Abstract
Background
The outcomes of relapsed or refractory acute myeloid leukemia (AML) remain poor. Although the concomitant use of granulocyte colony-stimulating factor (G-CSF) and anti-chemotherapeutic agents has been investigated to improve the antileukemic effect on AML, its usefulness remains controversial. This study aimed to investigate the effects of G-CSF priming as a remission induction therapy or salvage chemotherapy.
Methods
We performed a thorough literature search for studies related to the priming effect of G-CSF using PubMed, Ichushi-Web, and the Cochrane Library. A qualitative analysis of the pooled data was performed, and risk ratios (RRs) with confidence intervals (CIs) were calculated and summarized.
Results
Two reviewers independently extracted and accessed the 278 records identified during the initial screening, and 62 full-text articles were assessed for eligibility in second screening. Eleven studies were included in the qualitative analysis and 10 in the meta-analysis. A systematic review revealed that priming with G-CSF did not correlate with an improvement in response rate and overall survival (OS). The result of the meta-analysis revealed the tendency for lower relapse rate in the G-CSF priming groups without inter-study heterogeneity [RR, 0.91 (95% CI 0.82–1.01), p = 0.08; I2 = 4%, p = 0.35]. In specific populations, including patients with intermediate cytogenetic risk and those receiving high-dose cytarabine, the G-CSF priming regimen prolonged OS.
Conclusions
G-CSF priming in combination with intensive remission induction treatment is not universally effective in patients with AML. Further studies are required to identify the patient cohort for which G-CSF priming is recommended.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Although treatment outcomes have improved in patients with acute myeloid leukemia (AML), relapse and relapse mortality rates remain high [1]. Chemotherapy-resistant leukemic stem cells (LSCs) residing in the bone marrow niche facilitate disease recurrence [2,3,4]. Granulocyte colony-stimulating factor (G-CSF) is widely used to support the recovery from bone marrow suppression after chemotherapy for hematological malignancies [5] and mobilization of hematopoietic stem cells [6,7,8]. The concurrent use of G-CSF with anticancer chemotherapy against AML can induce leukemic blasts [9], including quiescent LSCs residing in the bone marrow niche [10], into the synthesis phase and increase susceptibility to cell cycle-dependent drugs. This is called the priming effect of G-CSF and contributes to enhancing the antileukemic effect in vivo, improving the outcomes of AML treatment. Several investigators have conducted randomized controlled trials (RCTs) regarding this [11,12,13,14,15,16]. In contrast, a combination of G-CSF and antileukemic chemotherapy can induce severe bone marrow suppression because G-CSF switches the status of normal hematopoietic stem cells from dormancy to self-renewal and may sensitize them to anticancer agents [17]. The American Society for Clinical Oncology guidelines do not describe the use of G-CSF in combination with chemotherapy aimed at a priming effect. The National Comprehensive Cancer Network guidelines recommend (category 2B) an intensive combination chemotherapy regimen using fludarabine, cytarabine, and G-CSF (FLAG) with/without anthracycline for patients with AML aged <60 years for standard or adverse risk [18]. In Japan, there has been no systematic review on the use of a G-CSF priming regimen to improve the remission induction rate and outcomes of patients with AML, and the importance of G-CSF priming remains to be determined. Therefore, we performed a systematic literature review to evaluate the effects and safety of priming malignant blasts with G-CSF for AML, which will assist in updating clinical guidelines for G-CSF use.
Methods
Search strategy
A systematic review of the literature was performed according to the “Medical Information Network Distribution Service (MINDS) Handbook for Clinical Practice Guideline Development 2014” [19] and “MINDS Clinical Practice Guideline Development Guide 2017” [20] using PubMed, Ichushi-Web, and the Cochrane Library. The search terms used in the combination of Medical Subject Headings and keywords were as follows: “leukemia, myeloid, acute/drug therapy,” “granulocyte colony-stimulating factor,” “prevent*, prevention, and control,” “prophylaxis*,” and “first, initial, induction” in all fields. Two reviewers (Y.N. and T.M.) of the systematic review team independently performed the initial screening based on the titles and abstracts of all articles for ineligible reports, followed by full-text screening (i.e., second screening) according to the inclusion and exclusion criteria. The reasons for exclusion were recorded, and duplicates were removed. Disagreements were resolved by consensus among the coauthors. These articles were examined for quality reporting data related to the selection criteria outlined in the following section.
Selection criteria
The inclusion criteria of this study were as follows: studies (1) designed as an RCT, a non-RCT, and a cohort or case–control trial; (2) aiming at adult patients diagnosed with AML; and (3) that included patients in the treatment group who received standard intensive induction therapy or salvage high-dose intensity treatment. Studies assessing treatments with low-dose cytarabine or similar low-intensity chemotherapy were excluded. We also excluded guidelines, reviews, letters, abstracts without articles, laboratory studies, systematic reviews, meta-analyses, and case reports.
