Abstract
Few studies have evaluated granulocyte colony-stimulating factor (G-CSF) priming in elderly patients with intensively treated acute myeloid leukemia (AML), and no data are available for genetically defined AML subgroups. We provide long-term results (median follow-up 7.6 years) of a randomized trial in which 183 patients (median age 67 years) received G-CSF prior to (G-CSF priming) or after two cycles of induction chemotherapy. CR rates with G-CSF priming and G-CSF post-chemotherapy were comparable (57 vs. 67 %, p = 0.153), with overall survival (OS) probabilities of 14 vs. 17 % at 10 years. Induction mortality was significantly higher with G-CSF priming (23 vs. 10 %, p = 0.015), primarily in normal karyotype (NK) AML. In this subgroup, a trend for better relapse-free survival (RFS) was observed with G-CSF priming (44 vs. 22 % at 10 years, p = 0.074) but did not translate into an OS benefit. G-CSF priming had no impact on AML with FLT3-ITD and NPM mutations and did not improve outcome in patients with adverse cytogenetics. In a landmark analysis, late consolidation with autologous stem cell transplantation or a second consolidation cycle significantly improved RFS compared with one consolidation cycle (21.0 vs. 12.8 months, p = 0.046). Future studies on G-CSF priming should be restricted to NK AML and used only in post-remission therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cytotoxic treatment for acute myeloid leukemia (AML) has not changed substantially since the 1980s and relies on the combination of cytarabine and an anthracycline (e.g., the 3 + 7 regimen); efforts to improve results by adding additional cytotoxic agents or increasing the intensity of induction chemotherapy have had no reproducible benefit [1]. Administration of the myeloid growth factors granulocyte colony-stimulating factor (G-CSF) or granulocyte-macrophage (GM)-CSF prior to and concurrently with induction chemotherapy (“priming”) has been explored based on preclinical data suggesting sensitization of leukemic stem and progenitor cells to the cytotoxic effects of chemotherapy [2–7]. Administration of CSF following induction and consolidation chemotherapy reduces the duration of neutropenia, frequency of hospitalization, and requirement for antibiotics, as shown in numerous randomized trials (reviewed in [8], [9]). While these studies had no impact on overall survival, growth factor support may benefit elderly patients in particular, given the high rate of morbidity and mortality associated with intensive chemotherapy in this population.
Data on the use of G-CSF priming specifically in elderly AML patients are limited with only two [10], [11] of seven published randomized trials [12–16], focusing on patients above 60 years of age. In addition to age, these studies differed in their inclusion of de novo vs. secondary and untreated vs. refractory or relapsed AML. The study reported by Estey et al. included patients with relapsed or refractory AML as well as advanced MDS and demonstrated an increased CR rate by G-CSF priming [10]. Follow-up was short, with no improvement of RFS at 6 months. G-CSF priming was also associated with an increased CR rate in the study by Amadori et al. but failed to improve either RFS or OS [11]. It was not evaluated whether subgroups of patients defined by molecular genetic parameters showed a differential effect of G-CSF priming, as nearly half of the patients did not have evaluable cytogenetic data. Importantly, all but one [11] of the seven trials compared concurrent use of G-CSF and chemotherapy with placebo or no growth factor treatment.
The present prospective, randomized clinical trial was initiated in 1999 and was designed to compare two strategies utilizing G-CSF as an adjunct to successive cycles of intensive induction and consolidation chemotherapy in elderly patients with newly diagnosed AML. G-CSF was administered either as supportive therapy, starting after each induction cycle, or as a priming agent given prior to and continuing throughout and after chemotherapy. In addition, we assessed the impact of G-CSF administration on the ability to collect peripheral blood stem cells for autologous stem cell transplantation (ASCT) as late consolidation and compared ASCT with reduced intensity Flag-Ida consolidation in terms of remission duration and survival. Lastly, we examined the impact of molecular genetic aberrations (FLT3 internaI tandem duplication (FLT3-ITD) and NPM1 mutations) and cytogenetics-based risk classification on treatment outcome in the setting of the two G-CSF administration schedules. We present here the long-term outcome with a median follow-up of surviving patients of more than 7 years.
Patients and methods
Patients
From January 2000 to August 2005, 196 previously untreated patients older than 60 years with a confirmed diagnosis of AML by current WHO criteria were enrolled in this prospective, randomized multicenter phase III trial. All subtypes of AML except acute promyelocytic leukemia were eligible. Patients were required to have an ECOG performance status of <3 and adequate organ function of kidney, liver, lung, and heart.
