Abstract
Currently, there is no consensus regarding the optimum iron supplementation during pregnancy. The aim of this study is to evaluate the effect of different iron supplementation doses (including no supplementation) during pregnancy on the iron status of the mother and on the health of the neonate. A longitudinal study was conducted involving 358 pregnant women and their newborns. Mothers were classified as non-supplemented, low iron supplemented (<60 mg/day), moderate iron supplemented (between 60 and 100 mg/day) or high iron supplemented (>100 mg/day). General clinical and obstetric histories, haemoglobin (Hb), serum ferritin (SF) and transferrin saturation were evaluated in the first, second, third trimesters, and at partum. SF and Hb decreased less sharply in the iron-supplemented groups compared to the non-supplemented group. The higher the doses of iron supplementation, the lower the percentages of iron depletion at partum (p < 0.001), iron deficiency anaemia (p < 0.001) and preterm deliveries (p = 0.009) as well as a higher birth weight of the newborn. However, the group with high supplementation had a greater percentage (27.6 %) of women at risk of haemoconcentration at partum. Our Mediterranean women began gestation with iron stores close to deficit (SF, 28.1 μg/L; 95 % CI 27.9–28.4). With these iron stores, supplementation with iron at daily doses of between 60 and 100 mg appears to be the most beneficial for the health of mother and child. These findings need to be confirmed in further randomised clinical trials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Iron requirements during pregnancy increase to cover the expansion of the erythrocyte mass (principally during the second trimester) and to enable placenta and foetal growth, especially in the third trimester [1]. These requirements do not appear to be compensated for by diet alone [2], and as such, the pregnant woman is at risk of iron deficit and its consequences. Iron deficiency is associated with significant problems of health for the mother and the foetus, such as maternal anaemia and increase in the rates of preterm birth, lower birth weight [3–5] or delayed maturation and cognitive development of the child [6].
To avoid iron deficit, international scientific organizations recommend prophylactic prenatal iron supplementation. There are wide differences in the recommended daily iron dose, varying between 30 and 120 mg [7, 8] according to whether or not anaemia is present during the pregnancy.
Although several studies in the developed world (especially in the USA and northern Europe) have evaluated the effect of iron supplementation during pregnancy on the biochemical levels of iron over the long term of pregnancy [3, 9, 10], not many compare the effect of different doses of iron supplementation [11], and equivalent studies have not been conducted in European women of the Mediterranean countries. Further, there is no consensus regarding the best level of iron supplementation during pregnancy for the prevention of iron deficit in the mother, nor has there been conclusive evidence of reduction in adverse clinical effects not only for the mother but also for the newborn in terms of preterm births or low birth weight infants [9].
Therefore, we proposed to evaluate, in Caucasian Mediterranean women, the effect of different iron supplementation doses during pregnancy, including non-supplementation, on the evolution of iron status in the mother and on the health of the neonate.
Materials and methods
We are presenting the results of the investigations conducted on pregnant women by the same research team in two different periods, between 1991 and 1995 and between 2005 and 2008. These studies had the same longitudinal design and the same methodology, which is explained below, and were performed in the same hospital.
Pregnant women were followed up from their first prenatal care appointment (gestational week 10) until delivery at the Obstetrics and Gynaecology Unit of the Hospital Universitari Sant Joan de Reus (Catalonia, Spain). The studies were approved by the ethics committee of the hospital. All the volunteers who entered into the study provided written informed consent in accordance with the Declaration of Helsinki.
The criteria for inclusion were: Caucasian pregnant women between the 8th and 10th week of gestation, and >18 years of age. The exclusion criteria were: to have a multiple pregnancy (more than one foetus), the presence of any chronic illness that could affect the overall health of the woman, a possible inflammation diagnosis based on high serum ferritin (SF) levels (SF >62 μg/L) [12] and low transferrin saturation (TS) levels (TS <16 %) and giving birth at a different hospital to where the pregnancy was being monitored.
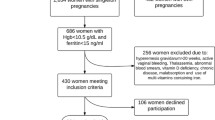
A total of 390 pregnant women were recruited (90 during the first period and 300 during the second). Three hundred fifty-eight complied with all the requirements of the study, 8 were excluded for giving birth at a different hospital, 6 had aborted, 3 had a possible inflammation and 15 had incomplete biochemical data.