Data extraction and quality assessment
After the second screening, the reviewer (Y.N.) of the systematic review team evaluated the articles and extracted data using standardized data abstraction forms. The evidence indicated by individual studies related to critical outcomes included within the clinical questions posed by the guideline creation team was divided into groups based on study design and quality. The following eight outcome indicators were evaluated: (1) infection-related mortality; (2) overall survival (OS); (3) disease progression/recurrence; (4) improvement in remission induction rate (priming effect); (5) duration of neutropenia or thrombocytopenia; (6) incidence of secondary cancer; (7) adverse events, such as musculoskeletal pain; and (8) quality of life (QOL). The authors determined the outcomes of the Population, Intervention, Comparator, and Outcome (PICO) framework on both the benefits and harms of concomitant G-CSF use with chemotherapy. The leader (S.Y.) of the guideline creation team resolved the conflicts and questions. The level of evidence was evaluated not for individual references but for each outcome for studies grouped by study design. The certainty of evidence was assessed based on the risk of bias, inconsistency, imprecision, indirectness, and publication bias. The literature quality and body of evidence were evaluated using the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) approach and then classified into four levels: “strong,” “medium,” “weak,” and “very weak.”
Statistical methodology
Review Manager (The Cochrane Collaboration, London, UK) version 5.41 was used for statistical analyses. After a qualitative analysis using Excel, studies were considered eligible and included in the meta-analysis if they were designed to compare the use of G-CSF combined regimens for AML with a control group. The risk ratio (RR) for each endpoint was calculated, and the effect size was described as a 95% confidence interval (CI) for each study. They were calculated using fixed- or random-effects models, depending on the level of heterogeneity. A forest plot was used to graphically present the results of the calculated RR for individual studies and overall meta-analyses. The degree of heterogeneity was assessed using the I2 and Chi-squared-based Q tests. A p value <0.05 in the Z test was considered significant. A funnel plot was used to graphically investigate the potential publication bias.
Results
Literature search
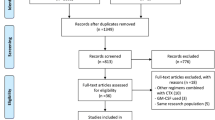
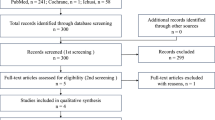
The initial literature search included 274 results: PubMed, 196; the Cochrane Library, 1; and Ichushi-Web, 77 (date of search: March 23, 2020). An additional four articles were manually selected and added. Among the 278 articles obtained, 216 were excluded after screening for the following criteria: human participants only, publication date ranging from January 1, 1990, to December 31, 2019, publications in English or Japanese, and selection criteria outlined in the section above, yielding 11 articles (Fig. 1). In most cases, the primary reason for exclusion was participants’ eligibility.
Modified PRISMA flow diagram of the literature search and study selection process. Number of studies included in the meta-analysis of disease progression/recurrence (*1), remission induction rate (*2), and adverse events, such as musculoskeletal pain (*3), were shown. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-analysis
Studies selected for the meta-analysis
Eleven studies [11,12,13,14,15,16, 21,22,23,24,25], six RCTs and five case–control studies, were included in the descriptive qualitative analysis, of which six RCTs [11,12,13,14,15,16] were examined in the meta-analysis. The six RCTs were conducted between 1994 and 2016. Meta-analyses of the study findings on the duration of neutropenia or OS were not feasible because of differences in the treatment benefit and harm assessment measurements. Three RCTs [11, 13, 16] were ultimately selected for the meta-analysis of disease progression/recurrence, 6 [11,12,13,14,15,16] for the G-CSF priming effect on the remission induction rate, and 2 [12, 15] for adverse events, such as musculoskeletal pain. Four case–control studies also performed meta-analyses on disease progression/recurrence [22,23,24,25].
Relationship between each outcome using the PICO framework and the G-CSF priming in AML
Relationship between infection-related mortality and the G-CSF priming
One evaluable RCT included 640 patients with newly diagnosed AML aged ≤60 years who received standard-dose remission induction chemotherapy with (n = 321) or without (n = 319) G-CSF priming [11]. This was not a placebo-controlled RCT; thus, there was a risk of bias. No significant differences were observed in infection-related mortality between patients who received chemotherapy with and without G-CSF priming [RR, 1.83 (95% CI 0.79–4.52); p = 0.175]. Although the report is high quality, involving over 300 patients in both the control and intervention groups, the evaluation depends on a single paper. According to the GRADE approach, the quality/certainty of evidence for this outcome was “medium.”
Relationship between OS and the G-CSF priming
Data from five RCTs [11,12,13,14, 16] and five case–control studies [21,22,23,24,25] were included in the qualitative analysis. Overall, 4,626 patients were included in the five RCTs: two RCTs which included 2347 patients of all ages [13, 14], two RCTs on 1557 patients aged ≤60 years [11, 16], and one RCT on 722 older patients aged ≥61 years [12]. Four RCTs studied newly diagnosed AML [11, 12, 14, 16], and one RCT focused on relapsed/refractory AML [13]. All five RCTs concluded that there was no significant difference in OS between groups with and without G-CSF priming. However, among the subgroups of patients with AML at standard risk [11] or those who received high-dose Ara-C [16], OS was significantly better in the G-CSF priming arm than in the control arm. A meta-analysis of this outcome was not performed because of differences in effect measures.