The trial was approved by the ethics committee of the Johann Wolfgang-Goethe University (Frankfurt, Germany) registered at ClinicalTrials.gov (NCT00199147). All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975 as revised in 2008. Patients were enrolled in four centers (Frankfurt, Hannover, Essen-Werden, and Mannheim).
Study objectives
The primary objective of the study was to compare the efficacy of intensive induction therapy with cytarabine, idarubicin, and etoposide (IdAV) given in parallel (G-CSF priming) and followed by G-CSF vs. the same IdAV chemotherapy followed by G-CSF (G-CSF post-chemo) to induce complete remission in elderly patients with newly diagnosed de novo or secondary AML. Secondary objectives included (a) comparison of the antileukemic efficacy of the IdAV regimen ± G-CSF priming in de novo AML vs. secondary AML, (b) assessment of the influence of prior G-CSF priming vs. no priming on PBSC mobilization for ASCT after consolidation therapy with dose-reduced fludarabine, cytarabine, and idarubicin (mini-Flag-Ida) chemotherapy for ASCT, and (c) investigation of the feasibility of high-dose chemotherapy with autologous peripheral stem cell (PBSC) support as late consolidation therapy in elderly patients.
Study design and treatment
Treatment schedules and the overall protocol design are summarized in Fig. 1. After informed consent, patients were randomly allocated in a 1:1 ratio to one of two induction treatments, consisting of an identical chemotherapy but differing in the schedule of G-CSF administration. To balance treatment assignment across known risk factors, patients were stratified according to the diagnosis of either (a) de novo AML or (b) AML secondary to chemo-/radiotherapy or an antecedent myelodysplastic syndrome. A separate randomization list was maintained for each stratum. Cycle 1 consisted of cytarabine 100 mg/m2 given by continuous infusion on days 1–7, idarubicin 10 mg/m2 IV on days 2, 4, and 6, and etoposide 100 mg/m2 IV on days 3–7 (IdAV I). Irrespective of whether the patients had already achieved a complete remission (CR) after the first induction course, a moderately dose-reduced cycle 2 was administered (cytarabine 100 mg/m2 given by continuous infusion on days 1–5, idarubicin 10 mg/m2 on days 1 and 3, etoposide 100 mg/m2 on days 1–5; IdAV II). G-CSF (Filgrastim, Neupogen®, Amgen) was given subcutaneously in one daily dose of 5 μg/kg body weight beginning one day before chemotherapy (day 0) in the G-CSF priming group and on the day after chemotherapy (day 8 of cycle 1 and day 6 of cycle 2, respectively) in the G-CSF post-chemo group and was continued until the absolute neutrophil count was 1,000/μL for three consecutive days. The administration of G-CSF was postponed or interrupted in the event of leukocytosis of more than 50,000/μL white blood cells.
Patients were randomized to receive two different schedules of G-CSF in conjunction with the same induction regimen. In the priming group, G-CSF was started 1 day before induction and patients assigned to growth factor support received G-CSF starting 1 day after the last dose of chemotherapy (G-CSF post-chemo). In both groups, G-CSF was continued until neutrophil recovery. Patients achieving a CR were scheduled to receive an early consolidation cycle consisting of an age-adapted Flag-Ida regimen, with G-CSF started on day 1 of the cycle irrespective of the original randomization. G-CSF was continued with the intent of mobilizing PBSC for subsequent ASCT. Patients without sufficient PBSC were scheduled to receive late consolidation with a second mini-Flag-Ida cycle
Patients in CR after two induction cycles who were considered to tolerate intensive post-remission therapy received a third chemotherapy cycle (early consolidation) consisting of an age-adapted, reduced intensity Flag-Ida regimen (mini-Flag-Ida) to improve remission quality. This consisted of fludarabine 30 mg/m2 on days 1–4, cytarabine 600 mg/m2 on days 1–4, idarubicin 8 mg/m2 on days 1 and 3, and G-CSF 5 μg/kg body weight beginning on day 0, irrespective of the patient′s initial randomization to either G-CSF priming or G-CSF post-chemo. During the regeneration phase following this treatment cycle, mobilization of PBSC was assessed and PBSC apheresis was to be performed when CD34+ cells exceeded 1 × 104/mL, with a goal of obtaining sufficient PBSC for at least two transplants of 2 × 106 CD34+ cells/kg body weight. Patients who failed to collect sufficient numbers of PBSC were scheduled to receive a second mini-Flag-Ida cycle.