The data were collected at four outpatient visits and at partum. At the first visit around week 10 of gestation, clinical–obstetric history was taken, as well as a fasting blood sample for laboratory analyses. Body mass index (BMI) was calculated at the first clinic visit as \( {\mathrm{weight} \mathrm{in} \mathrm{kilograms}} \div {\mathrm{height} \mathrm{in} \mathrm{square} \mathrm{meters} \text{(kilograms per square meter)}} \). The socioeconomic status of the family was evaluated according to the Hollingshead index [13]. The index enables the status of the individual to be determined using nine categories of the individual’s occupation (ranging from unemployed to highly qualified skilled work) and the individual’s educational level categorised into seven levels ranging from non-completed primary education up to completed tertiary level (university of equivalent). The status score was obtained by multiplying the value on the work scale by 5 and the value on the education scale by 3 and then combining the two scores. The highest possible score was divided by 3 with the aim of establishing three socioeconomic categories: low, medium and high. Based on this calculation, <5 % of the women in our study sample had a socioeconomic category described as “low”. For statistical purposes, these women were combined into the “medium” group, and hence, the study sample was segregated into two socioeconomic categories: low–medium vs. high.
In the second visit, between weeks 13 and 15 of gestation, the obstetrician advised all the women to take an iron supplement before breakfast of 120 mg/day in the form of iron sulphate in the first study and of 40 mg/day in the second. Another fasting blood sample for biochemical analyses was taken at visits 3 (week 24), 4 (week 34) and at partum.
A researcher not associated with the health-care personnel of the obstetrics team applied a semi-structured questionnaire designed by the research team to evaluate adherence to recommendations of iron supplementation. The interviewer recorded the initiation and continuance of oral iron supplementation, as well as the number of days per week that the supplements were usually taken. From this information, the total supplementary iron and the daily average of iron supplementation during pregnancy were calculated as:
Not all the pregnant women followed the recommendations; as such, four sub-groups were defined as a function of the type of iron supplementation taken during the pregnancy: no iron supplementation (17.0 %), low iron supplemented (<60 mg/day) [9] (47.2 %), moderate iron supplemented (between 60 and 100 mg/day) (10.6 %) and high iron supplemented (>100 mg/day) (25.1 %).
The blood samples were used to measure: Hb measured immediately with a Coulter GENS analyser (Coulter, Hialeah, FL, USA); SF by turbidimetric immunoassay as described [14]; serum transferrin and serum iron by spectrophotometry (Biokit S.A., Barcelona, Spain, and ITC Diagnostics S.A, Barcelona) using standard clinical chemistry techniques. Transferrin saturation (TS) was calculated [15] as: \( \text{TS}\left( {\text{inpercent}} \right)=(\text{serum iron in micromoles per litre}\div \text{serum transferrin in grams per litre})\times 3.9 \).
“Iron depletion (ID)” was defined as SF < 12μg/L, “anaemia” as Hb values <110 g/L in the first and third trimester, and at partum, and as Hb values <105 g/L in the second trimester [16]. “Iron deficiency anaemia (IDA)” was defined as anaemia and SF < 12μg/L simultaneously and “risk of haemoconcentration” as Hb values >130 g/L in the second and third trimester of gestation or at partum [9, 17].
The gestational age (weeks), weight of the newborn and the Apgar scores at the 1st, 5th and 10th minute of life were recorded. “Preterm” was defined as babies born before the 37th week of gestation and “low birth weight” as the newborn weighing <2,500 g. Adjusted birth weight was the birth weight adjusted by gestational age and gender of the baby.
Statistical analyses
All statistical analyses were performed with the SPSS package (version 19.0). All variables were examined for kurtosis (normal or skewed distributions), and all were observed to follow Gaussian distributions (except the values of SF) and were expressed as mean and 95 % confidence interval (CI). The distributions of SF values were observed to be non-Gaussian and were log transformed and presented as geometric mean and 95 % CI.
The adjusted birth weight was obtained from the predicted values of a multiple linear regression including the birth weight (dependent variable), the gestational age and gender of the baby.