In total, 687 patients were included in the five case–control studies: three studies on 510 newly diagnosed patients of all ages [21,22,23,24,25], one study on 71 relapsed/refractory AML [24], and one study on 106 patients with secondary AML [25]. Regarding intensity of intervention, two studies were designed to compare the asymmetry between purine analogs with high-dose Ara-C and standard-dose chemotherapy. Thus, there was a non-negligible bias among the evaluated studies.
In two studies on relapsed/refractory AML, patients who received G-CSF priming chemotherapy had significantly better OS than those in the control group [24, 25]. However, the effect on OS was inconsistent; there were no differences in OS between the intervention and control groups in other three studies [21,22,23]. Overall, the G-CSF priming did not affect patient survival. Although this can be beneficial in some selected groups, it is unclear because of inconsistencies between reports. The quality/certainty of evidence was “medium.”
Relationship between disease progression/recurrence and the G-CSF priming
Data from the following three RCT studies were included (n = 1913): two RCTs on patients aged ≤60 years (n = 1557) [11, 16] and one RCT on patients of all ages (n = 356) [13]. Two RCTs were conducted on newly diagnosed AML [11, 16], and one RCT on relapsed/refractory AML [13]. Combination treatment was standard remission induction therapy in one RCT (n = 640) [11], and two RCTs included randomization allocating high- or standard-dose cytarabine (n = 1273) [13, 16]. None of these RCTs was placebo-controlled; thus, there was a risk of bias. The relapse rate was significantly lower in the G-CSF priming group than in the control group (p = 0.04) in one RCT [11]; however, there was no difference in the other two studies [13, 16]. The result of meta-analysis revealed the tendency for lower relapse rate in the G-CSF priming groups without inter-study heterogeneity [RR, 0.91 (95% CI 0.82–1.01), p = 0.08; I2 = 4%, p = 0.35] (Fig. 2a). No apparent asymmetry was observed in the funnel plot (Fig. 2b).
Association between disease progression/recurrence and the G-CSF priming. (a) Forest plot shows treatment effects versus the study size estimated from the standard error of log (RR). Open circles indicate individual studies in this meta-analysis. The broken line is a pseudo 95% confidence interval of effect measures in the study. (b) Funnel plot showing the symmetrical distribution of studies indicating the absence of a publication bias. CI confidence interval, G-CSF granulocyte colony-stimulating factor, IV inverse variance method, RR risk ratio, SE standard error
In the four case–control studies (n = 581), patient characteristics varied: two were on older patients in their 80 s (n = 313) [21, 23], three studies on newly diagnosed AML (n = 510) [21,22,23] (one study was solely on the favorable chromosomal risk group (n = 114) [21]), and one study on relapsed/refractory AML (n = 71) [24]. As an intervention, one study included an asymmetric comparison of treatment arms with purine analogs and high-dose Ara-C vs. standard induction therapy [23]. Compared with the control group, patients in the G-CSF priming group had a lower cumulative incidence of relapse in one study [22] and longer relapse-free survival in two studies [23, 24]. Owing to the differences in the effect measures in each study, a meta-analysis could not be performed. Overall, G-CSF priming did not affect the progression. In some specific patient populations, G-CSF priming may be beneficial, but the results were inconsistent. Moreover, the background of the patient characteristics varied; thus, careful interpretation of this evaluation is required. The quality/certainty of evidence on this outcome was “strong.”
The G-CSF priming effect on remission induction rate
This effect was defined as complete remission induction rate. Data from six RCTs [11,12,13,14,15,16] on 4684 patients included various subjects: two studies included 2347 patients of all ages [13, 14], one study included elderly patients aged ≥61 years (n = 722) [12], two studies included those aged ≤60 years (n = 1557) [11, 16], and one study included those aged ≤66 years (n = 58) [15]. Four studies included 4270 patients with newly diagnosed AML [11, 12, 14, 16] and two included 414 patients with relapsed/refractory AML [13, 15]. For combination therapy with G-CSF priming, three studies used standard remission induction chemotherapy (n = 1420) [11, 12, 15] and three studies were randomized with high-dose cytarabine, standard chemotherapy, or other treatments (n = 3264) [13, 14, 16]. Only one study used a placebo control arm (n = 58) [15]; thus, there was some risk of bias. Only one out of six RCTs revealed the benefit of the G-CSF priming in remission induction rate [12]. The result of the meta-analysis had some heterogeneities, and there was no difference between the groups with and without G-CSF priming [RR, 1.03 (95% CI 0.96–1.10), p = 0.42; I2 = 55%, p = 0.05] (Fig. 3a). No apparent asymmetry was observed in the funnel plot; thus, publication bias was not evident (Fig. 3b).
