-asp during admission or at the nadir according to NCI-CTCAE version 3.0.
Statistical analysis
Clinicopathologic variables were compared between the groups by Pearson chi-square or Fisher exact tests, as appropriate. Overall survival (OS) was calculated from the date of diagnosis to the date of death or the last follow-up visit. Progression-free survival (PFS) time was measured from the date of initial treatment to the date of disease progression, death, or the last follow-up visit. Disease-free survival (DFS) was calculated from the date of CR to the first evidence of relapse. Survival curves were derived by the Kaplan–Meier method [13]. Comparison of survivals was performed using the log-rank test. Univariate and multivariate analyses of independent factors for survival were performed using the Cox proportional hazard model [14]. Variables with clinical significance and a significance level of <0.05 were used for covariate entry. Variables with a P value >0.10 were removed during a backward stepwise analysis. All statistical tests were two-sided, with significance defined as P < 0.05. All analyses were performed using SPSS, version 21.0 (IBM Corporation, Armonk, NY, USA).
Results
Patients’ characteristics
Patients’ characteristics are summarized in Table 1. The median age was 48.5 years (range, 18–73 years), and 48 patients (68.6 %) were male. Half of the patients presented with UAT involvement with dissemination to lymph nodes (n = 6), bone marrow (n = 15), skin (n = 5), gastrointestinal (GI) tract (n = 3), soft tissues (n = 5), central nervous system (n = 4), lung (n = 3), adrenal gland (n = 1), bone (n = 1), and orbit (n = 1). NUAT-NTCL involved lymph nodes (n = 9), bone marrow (n = 10), skin (n = 11), GI tract (n = 4), soft tissues (n = 4), central nervous system (n = 1), lung (n = 5), liver (n = 1), and adrenal gland (n = 1). Nearly two thirds of the patients had systemic symptoms and three fourths had high-intermediate and high IPI risk scores.
Treatment outcomes according to the first-line chemotherapy
Sixty-six patients were eligible for response evaluation. Among patients with chemotherapy without l-asp, there were no differences in overall response rate (ORR) and CR rates between anthracycline-treated and IMEP-treated groups (ORR, 26.3 vs. 40.7 %, P = 0.312; CR, 15.8 vs. 25.9 %, P = 0.488). Similar treatment outcomes were observed in patients treated with anthracycline-based regimens, regardless of the regimen (data not shown). However, higher ORR and CR rates were observed in patients treated with IMEP plus l-asp compared with those treated with chemotherapy without l-asp (ORR, 90.0 vs. 34.8 %, P < 0.0001; CR, 65.0 vs. 21.7 % P = 0.001). Clinical factors and IPI risks were relatively well balanced between the treatment groups except for higher frequencies of UAT presentations and better ECOG PS in the IMEP plus l-asp group (Table 2). Hematologic toxicities consisting of grade 3/4 leukopenia and neutropenia were the most frequent adverse events of the IMEP plus l-asp group. Nine patients (41 %) experienced ≥grade 3 febrile neutropenia without death events. Grade 3 or 4 allergic reactions due to l-asp were observed in four patients (18 %).
Survival analysis
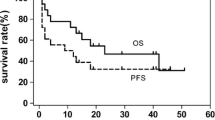
After a median follow-up of 12.8 months (range, 1.1–186.6 months), median OS, PFS, and DFS were 11.3, 5.6, and 8.0 months, respectively. The OS and PFS times were significantly higher for the IMEP plus l-asp group compared with the chemotherapy without l-asp group (Table 2, Fig. 1a, b). The IMEP plus l-asp group showed a tendency toward prolonged DFS (Fig. 1c). Although IMEP plus l-asp favorably affected OS and PFS in patients with UAT-NTCL (P = 0.004 and P < 0.001, respectively; Fig. 2a, b), it did not significantly prolong survival in patients with NUAT-NTCL (P = 0.219 and P = 0.799, respectively; data not shown). Regarding IPI risk groups, IMEP plus l-asp significantly prolonged OS and PFS in the high IPI risk group (3 to 5) (P = 0.007 and P < 0.001, respectively). However, it did not prolong survivals in the low IPI risk group (0 to 2) (P = 0.826 for OS and P = 0.990 for PFS). In patients treated with chemotherapy without l-asp, there were no survival differences between the anthracycline-treated and IMEP-treated groups (median OS, 5.2 vs. 6.2 months, P = 0.229; median PFS, 3.2 vs. 3.2 months, P = 0.432).
