Abstract
Background/Objectives:
Iron in high doses or when given to non-anaemic women may have adverse effects on pregnancy outcomes. This study aimed to estimate the supplemental iron intake in non-anaemic pregnant women attending an urban antenatal care setting in South India and examine the association of supplemental iron intake with birth outcomes.
Subjects/Methods:
A cohort of 1196 non-anaemic pregnant women was studied. Daily supplemental iron intake was calculated as total supplemental iron consumed (mg) during pregnancy divided by the total number of days the supplement was recommended. Association of tertiles of supplemental iron intake with term low birth weight (tLBW), preterm delivery and small for gestational age (SGA) was examined using log-binomial regression, adjusting for maternal age, height, body mass index at recruitment, parity, education and type of delivery.
Results:
Mean haemoglobin in trimester 1 was 12.4±0.9 g/dl and mean supplemental iron intake was 37.7±4.0 mg/day. Women in the highest tertile (>39.2 mg/day) of supplemental iron intake had an increased risk of tLBW as compared with the lowest tertile (⩽36.6 mg/day) (adjusted risk ratio: 1.89; 95% confidence interval: 1.26, 2.83). Although supplemental iron intake was negatively correlated with gestational age (r=−0.20, P<0.001) and birth weight (r=−0.07, P=0.011), there was no association between preterm delivery or SGA and supplemental iron intake.
Conclusions:
It appears that iron supplementation in non-anaemic pregnant women may not be beneficial, as we have observed the adverse effects with a prescribed dose of 45 mg/day. This may warrant the consideration of an individualized approach for antenatal iron supplementation, especially in non-anaemic women.
Similar content being viewed by others
Introduction
Globally, it is estimated that about 40% of pregnant women are anaemic,1 and in India this estimate is almost 60%, making it a serious public health problem.2 Anaemia during pregnancy, defined as a haemoglobin (Hb) <11 g/dl, 3 is associated with an increased risk of adverse birth outcomes such as low birth weight (LBW), intrauterine growth restriction, preterm delivery and small for gestational age (SGA) infants. In addition, severe anaemia can lead to maternal and infant mortality.1, 2, 3, 4
At least half of the anaemia burden during pregnancy is due to iron deficiency,2 which can affect the foetus through impaired delivery of oxygen to the uterus, placenta and developing foetus itself. Pregnant women require a greater amount of iron to account for the needs of pregnancy and delivery,5 and are therefore at an increased risk of developing iron deficiency anaemia. Thus, to correct this, they are started on prophylactic oral iron supplements as soon as possible. In India, although the national guideline recommends an equivalent of 100 mg of elemental iron and 0.5 mg of folic acid to be supplemented for a minimum of 100 days during pregnancy,2 in practice, the dose of iron supplemented varies from place to place.6, 7 It is to be noted that pregnant women generally visit the antenatal centre only by the end of first trimester, which allows the antenatal care to commence only at that time or in general, by the start of second trimester. Anaemic women (Hb<11 g/dl) are prescribed higher doses of iron, according to the severity of their anaemia.3
Iron in high daily doses could be responsible for cellular damage through oxidative stress.8 It is speculated that non-transferrin bound iron (NTBI) in the blood8, 9 can affect the placenta by the release of reactive oxygen species leading to lipid peroxidation and DNA damage, resulting in outcomes such as intrauterine growth restriction, LBW, preterm delivery and SGA, as well as other complications.8, 10, 11 Adverse effects of high dose iron supplementation, like an increased risk of haemoconcentration at delivery and SGA, have been reported earlier in non-anaemic, iron-replete women,12, 13 such that the individualization of supplementation, rather than its generalization, is now suggested.5, 13 These effects have yet to be studied or reported in India, despite high supplemental iron intake in some parts of the country.6 This is particularly worrying as the recommended daily supplemental dose of iron given to all women, regardless of Hb, has been increased since 2013.2 Therefore, the present study aimed to examine the association of supplemental iron intake (45 mg/day) with birth outcomes, such as term LBW (tLBW), preterm delivery and SGA, in non-anaemic, pregnant women attending an urban antenatal care setting in South India.
Subjects and methods
Study design
The current study was part of a prospective observational cohort study of pregnant women conducted at St. John’s Research Institute and St. John’s Medical College Hospital (SJMCH) in Bangalore, India. The details of the cohort have been previously described.14 All pregnant women aged 17–40 years registered for antenatal screening in their first trimester at the Department of Obstetrics and Gynaecology at SJMCH were invited to participate in the study. Women with multiple foetuses, those with a clinical diagnosis of chronic illness such as diabetes mellitus, hypertension, heart disease and thyroid disease, those who tested positive for Hepatitis B surface antigen/HIV/syphilis infection (venereal disease) or who anticipated moving out of the city before delivery were excluded from the study. Initially, 2558 women consented to participate and were recruited of whom 429 were lost to follow-up. The common reasons for loss to follow-up were mostly independent of the study protocol such as distance to the hospital from the subjects’ home or the Indian cultural practice of women in their third trimester visiting their native place for delivery. The subjects included were those who had deliveries at the hospital. From the 1838 women who had live births in the cohort, 1196 healthy, non-anaemic women (Hb≥11 g/dl when measured in trimester 1), who were prescribed 45 mg of elemental iron and 0.5 mg of folic acid per day from the beginning of the second trimester, were included in the current study. This study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the institutional ethical review board of SJMCH Bangalore. Written informed consent was obtained from all study participants at enrolment.