G-CSF priming effect on remission induction rate. (a) Forest and (b) funnel plots based on the RCTs and (c) forest and (d) funnel plots based on the case–control studies. Although there were significantly few studies and insufficient variations in standard errors to assess whether funnel plots were symmetric, there was no asymmetry visible in any of the funnel plots (b and d). CI confidence interval, G-CSF granulocyte colony-stimulating factor, IV inverse variance method, RCTs randomized controlled trials, RR risk ratio, SE standard errors
The patient characteristics in the selected four case–control studies (n = 573) were heterogeneous: three studies on 502 patients with newly diagnosed AML of all ages [22, 23, 25] and one study on 71 patients with relapsed/refractory AML aged ≤60 years [24]. One study consisted of an asymmetric comparison between treatment arms using a purine analog with high-dose cytarabine and standard chemotherapy (n = 199) [23]. Thus, there was a risk of bias. The results of the meta-analysis revealed that the remission induction rate was significantly better in the groups using combination therapy with G-CSF priming [RR, 1.27 (95% CI 1.12–1.43); p = 0.0002], and the result was consistent (I2 = 3%, p = 0.38) (Fig. 3c). Although the possibility of publication bias was not excluded owing to the small number of studies, no apparent asymmetry in the funnel plot was detected (Fig. 3d). The effect of the combined use of G-CSF priming on treatment effectiveness was insignificant in the evaluation using RCT studies, but it was significant in that using non-RCT studies. Thus, there was a divergence between the results of these meta-analyses. Overall, remission induction rates were significantly higher in the G-CSF priming group than in the control groups in one out of the six RCTs [12] and three of the four non-RCTs [23,24,25]. Although the G-priming group can be beneficial in some specific groups, including older patients with newly diagnosed disease [12] or patients harboring adverse prognostic factors [22,23,24,25], the benefit of G-priming has been inconsistent between reports and remains unclear. The quality/certainty of evidence on this outcome was “strong.”
Relationship between the duration of neutropenia and the G-CSF priming
Data from four RCTs [11, 13, 15, 16] included various participants (n = 1971): two included patients aged ≤60 years (n = 1557) [11, 16], one included patients aged ≤66 years (n = 58) [15], and one included patients across all age groups (n = 356) [13]. Two studies included newly diagnosed AML (n = 1557) [11, 16], and two included relapsed/refractory AML (n = 414) [13, 15]. The intensity of the intervention varied: two studies performed standard chemotherapy (n = 698) [11, 15] and two studies were designed to compare the asymmetry between purine analogs with high-dose Ara-C and standard-dose chemotherapy (n = 1273) [13, 16]. The duration of neutropenia was significantly shorter in the G-CSF priming group in two studies (n = 414) [13, 15], not significantly different between the G-CSF priming and control groups in one study (n = 640) [11], and significantly longer in the G-CSF priming group in one study (only cycle 2) (n = 917) [16]. Because of the divergence in the effect measures in each study, a meta-analysis could not be performed.
Two case–control studies (n = 303) [22, 25] were asymmetric in terms of patient characteristics and intervention: one study assessed patients with newly diagnosed AML and was designed to compare treatment arms with purine analog and high-dose Ara-C vs. standard chemotherapy (n = 106) [25]. In both studies, the G-CSF priming group had a shorter duration of neutropenia than the control group. Regarding the harmful effects of the G-CSF priming strategy on neutropenia, the results of the evaluated studies were inconsistent. Moreover, in most studies, G-CSF administration was designed to continue from the start of combination chemotherapy until neutrophil recovery. Thus, the relationship between G-CSF priming and the duration of neutropenia remains unclear. The quality/certainty of evidence on this outcome was “medium.”
Relationship between the incidence of secondary cancer and the G-CSF priming
Although the outcome was set before starting the evaluation, we could not find any studies aimed at this outcome; thus, we concluded that this relationship was unevaluable.
Relationship between adverse events, such as musculoskeletal pain, and the G-CSF priming
The following two RCTs were included in the meta-analysis (n = 780): an RCT on 722 elderly patients with newly diagnosed AML [12] and 58 patients with relapsed/refractory AML aged ≤66 years [15]. Only the latter was a placebo-controlled study; therefore, there was a risk of bias. The result of the meta-analysis was not heterogeneous, which revealed that there was no significant difference in adverse events, including musculoskeletal pain, between patients who received chemotherapy with the G-CSF priming and those who did not [RR, 1.39 (95% CI 0.26–7.31), p = 0.70; I2 = 0%, p = 0.54) (Fig. 4a). Although the small number of studies was a limitation, the funnel plot indicated no publication bias (Fig. 4b). The quality/certainty of evidence was “middle.”