Prediction of survival
Regarding OS, significant factors by univariate analysis were elevated LDH level, poor ECOG PS, two or more extranodal sites, high IPI scores, and treatment with chemotherapy without l-asp. In multivariate analysis, independent factors adversely affecting OS were age >60 years, poor performance status, two or more extranodal sites, and chemotherapy without l-asp (Table 3).
Factors associated with PFS by univariate analysis were poor ECOG PS and use of chemotherapy without l-asp. Multivariate analysis confirmed that poor performance status and use of chemotherapy without l-asp were independent predictors of reduced PFS (Table 3). There were no significant factors associated with DFS in univariate or multivariate analyses (data not shown).
Discussion
Our study demonstrates that chemotherapy with IMEP plus l-asp as frontline treatment is active against stage III/IV NTCL. In addition, IMEP plus l-asp significantly improved survival in patients with advanced NTCL compared with chemotherapy without l-asp. Poor ECOG PS and chemotherapy without l-asp were independent factors for reduced OS and PFS.
Since the first case report of l-asp treatment in relapsed NTCL [15], several subsequent retrospective studies and a few phase II studies have shown that an l-asp-containing regimen resulted in ORR of 67–81 % and CR rate of 45–66 %, which represented a survival benefit in relapsed or refractory NTCL (Table 4) [6–8, 16–18]. These favorable results were comparable to those of patients with stage III/IV NTCL who were treated with IMEP plus l-asp in this study. However, heterogeneity in patient populations existed across most studies and patients with stage III/IV NTCL accounted for only 27–71 % of the patient population. In contrast, all NTCL patients in our study were Ann Arbor stage III/IV, suggesting a relatively homogenous population. In addition, treatment outcomes were compared based on chemotherapeutic regimens, and there were no differences in survival outcomes between CHOP-like and IMEP groups before the l-asp era. This indicated that CHOP-like regimens were unsatisfactory for the treatment of advanced NTCL, as shown in previous studies [19, 20].
IMEP, a non-anthracycline-based combination chemotherapy regimen, was moderately effective for relapsed or refractory NTCL as a second-line treatment and achieved an ORR of 44 % [21]. In addition, frontline IMEP resulted in an ORR of 73 % (CR rate, 27 %) with favorable safety profiles in stage I/II NTCL in a prospective multicenter trial [22]. Therefore, IMEP is active and safe for patients with NTCL and was commonly used in combination with and without l-asp in this study. Although febrile neutropenia was observed in 41 % of patients treated with IMEP plus l-asp in our study, there were no treatment-related deaths. Therefore, IMEP plus l-asp might be a reasonable option for the treatment of advanced NTCL. Due to the significant toxicities of the dexamethasone, methotrexate, ifosfamide, l-asp, and etoposide (SMILE) regimen, a modified SMILE regimen was retrospectively investigated in advanced or relapsed/refractory NTCL [23] and showed efficacy similar to that of other l-asp-based regimens. Although CR, ORR, and PFS in the modified SMILE group were superior to those in the CHOP group, modified SMILE showed only a trend toward improved OS [23]. Similarly, frontline IMEP plus l-asp significantly improved treatment outcomes in stage III/IV NTCL compared with CHOP-like regimens in our study. However, IMEP plus l-asp did not seem to be beneficial to our patients with stage III/IV NUAT-NTCL and those with low IPI scores, and other active combination chemotherapy regimens should be investigated in these groups. Because NUAT-NTCL is a unique subset and is heterogeneous in terms of clinical prognostic factors and survival outcomes [10], treatment strategies might be explored separately from UAT-NTCL. However, because only five patients with NUAT-NTCL received IMEP plus l-asp and a wide array of chemotherapy regimens was given, it is cautious to compare the outcomes of IMEP plus l-asp with those of chemotherapy without l-asp in NTCL. In addition, changes in diagnostic criteria [1, 9], staging and re-staging [12, 24], and treatment strategy during the time lag between January 2000 and February 2013 should be taken into consideration to interpret our results.