Analysis of variance was applied, together with adjustment with the Bonferroni correction when comparing continuous variables. The χ2 test was used for comparing categorical variables.
Results
Table 1 summarises the general characteristics, socioeconomic status and iron supplementation in the women participating in the study. Of the women in the study, 83 % (n = 297) took iron supplementation during pregnancy and 17 % (n = 61) did not. Among the supplemented group of women, 8 % took the iron supplements with a frequency of 1–3 days per week, 19 % with a frequency of 4–5 days per week and the remaining 73 % with a frequency of 6–7 days per week. The weekly frequency of iron supplement intake did not differ between groups (p > 0.05). Of the women, 4.7 % took daily multivitamin supplements before the first obstetric visit, and only two women continued to take multivitamins until the end of gestation.
The women excluded from the present analysis owing to lack of blood samples at some trimester of pregnancy (n = 15) were no different statistically from the women finally included in the analysis, regarding obstetric characteristics, biochemical parameters and newborn characteristics.
Table 2 summarises the measured biochemical and haematological parameters related to iron as a function of the iron supplementation taken. In general, the mean biochemical values decreased in the course of the pregnancy and increased at partum. Although the women of the four groups (none, low, moderate and high dose) commenced pregnancy with similar SF and Hb values, these values at partum were significantly lower in the low iron-supplemented and in the non-supplemented groups than in the other two groups.
Table 3 summarises the frequencies of iron deficiency states and of risk of haemoconcentration by trimester of gestation and at partum, according to the dose of iron taken. The frequencies of ID and IDA increased as pregnancy advanced in all the groups and decreased at partum. At higher doses of iron supplementation, there were significant inverse linear associations with lower frequencies of ID (p < 0.001), anaemia (p <0.001) and IDA (p = 0.001) at partum. There was a significant direct linear relationship towards a higher risk of haemoconcentration at higher doses of iron supplementation (p < 0.001).
Table 4 summarises the characteristics of the newborn as a function of maternal iron supplementation. There were no differences with regard to birth weight. However, the birth weight adjusted for gestational age and gender of the baby was significantly greater in the high and moderate iron supplementation groups compared to the non-supplemented group (3,310 and 3,280 vs. 3,115 g; p < 0.050). There were no statistically significant differences in the frequencies of low birth weight and preterm newborns between groups. However, in the case of the preterm infants, a higher iron supplementation was significantly associated with a lower frequency of preterm births (p = 0.009).
Discussion
We observed that iron status decreased during pregnancy in all groups, whether or not supplemented with iron. However, the women receiving moderate-dose supplements had a better iron status at the end of gestation and greater benefits for the newborn (less preterm and higher weight) than the non-supplemented. With high-dose supplementation, although the same benefits as the moderate supplementation were observed, there was a greater risk of haemoconcentration at the end of the pregnancy.
Combining the data of the two studies performed by members of the same research team using the same study design and methodology enabled us to compare the group of pregnant women to whom low-dose iron had been prescribed relative to the more recent study for whom high-dose iron had been prescribed. The different doses of iron resulted from changes over time in expert guidelines and recommendations of iron dose prescription for pregnant women. Both studies contained homogenous samples of Mediterranean women with no known pathology and with a medium–high socioeconomic status. Very few women (4.9 %), by our calculation, had a low socioeconomic status. Overall, the anthropometric and obstetric data of the women are similar to those observed in pregnant women in other developed countries [18, 19]. The average infant birth weight and the percentage of preterm or low birth weight were also similar to those found in industrialised countries [20, 21].
Iron status was assessed with a battery of parameters recommended by international scientific organizations; variables that can be systematically assessed in standard, non-specialist, clinical practice.
Haemoglobin levels are often used as a proxy for iron deficiency in populations, despite not being either specific or sensitive indicators of iron status [22]. SF was used because it is considered to be the best biochemical parameter for monitoring iron status deficiency in pregnancy [23]. SF unequivocally identifies subjects without iron stores since this measure does not produce false-positive results. However, a known limitation of SF is that it increases not only with the iron content of the organism but also with acute or chronic inflammation, malignancy or liver disease, even in women with iron deficiency [24]. Since TS does not increase in the presence of inflammation [24], some authors suggest that TS should also be measured [25, 26] in order to resolve inconsistencies (high SF and low TS) such as an iron deficiency masked by inflammation.