Cytogenetic and molecular genetic analysis
At diagnosis, blood and bone marrow samples were examined for cytogenetic abnormalities with the use of standard banding techniques and classified according to the International System for Human Cytogenetic Nomenclature [17]. Cytogenetic abnormalities were grouped according to published criteria as core binding factor (CBF) AML, normal karyotype (NK) AML, and monosomal karyotype (MK) negative and MK positive [18]. If sufficient genomic DNA was available, mutations in NPM1 [19] and FLT3 [20] were retrospectively determined as previously described to facilitate regrouping according to the ELN classification [21].
Statistical analysis
Remission status was assessed after recovery from the second induction cycle according to the National Cancer Institute criteria [22]. Relapse-free survival (RFS) for patients achieving CR was calculated from date of CR to date of relapse, death in remission, or date when patient was last known to be in remission. Overall survival (OS) was defined from date of randomization to date of death or last follow-up. Survival curves were constructed according to the method of Kaplan and Meier [23], and differences in RFS and OS were assessed by the log-rank test. Patient characteristics and CR rates were compared using the Pearson χ 2 test for binary variables and the Student’s t test for continuous variables. Multivariate analysis was performed by Cox regression.
Results
Patient characteristics and treatment with G-CSF
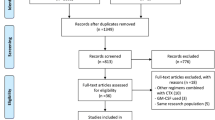
Between January 2000 and August 2005, 183 of 196 enrolled patients were eligible and started study treatment (CONSORT flow diagram, Online Resource 1). Demographics of patients randomly assigned to receive either G-CSF priming (n = 91) or G-CSF post-chemo (n = 92) are shown in Table 1. Patient characteristics were well balanced between both arms, except for fewer patients in the G-CSF priming group with CBF leukemias or classified as having a favorable genetic risk according to European LeukemiaNet guideline [21] than in the G-CSF post-chemo group.
In the priming group, G-CSF was administered for an average of 23.1 ± 0.9 days (mean±SEM) during induction I and 21.9 ± 1.1 days during induction II. In the G-CSF post-chemo group, G-CSF was given for 15.6 ± 0.6 (p < 0.001 vs. G-CSF priming group) and 15.8 ± 1.2 days (p = 0.001), respectively.
Response to induction therapy
Complete remission after one or two courses of induction chemotherapy was achieved in 114 of 183 patients (62 %). The causes of induction failure included resistant disease (29 %), death during hypoplasia (7 %), and unknown reasons (1 %). Responses were similar in the G-CSF priming and the G-CSF post-chemo group, with CR rates of 57 and 67 %, respectively (p = 0.153).
G-CSF priming was associated with a significantly increased induction mortality (23 vs. 10 %, p = 0.014). During induction I, we observed a higher rate of severe mucositis and significantly more life-threatening infectious complications in the G-CSF priming arm (41 vs. 28 %, p = 0.04; Tables 2 and 3). Importantly, time to recovery of neutrophils after induction cycle 1 (20.5 vs. 21.8 days) and cycle 2 (16.3 vs. 14.9 days) did not differ between both treatment groups. Induction mortality was highest during the first cycle of chemotherapy (28/30 patients).
Relapse and survival
Of the 114 complete responders to induction therapy, three patients died while in CR and 85 patients due to recurrence of leukemia, 40 out of 52 patients (77 %) in the G-CSF priming vs. 48 out of 62 patients (77 %) in the G-CSF post-chemo group (p = 0.95). AML relapse occurred predominantly within the first 2 years after achieving a CR. However, five out of seven late relapses were observed in the G-CSF post-chemo group.
The probability of RFS at 5 and 10 years in the G-CSF priming vs. G-CSF post-chemo groups was estimated to be 25 and 25 % vs. 22 and 14 %, respectively, with no significant differences between both treatment arms (Fig. 2a, p = 0.407). OS survival rates at 5 and 10 years in the G-CSF priming vs. G-CSF post-chemo group were calculated to be 15 and 14 % vs. 20 and 17 %, respectively (Fig. 2b, p = 0.205). The median follow-up of the surviving patients was 7.6 (range, 4.8–11.4) years.