Adverse events, such as musculoskeletal pain, associated with G-CSF priming. Forest (a) and funnel (b) plots of the RR of adverse events, such as musculoskeletal pain, comparing the G-CSF priming and control study arms for each study. Although there were remarkably few studies and insufficient variations in SE to assess whether funnel plots were symmetric, there was no asymmetry visible in the funnel plots. CI confidence interval, G-CSF granulocyte colony-stimulating factor, IV inverse variance method, RCTs randomized controlled trials, RR risk ratio, SE standard errors
Relationship between the QOL and the G-CSF priming
Although the outcome was initially set, there was no study evaluating this outcome. Thus, we concluded that this relationship could not be determined.
Discussion
In this review, we did not find a significant improvement in the OS and remission induction rates associated with the G-CSF priming strategy through RCT evaluation. However, the results of the meta-analysis of the three RCTs aimed at newly diagnosed AML on disease progression/recurrence revealed a tendency toward a lower relapse rate in the G-CSF priming group (Fig. 2a). Through the subgroup analysis of RCTs, patients in the standard-risk group [11] or those receiving high-dose cytarabine [16] showed a significant benefit in OS from G-CSF priming, although the reasons underlying this survival improvement remain unclear. Furthermore, the evaluation of two case–control studies for relapsed/refractory AML [24] or newly diagnosed secondary AML [25] revealed that G-CSF priming was associated with better OS. Moreover, meta-analysis of four case–control studies showed a significantly better remission induction rate in the G-CSF priming group than in the control group (Fig. 3c). Consequently, although some patients had survival benefits from G-CSF priming, the exact patient groups that benefited from this strategy are unclear. In terms of remission induction, the G-CSF priming group had better response rates than the control group in one RCT which only enrolled patients aged ≤61 with newly diagnosed AML [12], in a case–control study for secondary newly diagnosed AML [25], and in the specific subgroup harboring adverse chromosomal abnormalities in another case–control study [22]. Following standard remission induction regimens, patients in these subgroups tended to have lower response rates than those in the favorable/intermediate risk groups or younger populations; this may explain why the effects of G-CSF priming were more evident in unfavorable risk subgroups. Based on the above, there is room for future prospective evaluation.
Recent progress in the biology of AML has revealed that the mechanism of disease relapse can be caused by an increase in leukemic blasts originating from LSCs [3, 4]. Quiescent LSCs residing in the bone marrow niche have low chemosensitivity and survive through the lines of consolidation [2]. G-CSF priming sensitizes most leukemic blasts [26]. Moreover, it induces cell cycle quiescent LSCs into the cell cycling state, potentiates their sensitivity to cell cycle-dependent traditional antileukemic agents, and significantly enhances the induction of apoptosis and elimination of LSCs [10].
Although beyond the scope of the current evaluation, G-CSF priming with the most intensive treatment, the conditioning regimen before hematopoietic stem cell transplantation, is associated with better outcomes [27,28,29,30]. A nationwide prospective study is ongoing [31]. In contrast, several groups have reported the effectiveness of G-CSF priming combined with low-dose cytarabine or other low-intensity chemotherapeutic agents, including low-dose cytarabine and aclarubicin (CAG) [32] or CAG with decitabine [33]. Moreover, a recent study involving unfit or relapsed/refractory AML reported promising results of the combination with venetoclax, hypomethylating agents, and half-dose CAG [34], while interactions between these agents and the G-CSF receptor signal transduction pathway remain uncovered. Evaluation of G-CSF priming with these factors was not performed in this study. The G-CSF priming strategy in these settings, combined with a high-intensity preparative conditioning regimen or less toxic chemotherapy, awaits evaluation.
The current study revealed that G-CSF priming with remission induction treatment was not associated with an increase in the duration of neutropenia, incidence of infection-related mortality, disease progression/recurrence, and adverse events. Notably, most studies sequentially administered G-CSF as prophylaxis for neutropenia after the concomitant use of G-CSF with chemotherapy; thus, the true effect on prolonged period of neutropenia cannot be strictly evaluated.
It is important to consider Japan’s recent public approval of this strategy. The Japan Adult Leukemia Study Group conducted a phase II study to evaluate the efficacy of FLAG with a mitoxantrone regimen and revealed that it was an effective and safe salvage therapy to achieve complete remission in 73% of patients with relapsed/refractory AML [35]. Based on these evaluations, on February 4, 2022, a pre-evaluation of the public knowledge-based application (Kouchi-shinsei) of the combined use of G-CSF for chemotherapy in relapsed/refractory AML was completed. It was approved that lenograstim and filgrastim in combination with anticancer chemotherapy using fludarabine and cytarabine could be covered by Japanese insurance. Thus, the G-CSF priming regimen is now more prevalent in Japan than in the past.