In our study, we observed that SF and TS values decreased as pregnancy progressed in all groups, as observed in the majority of studies performed in pregnant women of industrialised countries, not only in the non-iron-supplemented women [3, 10, 27, 28] but also in women with low iron supplementation [3, 10, 11, 29, 30], moderate (60–80 mg/day) [11, 27, 31] and even higher doses (100–200 mg/day) [5, 28, 32].
Although some authors recommended low-dose iron supplementation (<60 mg/day) instead of higher iron doses [9, 30], in our study, we were able to observe that, of the three levels of supplementation, the low-dose supplementation was not recommendable since this was associated with a higher frequency of ID and IDA not only in the third trimester but also at partum and with a higher percentage of preterm births. This would indicate that, in our population, where pregnant women start pregnancy with relatively low iron stores (SF = 28.1 μg/L), the moderate- and high-dose supplementation with iron have the greater effectiveness in the prevention of ID.
This finding coincides with the results obtained by Milman et al. [31] in a study conducted in Denmark with 301 healthy pregnant women. In this study, they assessed the effectiveness of different iron supplementation doses (20, 40, 60, 60 mg/day) according to the initial iron store levels in the prevention of ID and IDA at the end of pregnancy. They suggested that women having SF ≤ 30 μg/L at early pregnancy, as in our study, should take 80–100 mg ferrous iron/day, while women with higher initial iron stores could take less iron supplementation in order to prevent iron deficiency states [31].
Several authors have highlighted that anaemia during pregnancy is associated with a decrease in the gestational age, a higher risk of preterm deliveries and a decrease in the birth weight [18, 33–35]; the detrimental effect could be due to the hypoxia that results from iron deficiency. In addition, Scholl et al. [36], in a prospective cohort study involving 826 pregnant women, compared the risk of preterm deliveries between pregnant women with IDA and women with anaemia from other causes. They found that the relationship between anaemia and preterm deliveries was specific to IDA [36].
The mechanism by which iron deficiency may increase the risk of preterm deliveries is not well established. A possible hypothesis is that iron deficiency, especially during pregnancy when the oxygen demands are particularly high because of the increased metabolism of the mother and foetus, may lead to an insufficient oxygenation of foetal tissues. This hypoxia could increase the norepinephrine concentration, which is a strong stimulus for the release of the corticotropin-releasing hormone (CRH), which is involved in the regulation of the process of delivery. High levels of CRH early in pregnancy could be related to a decrease in the gestational length and consequently to a higher risk of preterm delivery [37]. Therefore, in order to pre-empt preterm deliveries, iron deficiency during pregnancy should be prevented with iron supplementation.
The low iron supplementation and the non-supplementation groups of our study did not show any significant differences with respect to biochemical levels of iron in the mother and with respect to the newborn. This coincides with the observations in two recent reviews highlighting that women taking low-dose iron supplementation during gestation had similar percentages of preterm deliveries as non-iron-supplemented pregnant women [9, 38]. The only study with low-dose iron supplementation (30 mg/day) which showed a lower frequency of preterm deliveries compared to a group receiving placebo (7.5 vs. 13.9 %, p = 0.05) was the study conducted in Cleveland [10] with non-anaemic iron-replete pregnant women (geometric mean concentration of SF of 75 μg/L). The study was very similar to another study [3] also conducted in Cleveland and also with non-anaemic pregnant women but with lower initial iron stores (SF geometric mean of 47 μg/L) and with low-dose iron supplementation (30 mg/day) in early pregnancy. However, both groups in the study (iron supplemented and non-iron supplemented) completed the gestation period with similar percentages of iron deficiency and preterm deliveries. Low iron supplementation seems to have a protective effect on preterm deliveries only when the initial iron stores are high. Therefore, moderate iron doses could be more appropriate than low iron supplementation when serum ferritin is in the lower ranges and reinforces the importance of supplementation guidelines as a function of the initial iron status of the mother.