Poor ECOG PS adversely affected the OS of NTCL and was an independent predictor of reduced OS of UAT-NTCL in the largest Korean survey [10]. ECOG PS was an independent factor for DFS in relapsed or refractory NTCL in the Asia Lymphoma Study Group [6]. In addition, ECOG PS 1–2 was associated with reduced OS in a univariate analysis by the NK-Cell Tumor Study Group [7]. Similarly, ECOG PS ≥2 was an independent factor for reduced OS and PFS of stage III/IV NTCL in our study. Because patients who were treated with chemotherapy without l-asp had more NUAT presentations and a worse ECOG PS than those treated with IMEP plus l-asp, these factors might introduce a bias in interpreting the results and compromise survival outcomes in this group. The involvement of two or more extranodal sites was an independent predictor of reduced OS in NUAT-NTCL [10], as in our analysis for OS in stage III/IV NTCL.
In conclusion, IMEP plus l-asp is an independent predictor of improved survival in patients with Ann Arbor stage III/IV NTCL. In addition, this regimen was well tolerated without treatment-related deaths and showed comparable outcomes to other l-asp-containing regimens, including SMILE. However, IMEP plus l-asp should be prospectively evaluated in NTCL and new treatment strategies including the optimizing use of l-asp based on asparaginase activity [25] or antiasparaginase antibody [8] and alternative use of Erwinia asparaginase in case of hypersensitivity might be investigated, especially in NUAT-NTCL. Taken together, our data indicate that l-asp-containing regimens might be useful as a first-line treatment for stage III/IV NTCL.
References
Chan JK, Quintanilla-Martinez L, Ferry JA, Peh S-C (2008) Extranodal NK/T-cell lymphoma, nasal type. In: Swerdlow SH, Campo E, Harris NL et al (eds) WHO classification of tumours of haematopoietic and lymphoid tissues. IARC, Lyon, pp 285–288
Kwong YL, Chan AC, Liang R, Chiang AK, Chim CS, Chan TK, Todd D, Ho FC (1997) CD56+ NK lymphomas: clinicopathological features and prognosis. Br J Haematol 97(4):821–829
Yamaguchi M, Kita K, Miwa H, Nishii K, Oka K, Ohno T, Shirakawa S, Fukumoto M (1995) Frequent expression of P-glycoprotein/MDR1 by nasal T-cell lymphoma cells. Cancer 76(11):2351–2356
Lee KW, Yun T, Kim DW, Im SA, Kim TY, Yoon SS, Heo DS, Bang YJ, Park S, Kim BK, Kim NK (2006) First-line ifosfamide, methotrexate, etoposide and prednisolone chemotherapy +/− radiotherapy is active in stage I/II extranodal NK/T-cell lymphoma. Leuk Lymphoma 47(7):1274–1282. doi:10.1080/10428190600562823
Ando M, Sugimoto K, Kitoh T, Sasaki M, Mukai K, Ando J, Egashira M, Schuster SM, Oshimi K (2005) Selective apoptosis of natural killer-cell tumours by l-asparaginase. Br J Haematol 130(6):860–868. doi:10.1111/j.1365-2141.2005.05694.x
Kwong YL, Kim WS, Lim ST, Kim SJ, Tang T, Tse E, Leung AY, Chim CS (2012) SMILE for natural killer/T-cell lymphoma: analysis of safety and efficacy from the Asia Lymphoma Study Group. Blood 120(15):2973–2980. doi:10.1182/blood-2012-05-431460