Kaplan–Meier analysis of a relapse-free survival and b overall survival according to the assigned treatment arm. The probability of RFS with G-CSF priming was 25 % at 5 and 10 years vs. 22 and 14 % in the G-CSF post-chemo group (p = 0.407, log-rank test). All events observed beyond 2 years after CR were relapses (n = 7), five of which occurring in the G-CSF post-chemo group. (Panel A) Probability of OS with G-CSF priming was 15 and 14 % at 5 and 10 years vs. 20 and 17 % in the G-CSF post-chemo group (p = 0.205, log-rank test). The median follow-up of the surviving patients was 7.6 (range, 4.8–11.4) years (Panel B)
Effect of G-CSF priming in genetically defined subgroups
In younger AML patients, priming with G-CSF was shown to confer an RFS benefit in standard risk AML [13]. Thus, we evaluated the impact of G-CSF priming in genetically defined subgroups with well proven prognostic value, i.e., CBF AML, AML with NK, various cytogenetic abnormalities which are MK negative, and a MK positive [18]. Pretreatment cytogenetic results were available for all but six patients (97 %), and 49 % of patients presented with abnormal cytogenetics classified as MK negative or positive [18].
Among patients with NK AML, induction mortality was significantly higher with G-CSF priming than in the G-CSF post-chemo arm (25 vs. 2 %, p = 0.003). The ED rate in the two treatment arms did not differ significantly in patients with aberrant cytogenetics (MK positive or negative), and no case of ED was observed in patients with CBF leukemia. On the other hand, G-CSF priming conferred a trend to a better RFS (p = 0.074) only among patients with an NK (Fig. 3). In the G-CSF priming and post-chemo arms, probability of RFS was 44 and 26 % at 5 years, respectively, and 44 and 22 % at 10 years. To refine the impact of G-CSF in the large cohort of NK AML, patient samples were retrospectively analyzed for FLT3-ITD and NPM1 mutations to allow for reassignment to the recently published ELN genetic risk groups [21] (Table 1). G-CSF priming did not influence RFS or OS, irrespective of the ELN genetic risk group (data not shown).
Prognostic factors for achievement of CR and survival
When correlating the cytogenetic risk with the antileukemic effect of induction therapy, CR rates in CBF AML, NK AML, and MK negative and MK positive AML with aberrant cytogenetics were 88, 71, 58, and 38 %, respectively. Patients with an MK had a significantly inferior CR rate compared to all others (p = 0.002), but the described differences were also significant between MK positive or MK negative AML with aberrant cytogenetics vs. CBF or NK AML (p = 0.003). A binary logistic regression analysis for independent risk factors of induction mortality identified a monosomal karyotype (OR 6.78, 95 % CI, 1.52–30.28), G-CSF priming (OR 3.95, 95 % CI, 1.43–10.87), and age ≥75 years (OR 19.80, 95 % CI, 2.39–164.36). Gender, de novo vs. secondary AML and cytogenetics according to the ELN classification did not contribute further prognostic information.
RFS of patients with CBF or NK AML was significantly superior to RFS in patients with an abnormal karyotype (median RFS 13.1 vs. 7.0 months; p < 0.001) (Fig. 4a). Accordingly, patients with CBF or NK AML had a significantly superior OS compared to patients with MK negative or positive aberrant cytogenetics (median OS 10.2 vs. 18.4 months; p < 0.001) (Fig. 4b). The respective OS probabilities were 38, 29, 6, and 6 % at 5 years and 38, 27, 0, and 0 % at 10 years.
Impact of cytogenetic risk group as published by Breems et al. [18] on outcome by Kaplan–Meier analysis. Patients with cytogenetic aberrations other than CBF leukemia had an extremely poor relapse-free (Panel A) and overall survival (Panel B) irrespective of whether they were MK positive (median RFS 6.9 months, OS 5.6 months) or MK negative (median RFS 9.4 months, OS 11.8 months). There was no significant difference between CBF and NK AML in terms of RFS (102.4 vs. 15.2 months) and OS (29.8 vs. 13.3 months). RFS of patients with CBF leukemias or NK AML was significantly superior to RFS in patients with an abnormal karyotype (median RFS 13.1 vs. 7.0 months; p < 0.001). OS of patients with CBF leukemias or NK AML was significantly superior to OS of patients with an abnormal karyotype (median OS 10.2 vs. 18.4 months; p < 0.001)
Post-remission therapy
Mini-Flag-Ida consolidation was administered to 75 of the 114 CR patients (Suppl. Figure 1) considered to tolerate intensive post-remission therapy. Following mini-Flag-Ida I, collection of at least 2 × 106 CD34+ PBSC/kg was feasible in 36 out of 66 patients in whom mobilization of CD34+ cells was monitored corresponding to 45 % of patients in the G-CSF priming and 63 % of patients in G-CSF post-chemo group (p = 0.15). Of these, 19 patients actually proceeded to ASCT, i.e., 53 % of eligible patients. High-dose therapy consisted of thiotepa and melphalan (n = 8), melphalan (n = 5), busulphan and cyclophosphamide (n = 4), busulphan and melphalan (n = 1), and total body irradiation and cyclophosphamide (n = 1). ASCT proved to be safe without transplantation-related deaths.