With respect to the risk of haemoconcentration, our results indicate that a higher supplementation has significantly higher risk of haemoconcentration at partum (p < 0.001). These findings coincide with those of other studies [9, 17]. However, Scanlon et al. [18] suggest that iron supplementation cannot increase the Hb levels beyond what is optimal for a given person and thus cannot be regarded as one of the causes of high Hb levels [18]. Previous studies have described adverse effects from haemoconcentration during pregnancy related to increased oxidative stress and blood pressure [39] as well as risk of low birth weight and preterm deliveries [17, 35, 40]. The mechanism by which haemoconcentration may be detrimental is not fully understood, but increased blood viscosity could impair uteroplacental blood flow, decrease placental perfusion and increase placental infarction. Supplementation with high-dose iron presents a slightly increased risk of haemoconcentration at partum than moderate-dose supplementation (27.6 vs. 19.4 %) and does not present significantly better benefits in the prevention of IDA at the end of pregnancy (6.1 vs. 2.9 %). Hence, the moderate-dose supplementation (60–100 g/d) would be more recommendable than high dose.
One of the limitations of the study is that the women included in the present analysis form part of two different periods. However, these two groups of women are from the same geographic zone and were treated according to the same methodology, and the biochemical analyses were performed by the same hospital laboratory. Combining the data from the two groups of women enabled us to compare the recommended doses of iron supplementation which, currently, is lower than that recommended in the past. Another limitation is that all the women were volunteers and could therefore not be representative of our environment, but all of them were healthy and with similar characteristics of the pregnant women from other industrialised countries, suggesting that they were not different from the rest. Another limitation is that pregnant women do not always adhere exactly to the recommendations; therefore, we tried to assess the iron supplementation doses taken by a questionnaire administered by a member of the research team who was not involved in the health-care provision, so that the responses of the women in the study were more sincere (or less influenced by the presence of the caregiver). In addition, our study design cannot eliminate the influence of some non-controlled variables such as maternal stress during pregnancy, strenuous work or some other factors related to preterm births which, if distributed non-homogeneously in the study sample, might influence the incidence of preterm deliveries.
Conclusions
In summary, we can conclude that, in all the women, the levels of iron decreased over the course of advancing pregnancy despite iron supplementation. At high-dose supplementation, the women had higher iron levels, lower percentage of ID and IDA at the end of gestation as well as better gestational age, lower percentage of preterm infants and better infant birth weight, having initiated gestation with iron stores of around 28.1 μg/L. However, high-dose supplementation significantly increased the risk of haemoconcentration, a condition associated with detrimental effects for the mother and the newborn such as pre-eclampsia, oxidative stress, preterm deliveries and low birth weight infants. Hence, in women who begin pregnancy with iron reserves close to ID, a supplementation of between 60 and 100 mg appears recommendable. These findings need to be confirmed in clinical trials.
References
Bothwell TH (2000) Iron requirements in pregnancy and strategies to meet them. Am J Clin Nutr 72(Suppl 1):257S–264S
Hallberg L (2001) Perspectives on nutritional iron deficiency. Annu Rev Nutr 21:1–21
Cogswell ME, Parvanta I, Ickes L, Yip R, Brittenham GM (2003) Iron supplementation during pregnancy, anemia, and birth weight: a randomized controlled trial. Am J Clin Nutr 78:773–781