Yamaguchi M, Kwong YL, Kim WS, Maeda Y, Hashimoto C, Suh C, Izutsu K, Ishida F, Isobe Y, Sueoka E, Suzumiya J, Kodama T, Kimura H, Hyo R, Nakamura S, Oshimi K, Suzuki R (2011) Phase II study of SMILE chemotherapy for newly diagnosed stage IV, relapsed, or refractory extranodal natural killer (NK)/T-cell lymphoma, nasal type: the NK-Cell Tumor Study Group study. J Clin Oncol 29(33):4410–4416. doi:10.1200/JCO.2011.35.6287
Jaccard A, Gachard N, Marin B, Rogez S, Audrain M, Suarez F, Tilly H, Morschhauser F, Thieblemont C, Ysebaert L, Devidas A, Petit B, de Leval L, Gaulard P, Feuillard J, Bordessoule D, Hermine O (2011) Efficacy of l-asparaginase with methotrexate and dexamethasone (AspaMetDex regimen) in patients with refractory or relapsing extranodal NK/T-cell lymphoma, a phase 2 study. Blood 117(6):1834–1839. doi:10.1182/blood-2010-09-307454
Chan JK, Jaffe ES, Ralfkiaer E (2001) Extranodal NK/T-cell lymphoma, nasal type. In: Jaffe ES, Harris NL, Stein H, Vardiman JW (eds) WHO classification of tumours of haematopoietic and lymphoid tissues. IARC, Lyon, pp 204–207
Kim TM, Lee SY, Jeon YK, Ryoo BY, Cho GJ, Hong YS, Kim HJ, Kim SY, Kim CS, Kim S, Kim JS, Sohn SK, Song HH, Lee JL, Kang YK, Yim CY, Lee WS, Yuh YJ, Kim CW, Heo DS (2008) Clinical heterogeneity of extranodal NK/T-cell lymphoma, nasal type: a national survey of the Korean Cancer Study Group. Ann Oncol 19(8):1477–1484. doi:10.1093/annonc/mdn147
Shipp MA, Harrington DP, Anderson JR, Armitage JO, Bonadonna G, Brittinger G, Cabanillas F, Canellos GP, Coiffier B, Connors JM, Cowan RA, Crowther D, Dahlberg S, Engelhard M, Fisher RI, Gisselbrecht C, Horning SJ, Lepage E, Lister TA, Meerwaldt JH, Monterrat E, Nissen NI, Oken MM, Peterson BA, Tondini C, Velasquez WA, Yeap BY (1993) A predictive model for aggressive non-Hodgkin’s lymphoma. The International Non-Hodgkin’s Lymphoma Prognostic Factors Project. N Engl J Med 329(14):987–994
Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, Coiffier B, Fisher RI, Hagenbeek A, Zucca E, Rosen ST, Stroobants S, Lister TA, Hoppe RT, Dreyling M, Tobinai K, Vose JM, Connors JM, Federico M, Diehl V, International Harmonization Project on L (2007) Revised response criteria for malignant lymphoma. J Clin Oncol 25(5):579–586. doi:10.1200/JCO.2006.09.2403
Kaplan EL, Meier P (1958) Nonparametric estimation from incomplete observation. J Am Stat Assoc 53:457–481
Cox DR (1972) Regression models and life-table. J R Stat Soc Ser B 34:187–120
Nagafuji K, Fujisaki T, Arima F, Ohshima K (2001) l-asparaginase induced durable remission of relapsed nasal NK/T-cell lymphoma after autologous peripheral blood stem cell transplantation. Int J Hematol 74(4):447–450
Lin N, Song Y, Zheng W, Tu M, Xie Y, Wang X, Ping L, Ying Z, Zhang C, Deng L, Liu W, Zhu J (2013) A prospective phase II study of l-asparaginase- CHOP plus radiation in newly diagnosed extranodal NK/T-cell lymphoma, nasal type. J Hematol Oncol 6(1):44. doi:10.1186/1756-8722-6-44
Yong W, Zheng W, Zhu J, Zhang Y, Wang X, Xie Y, Lin N, Xu B, Lu A, Li J (2009) l-asparaginase in the treatment of refractory and relapsed extranodal NK/T-cell lymphoma, nasal type. Ann Hematol 88(7):647–652. doi:10.1007/s00277-008-0669-3