Patients who did not undergo stem cell mobilization (n = 9) or failed to collect sufficient stem cell numbers (n = 30) were eligible for a second course of mini-Flag-Ida, which was actually given to 15 patients. Three additional patients received an allogeneic stem cell transplantation. The impact of any late consolidation therapy was assessed by a landmark analysis. Only patients with a CR lasting 114 days or more were included in the control cohort (n = 34) in order to account for the median time from CR to mini-Flag-Ida II or ASCT in the patient group who actually received a late consolidation (n = 34). A late consolidation resulted in a significantly superior median RFS compared to no further treatment (21.0 vs. 12.8 months, p = 0.046). The probabilities of RFS were calculated to 35 vs. 21 % at 5 years and 31 vs. 17 % at 10 years in the mini-Flag-Ida II or ASCT vs. control group (Fig. 5). Compared to mini-Flag-Ida II, ASCT was not associated with an improved overall or relapse-free survival.
Landmark analysis of RFS in patients receiving late consolidation with ASCT or mini-Flag-Ida II in comparison with patients receiving only one consolidation cycle. Probabilities of RFS were 35 vs. 21 % at 5 years and 31 vs. 17 % at 10 years in patients receiving two vs. one cycle of post-remission therapy. Prolonged post-remission therapy significantly improved the median RFS (21.0 vs. 12.8 months, p = 0.046)
Discussion
In our study, G-CSF priming during induction therapy significantly increased the early death (ED) rate and failed to improve survival in elderly patients with previously untreated AML. We further demonstrate that G-CSF priming did not adversely affect the ability to collect autologous stem cells and shows that patients significantly benefitted from a second post-remission therapy with either ASCT or Flag-Ida as late consolidation. As a caveat, the landmark analysis does not take into account the reason why patients did not receive a late consolidation. Therefore, a selection bias cannot be excluded.
In contrast to the only other study comparable in design, patient characteristics and schedules of G-CSF administration [11], we provide complete data on cytogenetics for 97 % and on NPM1 or FLT3-ITD mutations for 86 % of our patients. In our study, the high ED rate associated with G-CSF priming was most pronounced in patients with a normal karyotype. To minimize potential confounding factors, we calculated the risk of ED within 60 days after start of induction chemotherapy for each individual patient according to a recently published prognostic scoring system for elderly AML patients [24] resulting in 22 vs. 19 % in the G-CSF priming and G-CSF post-chemo group (p=ns). Compared to this projected probability of early mortality, the ED rate was unexpectedly low (10 %) in the G-CSF post-chemo group. This finding is potentially attributable to the use of G-CSF as supportive therapy even though this has not been a consistent finding in the published studies [25]. Despite the higher ED rate, we observed a trend for improved RFS in the G-CSF priming arm, but only among patients with NK AML. This is consistent with the concept that G-CSF priming may enhance the antileukemic activity of chemotherapy during induction, but does not counterbalance the excess toxicity and mortality incurred during induction cycle I.
In patients below 60 years of age, Löwenberg et al. similarly reported improved RFS at 4 years with G-CSF priming despite increased induction mortality [13]. The lower relapse rate and improved RFS and OS were observed in the subset of patients with standard risk AML, a subgroup likely to encompass primarily patients with normal cytogenetics, as in our study. Conversely, no improvement of DSF or OS was demonstrated in a subsequent study of G-CSF priming in younger patients with similar characteristics who received standard 7 + 3 induction chemotherapy [16]. However, 5-year RFS and OS were superior in a subset of patients who received G-CSF priming in conjunction with high-dose cytarabine. None of the other G-CSF priming studies demonstrated differences in terms of survival endpoints, including the Amadori trial [11] which used similar G-CSF schedules as in our study, i.e., priming vs. supportive.