In the current study, we evaluated the outcomes of G-CSF priming, including the response rate, OS, and relapse rate through 6 RCTs. Four of the included RCTs studied newly diagnosed AML (n = 4270) [11, 12, 14, 16], while two focused on relapsed/refractory cases (n = 414) [13, 15]. Considering that G-CSF priming for AML is currently approved only for relapsed/refractory cases with FLAG-based intensive regimens, further studies are required to determine whether G-CSF priming is also beneficial for newly diagnosed AML in Japan.
This systematic review and meta-analysis has some limitations. Patient characteristics, disease status, and treatment intensity varied. Moreover, we included articles from a wide range of published years; thus, the efficacy of supportive care may have influenced the response rate and survival. In addition, although we assessed the publication bias through funnel plot analyses and did not detect any bias, the number of studies included was small.
Conclusions
The current evaluation cannot confirm a clear benefit of G-CSF priming with concurrent chemotherapy in all patients with AML. However, the benefits of this strategy in improving the response rate and OS have been suggested for some specific subgroups. Further studies are required to identify the patient cohort for which G-CSF priming is recommended.
Data availability
Data associated with this systematic review may be accessed from the corresponding author upon reasonable request.
References
Döhner H, Weisdorf DJ, Bloomfield CD (2015) Acute myeloid leukemia. N Engl J Med 373(12):1136–1152. https://doi.org/10.1056/NEJMra1406184
Ishikawa F, Yoshida S, Saito Y et al (2007) Chemotherapy-resistant human AML stem cells home to and engraft within the bone-marrow endosteal region. Nat Biotechnol 25(11):1315–1321. https://doi.org/10.1038/nbt1350
Lapidot T, Sirard C, Vormoor J et al (1994) A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature 367(6464):645–648. https://doi.org/10.1038/367645a0
Bonnet D, Dick JE (1997) Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med 3(7):730–737. https://doi.org/10.1038/nm0797-730
Akiyama N, Okamura T, Yoshida M et al (2022) Difference of compliance rates for the recommendations in Japanese Guideline on Febrile Neutropenia according to respondents’ attributes: the second report on a questionnaire survey among hematology-oncology physicians and surgeons. Support Care Cancer 30(5):4327–4336. https://doi.org/10.1007/s00520-022-06834-9
Pahnke S, Egeland T, Halter J et al (2019) Current use of biosimilar G-CSF for haematopoietic stem cell mobilisation. Bone Marrow Transplant 54(6):858–866. https://doi.org/10.1038/s41409-018-0350-y
Arora S, Majhail NS, Liu H (2019) Hematopoietic progenitor cell mobilization for autologous stem cell transplantation in multiple myeloma in contemporary era. Clin Lymphoma Myeloma Leuk 19(4):200–205. https://doi.org/10.1016/j.clml.2018.12.010
Hartmann T, Hübel K, Monsef I et al (2015) Additional plerixafor to granulocyte colony-stimulating factors for haematopoietic stem cell mobilisation for autologous transplantation in people with malignant lymphoma or multiple myeloma. Cochrane Database Syst Rev 2015(10):Cd010615. https://doi.org/10.1002/14651858.CD010615.pub2
Terpstra W, Löwenberg B (1997) Application of myeloid growth factors in the treatment of acute myeloid leukemia. Leukemia 11(3):315–327. https://doi.org/10.1038/sj.leu.2400561
Saito Y, Uchida N, Tanaka S et al (2010) Induction of cell cycle entry eliminates human leukemia stem cells in a mouse model of AML. Nat Biotechnol 28(3):275–280. https://doi.org/10.1038/nbt.1607
Löwenberg B, van Putten W, Theobald M et al (2003) Effect of priming with granulocyte colony-stimulating factor on the outcome of chemotherapy for acute myeloid leukemia. N Engl J Med 349(8):743–752. https://doi.org/10.1056/NEJMoa025406
Amadori S, Suciu S, Jehn U et al (2005) Use of glycosylated recombinant human G-CSF (lenograstim) during and/or after induction chemotherapy in patients 61 years of age and older with acute myeloid leukemia: final results of AML-13, a randomized phase-3 study. Blood 106(1):27–34. https://doi.org/10.1182/blood-2004-09-3728
Milligan DW, Wheatley K, Littlewood T et al (2006) Fludarabine and cytosine are less effective than standard ADE chemotherapy in high-risk acute myeloid leukemia, and addition of G-CSF and ATRA are not beneficial: results of the MRC AML-HR randomized trial. Blood 107(12):4614–4622. https://doi.org/10.1182/blood-2005-10-4202