Jaccard A, Petit B, Girault S, Suarez F, Gressin R, Zini JM, Coiteux V, Larroche C, Devidas A, Thieblemont C, Gaulard P, Marin B, Gachard N, Bordessoule D, Hermine O (2009) l-asparaginase-based treatment of 15 western patients with extranodal NK/T-cell lymphoma and leukemia and a review of the literature. Ann Oncol 20(1):110–116. doi:10.1093/annonc/mdn542
Kim BS, Kim TY, Kim CW, Kim JY, Heo DS, Bang YJ, Kim NK (2003) Therapeutic outcome of extranodal NK/T-cell lymphoma initially treated with chemotherapy—result of chemotherapy in NK/T-cell lymphoma. Acta Oncol 42(7):779–783
Suzuki R, Suzumiya J, Yamaguchi M, Nakamura S, Kameoka J, Kojima H, Abe M, Kinoshita T, Yoshino T, Iwatsuki K, Kagami Y, Tsuzuki T, Kurokawa M, Ito K, Kawa K, Oshimi K (2010) Prognostic factors for mature natural killer (NK) cell neoplasms: aggressive NK cell leukemia and extranodal NK cell lymphoma, nasal type. Ann Oncol 21(5):1032–1040. doi:10.1093/annonc/mdp418
Kim BS, Kim DW, Im SA, Kim CW, Kim TY, Yoon SS, Heo DS, Bang YJ, Park S, Kim BK, Kim NK (2009) Effective second-line chemotherapy for extranodal NK/T-cell lymphoma consisting of etoposide, ifosfamide, methotrexate, and prednisolone. Ann Oncol 20(1):121–128. doi:10.1093/annonc/mdn551
Kim TM, Kim D-W, Kang Y-K, Song H-S, Kim HJ, Kim BS, Heo DS (2013) A phase II trial of ifosfamide, methotrexate, etoposide, and prednisolone (IMEP) for previously untreated stage I, II extranodal natural killer/T-cell lymphoma, nasal type (NTCL): a multicenter study of the Korean Cancer Study Group. ASCO Meet Abstr 31:8521
Yang L, Liu H, Xu XH, Wang XF, Huang HM, Shi WY, Jiang SH (2013) Retrospective study of modified SMILE chemotherapy for advanced-stage, relapsed, or refractory extranodal natural killer (NK)/T cell lymphoma, nasal type. Med Oncol 30(4):720. doi:10.1007/s12032-013-0720-7
Cheson BD, Horning SJ, Coiffier B, Shipp MA, Fisher RI, Connors JM, Lister TA, Vose J, Grillo-Lopez A, Hagenbeek A, Cabanillas F, Klippensten D, Hiddemann W, Castellino R, Harris NL, Armitage JO, Carter W, Hoppe R, Canellos GP (1999) Report of an international workshop to standardize response criteria for non-Hodgkin's lymphomas. NCI Sponsored International Working Group. J Clin Oncol 17(4):1244–1253
Schrey D, Borghorst S, Lanvers-Kaminsky C, Hempel G, Gerss J, Moricke A, Schrappe M, Boos J (2010) Therapeutic drug monitoring of asparaginase in the ALL-BFM 2000 protocol between 2000 and 2007. Pediatr Blood Cancer 54(7):952–958. doi:10.1002/pbc.22417
Acknowledgments
This work was supported by a grant from the Innovative Research Institute for Cell Therapy, Republic of Korea (A062260). We thank Juyoun Kim for the assistance with clinical data collection.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, M., Kim, T.M., Kim, K.H. et al. Ifosfamide, methotrexate, etoposide, and prednisolone (IMEP) plus l-asparaginase as a first-line therapy improves outcomes in stage III/IV NK/T cell-lymphoma, nasal type (NTCL). Ann Hematol 94, 437–444 (2015). https://doi.org/10.1007/s00277-014-2228-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-014-2228-4