Thomas et al. recently showed that GM-CSF priming conferred an RFS benefit to AML patients up to 50 years of age and with predominantly unfavorable cytogenetic and molecular genetic markers [26]. This differs from the results of our study in which G-CSF priming clearly did not improve any outcome parameter in patients with adverse risk cytogenetics or normal karyotype AML with FLT3-ITD. Thus, neither our study nor that of Löwenberg et al. [13] are consistent with the concept that G-CSF priming improves antileukemic efficacy across all AML subsets, as suggested by the observation that G-CSF stimulates cycling and thus sensitization of quiescent LSC to cytarabine in an AML xenograft model [7]. Rather, our data and the reports by the HOVON/SAKK study group [13], [16] show an association between the effect of priming, risk group and cytarabine dose, providing circumstantial evidence that patients with standard risk cytogenetics, who benefit most from high-dose cytarabine, could derive the greatest benefit from G-CSF priming. However, this necessitates avoiding the excess induction mortality associated with G-CSF priming that was observed both in our study and the trial reported by Löwenberg, as well as in a murine xenotransplantation model [7]. Conceptually, this could be achieved by delaying G-CSF priming until a second induction cycle and/or high-dose cytarabine-based consolidation cycles, while administering the first induction cycle without growth factors or with G-CSF administered as supportive therapy after chemotherapy.
Another potential concern of G-CSF priming is a deleterious effect on normal hematopoietic stem cells that might also be sensitized to the cytotoxicity of chemotherapy. This could become particularly relevant in the setting of ASCT, which has been employed repeatedly as an alternative to standard high-dose cytarabine-based consolidation in elderly AML patients [27–31]. As judged from our data on mobilization of PBSC and the encouraging long-term survival data following late consolidation with ASCT, our results provide no evidence that number or function of stem cells is compromised by G-CSF priming. Our protocol recommended ASCT as the preferred late consolidation for all patients with sufficient numbers of collected PBSCs, but the reasons why patients did not proceed to ASCT or receive Flag-Ida as final consolidation were not documented. These results are in line with reports from two other large prospective trials [30] [32] in which only a minority (12 %) of enrolled patients actually underwent ASCT.
The value of intensive treatment of elderly AML patients and the decision algorithm are controversial, as results are poor in the vast majority of patients. We present here the long-term outcome data from a cohort of 183 AML patients with a median age of 67 years, 30 % of whom were 70 years or older. Cytogenetic risk classification rather than age was the principal determinant of survival with our intensive treatment regimen. Patients with normal cytogenetics or a CBF AML comprised 50 % of patients in our study and had a 10 year survival probability of 27 and 38 %, respectively. A monosomal karyotype conveyed a particularly unfavorable prognosis, extending the limited data available for elderly patients [33], [34].
In conclusion, our study demonstrates that elderly patients with newly diagnosed AML have no survival benefit from G-CSF priming if this is started already with the first induction cycle. This is due to excess mortality primarily in patients with NK AML. Superior RFS in this subset of patients suggests that further exploration of G-CSF priming in elderly patients is warranted only if started during post-remission therapy, particularly with high-dose cytarabine-based consolidation cycles. Conversely, G-CSF priming should not be offered to elderly patients with unfavorable cytogenetic features, as there is no evidence that priming improves the extremely poor outcome associated with intensive chemotherapy.