Krug U, Berdel WE, Gale RP et al (2016) Increasing intensity of therapies assigned at diagnosis does not improve survival of adults with acute myeloid leukemia. Leukemia 30(6):1230–1236. https://doi.org/10.1038/leu.2016.25
Ohno R, Naoe T, Kanamaru A et al (1994) A double-blind controlled study of granulocyte colony-stimulating factor started two days before induction chemotherapy in refractory acute myeloid leukemia. Kohseisho Leukemia Study Group Blood 83(8):2086–2092
Pabst T, Vellenga E, van Putten W et al (2012) Favorable effect of priming with granulocyte colony-stimulating factor in remission induction of acute myeloid leukemia restricted to dose escalation of cytarabine. Blood 119(23):5367–5373. https://doi.org/10.1182/blood-2011-11-389841
Wilson A, Laurenti E, Oser G et al (2008) Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell 135(6):1118–1129. https://doi.org/10.1016/j.cell.2008.10.048
National Comprehensive Cancer Network Acute Myeloid Leukemia (Version 4.2023). https://www.nccn.org/professionals/physician_gls/pdf/aml.pdf. Accessed 7 Aug 2023
Morizane T, Yoshida M, Kojimahara N et al (2014) Minds Handbook for Clinical Practice Guideline Development 2014. Japan Council for Quality Health Care, Tokyo. https://minds.jcqhc.or.jp/s/developer_manual(in Japanese)
Kojimahara N, Nakayama T, Morizane T et al (2017) Minds Manual for Guideline Development 2017. Japan Council for Quality Health Care, Tokyo
Borthakur G, Kantarjian H, Wang X et al (2008) Treatment of core-binding-factor in acute myelogenous leukemia with fludarabine, cytarabine, and granulocyte colony-stimulating factor results in improved event-free survival. Cancer 113(11):3181–3185. https://doi.org/10.1002/cncr.23927
Estey E, Thall P, Andreeff M et al (1994) Use of granulocyte colony-stimulating factor before, during, and after fludarabine plus cytarabine induction therapy of newly diagnosed acute myelogenous leukemia or myelodysplastic syndromes: comparison with fludarabine plus cytarabine without granulocyte colony-stimulating factor. J Clin Oncol 12(4):671–678. https://doi.org/10.1200/jco.1994.12.4.671
Halpern AB, Othus M, Huebner EM et al (2018) Phase 1/2 trial of GCLAM with dose-escalated mitoxantrone for newly diagnosed AML or other high-grade myeloid neoplasms. Leukemia 32(11):2352–2362. https://doi.org/10.1038/s41375-018-0135-8
Martin MG, Augustin KM, Uy GL et al (2009) Salvage therapy for acute myeloid leukemia with fludarabine, cytarabine, and idarubicin with or without gemtuzumab ozogamicin and with concurrent or sequential G-CSF. Am J Hematol 84(11):733–737. https://doi.org/10.1002/ajh.21545
Vulaj V, Perissinotti AJ, Uebel JR et al (2018) The FOSSIL Study: FLAG or standard 7+3 induction therapy in secondary acute myeloid leukemia. Leuk Res 70:91–96. https://doi.org/10.1016/j.leukres.2018.05.011
te Boekhorst PA, Löwenberg B, Vlastuin M et al (1993) Enhanced chemosensitivity of clonogenic blasts from patients with acute myeloid leukemia by G-CSF, IL-3 or GM-CSF stimulation. Leukemia 7(8):1191–1198
Konuma T, Kato S, Isobe M et al (2019) Reduced-toxicity myeloablative conditioning consisting of fludarabine/busulfan/low-dose total body irradiation/granulocyte colony-stimulating factor-combined cytarabine in single cord blood transplantation for elderly patients with nonremission myeloid malignancies. Biol Blood Marrow Transplant 25(4):764–770. https://doi.org/10.1016/j.bbmt.2018.12.004
Konuma T, Takahashi S, Uchida N et al (2015) Effect of Granulocyte Colony-Stimulating Factor-Combined Conditioning in Cord Blood Transplantation for Myelodysplastic Syndrome and Secondary Acute Myeloid Leukemia: A Retrospective Study in Japan. Biol Blood Marrow Transplant 21(9):1632–1640. https://doi.org/10.1016/j.bbmt.2015.05.009
Mori T, Aisa Y, Watanabe R et al (2008) Long-term follow-up of allogeneic hematopoietic stem cell transplantation for de novo acute myelogenous leukemia with a conditioning regimen of total body irradiation and granulocyte colony-stimulating factor-combined high-dose cytarabine. Biol Blood Marrow Transplant 14(6):651–657. https://doi.org/10.1016/j.bbmt.2008.03.006
Takahashi S, Iseki T, Ooi J et al (2004) Single-institute comparative analysis of unrelated bone marrow transplantation and cord blood transplantation for adult patients with hematologic malignancies. Blood 104(12):3813–3820. https://doi.org/10.1182/blood-2004-03-1001
Terakura S, Konuma T, Tanaka M et al (2020) Randomised controlled trial of conditioning regimen for cord blood transplantation for adult myeloid malignancies comparing high-dose cytarabine/cyclophosphamide/total body irradiation with versus without G-CSF priming: G-CONCORD study protocol. BMJ Open 10(12):e040467. https://doi.org/10.1136/bmjopen-2020-040467