Shah PS, Ohlsson A (2009) Knowledge synthesis group on determinants of low birth weight and preterm births: effects of prenatal multimicronutrient supplementation on pregnancy outcomes: a meta-analysis. CMAJ 180:E99–E108
Aranda N, Ribot B, Garcia E, Viteri FE, Arija V (2011) Pre-pregnancy iron reserves, iron supplementation during pregnancy, and birth weight. Early Hum Dev 87:791–797
Hernandez-Martinez C, Canals J, Aranda N, Ribot B, Escribano J, Arija V (2011) Effects of iron deficiency on neonatal behavior at different stages of pregnancy. Early Hum Dev 87:165–169
Dirección General de Cohesión del Sistema Nacional de Salud y Alta Inspección (2006) Guía de prevención de defectos congénitos. Ministerio de Sanidad y Consumo, Madrid
World Health Organization (2006) Iron and folate supplementation. Standards for maternal and neonatal care. Integrated Management of Pregnancy and Childbirth (IMPAC), Geneva
Pena-Rosas JP, Viteri FE (2009) Effects and safety of preventive oral iron or iron + folic acid supplementation for women during pregnancy. Cochrane Database Syst Rev 4:CD004736
Siega-Riz AM, Hartzema AG, Turnbull C, Thorp J, McDonald T, Cogswell ME (2006) The effects of prophylactic iron given in prenatal supplements on iron status and birth outcomes: a randomized controlled trial. Am J Obstet Gynecol 194:512–519
Milman N, Bergholt T, Eriksen L, Byg KE, Graudal N, Pedersen P, Hertz J (2005) Iron prophylaxis during pregnancy—how much iron is needed? A randomized dose–response study of 20–80 mg ferrous iron daily in pregnant women. Acta Obstey Gynecol Scand 84:238–247
Chen X, Scholl TO, Stein TP (2006) Association of elevated serum ferritin levels and the risk of gestational diabetes mellitus in pregnant women: the Camden study. Diabetes Care 29:1077–1082
Hollingshead AB (2011) Four factor index of social status. Yale Journal of Sociology 8:21–52, http://www.yale.edu/sociology/yjs/yjs_fall_2011.pdf. Accessed 19 June 2012
Gomez F, Simo JM, Camps J, Cliville X, Bertran N, Ferre N, Bofill C, Joven J (2000) Evaluation of a particle-enhanced turbidimetric immunoassay for the measurement of ferritin: application to patients participating in an autologous blood transfusion program. Clin Biochem 33:191–196
Fairbanks VF, Klee GG (1999) Biochemical aspects of haematology. In: Burtis CA, Ashwood ER (eds) Tietz textbook of clinical chemistry. WB Saunders, Philadelphia, pp 1698–1705
Centers for Disease Control and Prevention (1998) Recommendations to prevent and control iron deficiency in the United States. MMWR Recomm. Rep 47:1–29
Casanueva E, Viteri FE, Mares-Galindo M, Meza-Camacho C, Loria A, Schnaas L, Valdes-Ramos R (2006) Weekly iron as a safe alternative to daily supplementation for nonanemic pregnant women. Arch Med Res 37:674–682
Scanlon KS, Yip R, Schieve LA, Cogswell ME (2000) High and low hemoglobin levels during pregnancy: differential risks for preterm birth and small for gestational age. Obstet Gynecol 96:741–748
Reinold C, Dalenius K, Smith B, Brindley P, Grummer-Straw L (2011) Pregnancy nutrition surveillance 2009 report. Department of Health and Human Services. Centers for Disease Control and Prevention, Atlanta
Carrillo SM, Pérez Guillén A, Hernández Hernández RA, Herrera Mogollón HA (2010) Anthropometric nutritional evaluation of the pregnant women and its relation with the product of the gestation. Nutr Hosp 25:832–837
Río I, Castelló A, Jané M, Prats R, Barona C, Más R, Rebagliato M, Zurriaga O, Bolúmar F (2010) Reproductive and perinatal health indicators in immigrant and Spanish-born women in Catalonia and Valencia (2005–2006). Gac Sanit 24:123–127
Simpson JL, Bailey LB, Pietrzik K, Shane B, Holzgreve W (2011) Micronutrients and women of reproductive potential: required dietary intake and consequences of dietary deficiency or excess. Part II—vitamin D, vitamin A, iron, zinc, iodine, essential fatty acids. J Matern Fetal Neonatal Med 24:1–24
Walsh T, O’Broin SD, Cooley S, Donnelly J, Kennedy J, Harrison RF, McMahon C, Geary M (2011) Laboratory assessment of iron status in pregnancy. Clin Chem Lab Med 49:1225–1230