References
Rowe JM, Tallman MS (2010) How I treat acute myeloid leukemia. Blood 116:3147–3156
Bhalla K, Birkhofer M, Arlin Z, Grant S, Lutzky J, Graham G (1988) Effect of recombinant GM-CSF on the metabolism of cytosine arabinoside in normal and leukemic human bone marrow cells. Leukemia 2:810–813
Miyauchi J, Kelleher CA, Wang C, Minkin S, McCulloch EA (1989) Growth factors influence the sensitivity of leukemic stem cells to cytosine arabinoside in culture. Blood 73:1272–1278
Smith MA, Singer CR, Pallister CJ, Smith JG (1995) The effect of haemopoietic growth factors on the cell cycle of AML progenitors and their sensitivity to cytosine arabinoside in vitro. Br J Haematol 90:767–773
Braess J, Voss S, Jahns-Streubel G, Schoch C, Haferlach T, Kern W et al (2000) The pharmacodynamic basis for the increased antileukaemic efficacy of cytosine arabinoside-based treatment regimens in acute myeloid leukaemia with a high proliferative activity. Br J Haematol 110:170–179
Beekman R, Touw IP (2010) G-CSF and its receptor in myeloid malignancy. Blood 115:5131–5136
Saito Y, Uchida N, Tanaka S, Suzuki N, Tomizawa-Murasawa M, Sone A et al (2010) Induction of cell cycle entry eliminates human leukemia stem cells in a mouse model of AML. Nat Biotechnol 28:275–280
Ottmann OG, Bug G, Krauter J (2007) Current status of growth factors in the treatment of acute myeloid and lymphoblastic leukemia. Semin Hematol 44:183–192
Heuser M, Zapf A, Morgan M, Krauter J, Ganser A (2011) Myeloid growth factors in acute myeloid leukemia: systematic review of randomized controlled trials. Ann Hematol 90:273–281
Estey EH, Thall PF, Pierce S, Cortes J, Beran M, Kantarjian H et al (1999) Randomized phase II study of fludarabine + cytosine arabinoside + idarubicin +/− all-trans retinoic acid +/− granulocyte colony-stimulating factor in poor prognosis newly diagnosed acute myeloid leukemia and myelodysplastic syndrome. Blood 93:2478–2484
Amadori S, Suciu S, Jehn U, Stasi R, Thomas X, Marie JP et al (2005) Use of glycosylated recombinant human G-CSF (lenograstim) during and/or after induction chemotherapy in patients 61 years of age and older with acute myeloid leukemia: final results of AML-13, a randomized phase-3 study. Blood 106:27–34
Ohno R, Naoe T, Kanamaru A, Yoshida M, Hiraoka A, Kobayashi T et al (1994) A double-blind controlled study of granulocyte colony-stimulating factor started two days before induction chemotherapy in refractory acute myeloid leukemia. Kohseisho Leukemia Study Group. Blood 83:2086–2092
Löwenberg B, van Putten W, Theobald M, Gmur J, Verdonck L, Sonneveld P et al (2003) Effect of priming with granulocyte colony-stimulating factor on the outcome of chemotherapy for acute myeloid leukemia. N Engl J Med 349:743–752
Buchner T, Berdel WE, Hiddemann W (2004) Priming with granulocyte colony-stimulating factor—relation to high-dose cytarabine in acute myeloid leukemia. N Engl J Med 350:2215–2216
Milligan DW, Wheatley K, Littlewood T, Craig JI, Burnett AK (2006) Fludarabine and cytosine are less effective than standard ADE chemotherapy in high-risk acute myeloid leukemia, and addition of G-CSF and ATRA are not beneficial: results of the MRC AML-HR randomized trial. Blood 107:4614–4622
Pabst T, Vellenga E, van Putten W, Schouten HC, Graux C, Vekemans MC et al (2012) Favorable effect of priming with granulocyte colony-stimulating factor in remission induction of acute myeloid leukemia restricted to dose escalation of cytarabine. Blood 119:5367–5373
Mitelman F (1995) An international system for human cytogenetic nomenclature. S. Karger, Basel
Breems DA, Van Putten WL, De Greef GE, Van Zelderen-Bhola SL, Gerssen-Schoorl KB, Mellink CH et al (2008) Monosomal karyotype in acute myeloid leukemia: a better indicator of poor prognosis than a complex karyotype. J Clin Oncol 20082(6):4791–4797
Dohner K, Schlenk RF, Habdank M, Scholl C, Rucker FG, Corbacioglu A et al (2005) Mutant nucleophosmin (NPM1) predicts favorable prognosis in younger adults with acute myeloid leukemia and normal cytogenetics: interaction with other gene mutations. Blood 106:3740–3746
Frohling S, Schlenk RF, Breitruck J, Benner A, Kreitmeier S, Tobis K et al (2002) Prognostic significance of activating FLT3 mutations in younger adults (16 to 60 years) with acute myeloid leukemia and normal cytogenetics: a study of the AML Study Group Ulm. Blood 100:4372–4380
Dohner H, Estey EH, Amadori S, Appelbaum FR, Buchner T, Burnett AK et al (2010) Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood 115:453–474