Yamada K, Furusawa S, Saito K et al (1995) Concurrent use of granulocyte colony-stimulating factor with low-dose cytosine arabinoside and aclarubicin for previously treated acute myelogenous leukemia: a pilot study. Leukemia 9(1):10–14
Huang J, Hong M, Zhu Y (2018) Decitabine in combination with G-CSF, low-dose cytarabine and aclarubicin is as effective as standard dose chemotherapy in the induction treatment for patients aged from 55 to 69 years old with newly diagnosed acute myeloid leukemia. Leuk Lymphoma 59(11):2570–2579. https://doi.org/10.1080/10428194.2018.1443328
Chen X, Zhao Y, Li Q et al (2023) Single-center retrospective clinical evaluation of venetoclax combined with HMAs and half-dose CAG for unfit or refractory/relapsed AML. Onco Targets Ther 16:409–419. https://doi.org/10.2147/ott.S405611
Hatsumi N, Miyawaki S, Yamauchi T et al (2019) Phase II study of FLAGM (fludarabine + high-dose cytarabine + granulocyte colony-stimulating factor + mitoxantrone) for relapsed or refractory acute myeloid leukemia. Int J Hematol 109(4):418–425. https://doi.org/10.1007/s12185-019-02606-0
Acknowledgements
The authors are grateful to Ms. Natsuki Narita for her contribution to the initial literature search. The authors thank Ms. Natsuki Fukuda for her valuable comments and suggestions. We would like to thank Editage (www.editage.jp) for English language editing.
Funding
This study was partially supported by the Japan Society for the Promotion of Science, KAKENHI (grant number: JP22K15615).
Author information
Authors and Affiliations
Contributions
All the authors contributed to the conception and design of this present study. Y.N. wrote the draft of the manuscript, and all authors reviewed and commented on the manuscript. All the authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
T. Maeda reports scholarship donation from Chugai Pharmaceutical. SN reports honoraria from Kyowa Kirin. YO reports honoraria from Daiichi Sankyo, Pfizer, Chugai Pharmaceutical, Eli Lilly and Company, and Kyowa Kirin. KT reports honoraria from Ono Pharmaceutical, Chugai Pharmaceutical, Taiho Pharmaceutical, and Novartis Pharma. EI reports honoraria from Eli Lilly and Company, research funding from MSD, Ono Pharmaceutical, Jannsen Pharma, and Takeda Pharmaceutical. YM reports honoraria from Ono Pharmaceutical, MSD, Takeda Pharmaceutical, Eisai, Bristol Myers Squibb, research funding from MSD, and Ono Pharmaceutical. DM reports honoraria from Ono Pharmaceutical, Janssen Pharma, Nippon Shinyaku, Eisai, Mundipharma, Kyowa Kirin, Chugai Pharmaceutical, Zenyaku, MSD, SymBio Pharmaceuticals, Sanofi, AbbVie, Takeda Pharmaceutical, AstraZeneca, Bristol Myers Squibb, Genmab, research funding from Amgen Astellas Biopharma, Novartis Pharma, Kyowa Kirin, Ono Pharmaceutical, Chugai Pharmaceutical, Janssen Pharma, Takeda Pharmaceutical, Otsuka Pharmaceutical, Sanofi, Astellas, Bristol Myers Squibb, AbbVie, Eisai, MSD, Taiho Pharmaceutical, AstraZeneca, Eli Lilly and Company, and Genmab. TY reports honoraria from Kyowa Kirin, Pfizer, Chugai Pharmaceutical, Eli Lilly and Company, MSD, AstraZeneca, and Eisai. T. Motohashi reports honoraria from AstraZeneca, Chugai Pharmaceutical, and Myriad Genetics. EB reports honoraria from Chugai Pharmaceutical, Daiichi Sankyo, research funding from Taiho Pharmaceutical, and Chugai Pharmaceutical. T. Kubo reports honoraria from Chugai Pharmaceutical. T. Kimura reports honoraria from Sanofi. AS reports honoraria and research funding from Chugai Pharmaceutical, and Taiho Pharmaceutical. TT reports honoraria from Daiichi Sankyo, Chugai Pharmaceutical, and Eli Lilly and Company. SY reports research funding from Otsuka Pharmaceutical. The other authors declare that there are no conflicts of interest associated with this manuscript.
Ethical approval
Not applicable.
Informed consent
Formal consent was not required for this type of study.
Consent to participate
Not applicable.
Consent for publication
All authors consented to the publication of this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Najima, Y., Maeda, T., Kamiyama, Y. et al. Effectiveness and safety of granulocyte colony-stimulating factor priming regimen for acute myeloid leukemia: A systematic review and meta-analysis of the Clinical Practice Guideline for the use of G-CSF 2022 from the Japan Society of Clinical Oncology. Int J Clin Oncol 29, 899–910 (2024). https://doi.org/10.1007/s10147-023-02461-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-023-02461-4