Zimmermann MB (2008) Methods to assess iron and iodine status. Br J Nutr 99(Suppl 3):S2–S9
Muñoz M, García-Erce JA, Remacha ÁF (2011) Disorders of iron metabolism. Part II: iron deficiency and iron overload. J Clin Pathol 64:287–296
Rambod M, Kovesdy CP, Kalantar-Zadeh K (2008) Combined high serum ferritin and low iron saturation in hemodialysis patients: the role of inflammation. Clin J Am Soc Nephrol 3:1691–1701
Milman N, Agger AO, Nielsen OJ (1994) Iron status markers and serum erythropoietin in 120 mothers and newborn infants. Effect of iron supplementation in normal pregnancy. Acta Obstet Gynecol Scand 73:200–204
Svanberg B, Arvidsson B, Norrby A, Rybo G, Solvell L (1975) Absorption of supplemental iron during pregnancy—a longitudinal study with repeated bone-marrow studies and absorption measurements. Acta Obstet Gynecol Scand Suppl 48:87–108
Soares NN, Mattar R, Camano L, Torloni MR (2010) Iron deficiency anemia and iron stores in adult and adolescent women in pregnancy. Acta Obstet Gynecol Scand 89:343–349
Ribot B, Aranda N, Viteri F, Hernández-Martínez C, Canals J, Arija V (2012) Depleted iron stores without anaemia early in pregnancy carries increased risk of lower birthweight even when supplemented daily with moderate iron. Hum Reprod 27:1260–1266
Milman N, Byg KE, Bergholt T, Eriksen L, Hvas AM (2006) Body iron and individual iron prophylaxis in pregnancy—should the iron dose be adjusted according to serum ferritin? Ann Hematol 85:567–573
Romslo I, Haram K, Sagen N, Augensen K (1983) Iron requirement in normal pregnancy as assessed by serum ferritin, serum transferrin saturation and erythrocyte protoporphyrin determinations. Br J Obstet Gynaecol 90:101–107
Zhang Q, Ananth CV, Li Z, Smulian JC (2009) Maternal anaemia and preterm birth: a prospective cohort study. Int J Epidemiol 38:1380–1389
Wahed F, Latif SA, Nessa A, Bhuiyan MR, Hossain MB, Akther A, Mahmud MM (2010) Gestational anemia. Mymensingh Med J 19:462–468
Scholl TO (2005) Iron status during pregnancy: setting the stage for mother and infant. Am J Clin Nutr 81:1218S–1222S
Scholl TO, Hediger ML, Fischer RL, Shearer JW (1992) Anemia vs iron deficiency: increased risk of preterm delivery in a prospective study. Am J Clin Nutr 55:985–988
Allen LH (2001) Biological mechanisms that might underlie iron’s effects on fetal growth and preterm birth. J Nutr 131(Suppl 2):581S–589S
Macedo A, Cardoso S (2010) Routine iron supplementation in pregnancy. Acta Med Port 23:785–792
Ziaei S, Norrozi M, Faghihzadeh S, Jafarbegloo E (2007) A randomised placebo-controlled trial to determine the effect of iron supplementation on pregnancy outcome in pregnant women with haemoglobin > or = 13.2 g/dl. BJOG 114:684–688
von Tempelhoff GF, Heilmann L, Rudig L, Pollow K, Hommel G, Koscielny J (2008) Mean maternal second-trimester hemoglobin concentration and outcome of pregnancy: a population-based study. Clin Appl Thromb Hemost 14:19–28
Acknowledgments
This study was financially supported by a grant (PI052462) from the Health Research Fund of the Ministry of Health and Consumption (Madrid, Spain) [Instituto de Salud Carlos III, Fondo de Investigación Sanitaria, Ministerio de Sanidad y Consumo]. The authors would like to thank all the participating pregnant women for their enthusiastic collaboration. We thank the Clinical Chemistry Laboratories of the Hospital Sant Joan de Reus (Catalunya, Spain) for help with the blood analyses. We thank Fernando Viteri, iron expert, professor emeritus at the University of California, Berkeley, and research scientist at the Children’s Hospital Oakland Research Institute, for his expert assistance with the study and Ginny Gildengorin for the discussion of the analysis.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ribot, B., Aranda, N., Giralt, M. et al. Effect of different doses of iron supplementation during pregnancy on maternal and infant health. Ann Hematol 92, 221–229 (2013). https://doi.org/10.1007/s00277-012-1578-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-012-1578-z