Cheson BD, Cassileth PA, Head DR, Schiffer CA, Bennett JM, Bloomfield CD et al (1990) Report of the National Cancer Institute-sponsored workshop on definitions of diagnosis and response in acute myeloid leukemia. J Clin Oncol 8:813–819
Kaplan E, Meier P (1958) Nonparametric estimation of from incomplete observations. J Am Stat Assoc 53:457–481
Krug U, Rollig C, Koschmieder A, Heinecke A, Sauerland MC, Schaich M et al (2010) Complete remission and early death after intensive chemotherapy in patients aged 60 years or older with acute myeloid leukaemia: a web-based application for prediction of outcomes. Lancet 376:2000–2008
Gurion R, Belnik-Plitman Y, Gafter-Gvili A, Paul M, Vidal L, Ben-Bassat I et al (2012) Colony-stimulating factors for prevention and treatment of infectious complications in patients with acute myelogenous leukemia. Cochrane Database Syst Rev 6, CD008238
Thomas X, Raffoux E, Botton S, Pautas C, Arnaud P, de Revel T et al (2007) Effect of priming with granulocyte-macrophage colony-stimulating factor in younger adults with newly diagnosed acute myeloid leukemia: a trial by the Acute Leukemia French Association (ALFA) Group. Leukemia 21:453–461
Cahn JY, Labopin M, Mandelli F, Goldstone AH, Eberhardt K, Reiffers J et al (1995) Autologous bone marrow transplantation for first remission acute myeloblastic leukemia in patients older than 50 years: a retrospective analysis of the European Bone Marrow Transplant Group. Blood 85:575–579
Olivieri A, Capelli D, Montanari M, Brunori M, Massidda D, Poloni A et al (2001) Very low toxicity and good quality of life in 48 elderly patients autotransplanted for hematological malignancies: a single center experience. Bone Marrow Transplant 27:1189–1195
Ferrara F, Venditti A, Carellajr AM, Cantore N, Buccisano F, Tamburini A et al (2002) Autologous stem-cell transplantation for patients with acute myeloid leukemia aged over 60 years. Eur J Haematol 69:200–204
Oriol A, Ribera JM, Esteve J, Guardia R, Brunet S, Bueno J et al (2004) Feasibility and results of autologous stem cell transplantation in de novo acute myeloid leukemia in patients over 60-years-old. Results of the CETLAM AML-99 protocol. Haematologica 89:791–800
Ferrara F, Palmieri S, Annunziata M, Viola A, Pocali B, Califano C et al (2004) Continuous infusion idarubicin and oral busulfan as conditioning for patients with acute myeloid leukemia aged over 60 years undergoing autologous stem cell transplantation. Bone Marrow Transplant 34:573–576
Thomas X, Suciu S, Rio B, Leone G, Broccia G, Fillet G et al (2007) Autologous stem cell transplantation after complete remission and first consolidation in acute myeloid leukemia patients aged 61–70 years: results of the prospective EORTC-GIMEMA AML-13 study. Haematologica 92:389–396
Perrot A, Luquet I, Pigneux A, Mugneret F, Delaunay J, Harousseau JL et al (2011) Dismal prognostic value of monosomal karyotype in elderly patients with acute myeloid leukemia: a GOELAMS study of 186 patients with unfavorable cytogenetic abnormalities. Blood 118:679–685
Medeiros BC, Othus M, Fang M, Roulston D, Appelbaum FR (2010) Prognostic impact of monosomal karyotype in young adult and elderly acute myeloid leukemia: the Southwest Oncology Group (SWOG) experience. Blood 116:2224–2228
Acknowledgments
The authors would like to thank Caroline Zander, Gabriele Samson, and Emilia Januschewski for the excellent data management. This study was supported by an unrestricted grant from Amgen GmbH and Pfizer GmbH by the Deutsche Krebshilfe e.V (grant no. 109003) and grant no. DJCLS R 10/22 from the Deutsche-José-Carreras Leukämie-Stiftung e.V (DJCLS). OGO holds an endowed professorship of the DJCLS.
Conflict of interest
The authors declare no conflict of interest.
Informed consent
Informed consent was obtained from all patients for being included in the study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Gesine Bug and Steffen Koschmieder contributed equally.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplemental figure 1
Consort diagram of the trial. (DOC 37 kb)
Rights and permissions
About this article
Cite this article
Bug, G., Koschmieder, S., Krauter, J. et al. Long-term results of a prospective randomized trial evaluating G-CSF priming in intensive induction chemotherapy followed by autologous stem cell transplantation in elderly patients with acute myeloid leukemia. Ann Hematol 93, 193–202 (2014). https://doi.org/10.1007/s00277-013-1873-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-013-1873-3