Socio-demographic and anthropometric information
Socio-demographic data were collected at the end of the first trimester. Information on maternal anthropometry, clinical status and routine antenatal Hb was collected at the first, second and third trimesters. At the first visit, trained research assistants interviewed study subjects to obtain information on age, obstetric history and socioeconomic status. Gestational age was confirmed through ultrasonographic measurements measured on the ultrasound scanning machine (GE Voluson 730 Expert, probe 4C-A, Via Del Rio, Yorba Linda, CA, USA). A digital balance (Soehnle, Reutlingen, Germany) was used to record maternal weight to the nearest 100 g during each antenatal visit. The digital weighing scale was calibrated using standard weights once every month. Height was measured with a stadiometer to the nearest 0.1 cm.
Iron and folic acid supplementation
Folic acid (5 mg) supplementation was started at recruitment and continued until the end of first trimester. From the beginning of the second trimester until delivery, one tablet consisting of 150 mg ferrous sulfate (equivalent to 45 mg of elemental iron) along with 0.5 mg folic acid was prescribed per day. No other multivitamin or multi-mineral preparation was prescribed. Compliance with the prescribed iron supplement was recorded by a pill count from the empty blister packs that the study subjects returned at each visit. Daily supplemental iron intake was calculated as the total supplemental iron intake (mg) divided by the total number of days the supplement was recommended. Supplemental iron intake as a percentage of the prescribed amount was calculated as the total supplemental iron intake (mg) divided by the recommended supplemental iron intake (mg). Recommended supplemental iron intake is calculated as the amount of iron the woman was expected to consume from the beginning of second trimester till the end of her pregnancy. Average daily supplemental folic acid intake was calculated as a mean of the supplemental intake in trimesters 1, 2 and 3.
Dietary iron intake
A food frequency questionnaire was administered at each trimester visit to obtain information on habitual dietary intake for the preceding 3 months, as described in the previous publication.14
Blood sample collection and Hb analysis
Venous whole blood samples were collected into EDTA-coated anticoagulant tubes (Becton Dickenson, Franklin Lakes, NJ, USA) by trained laboratory assistants once every trimester. Blood Hb concentration was analyzed using an automated cyanmethaemoglobin technique (ABX Pentra 60 C+, Haematology analyzer, Horiba ABX diagnostics, Darmstadt, Germany). The measuring range was between 8 and 18 g/dl with a within run precision of >99%.
Delivery and birth information
Infants were weighed to the nearest 10 g on an electronic weighing scale (Salter Housewares 914 Electronic Baby and Toddler Scale, Oak Brook, IL, USA) immediately after birth. Infants born before 37 completed weeks of gestation were considered preterm. Infants weighing less than the 10th percentile for gestational age at delivery were defined as SGA.15 tLBW was defined as birth weight less than 2500 g among infants with gestational age greater than or equal to 37 weeks. Although the cutoff of <2500 g is applicable to infants born prematurely, we restricted our analysis to tLBW to keep the exposure of iron constant for the subjects and to remove the possible effect modification by prematurity.
Statistical analysis
The effects of daily supplemental iron intake during pregnancy (henceforth referred to as ‘supplemental iron intake’) on outcomes of tLBW, preterm delivery and SGA were examined using log-binomial regression. Tertiles of supplemental iron intake were considered as independent variables for all analyses, with the lowest tertile as the reference category. All potential confounders that were associated with tLBW, preterm delivery or SGA in the bivariate analysis at P<0.1 were included in the multivariate analysis. The crude risk ratio and adjusted risk ratio with 95% confidence interval (95% CI) of tLBW, preterm delivery and SGA were calculated. The association of supplemental iron intake with birth weight (in term infants) and gestational age at birth (GAB) was also examined using linear regression. For all analyses, two-sided P-values (P<0.05) were considered statistically significant. All statistical analyses were performed using the Statistical Analysis System (version 9.2; SAS, Cary, NC, USA) and the Statistical Package for Social Sciences V18 (IBM SPSS Statistics for Windows, Version 18.0. IBM Corp., Armonk, NY, USA). Log-binomial regression analysis was performed using the PROC GENMOD program in SAS.
Results
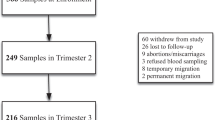
Of the 1838 live births, 1196 non-anaemic women who were prescribed 45 mg of elemental iron per day are included in the current study (Figure 1). Table 1 summarizes the general characteristics, iron supplementation and birth outcomes of the women participating in the study. The mean age of the subjects was approximately 25 years and 60% were nulliparous. About one-fifth were underweight with BMI<18.5 kg/m2 at recruitment. Approximately 1.6% of the subjects had a supplemental iron intake greater than 45 mg/day. The average supplemental iron intake in each tertile was 33.6, 37.8 and 41.5 mg/day, corresponding to 74, 84 and 95% of the prescribed amount, in tertile 1, 2 and 3, respectively. The dietary intake of iron between the tertiles of supplemental iron intake was comparable (15.0±4.3, 14.7±4.2, 14.5±3.8 mg/day; P=0.308). The socio-demographic profile of mothers excluded from this study because of lack of data on supplemental iron intake was comparable to those who were included; however, the gestational age at delivery was lower, as this excluded group also had women with foetal loss early in pregnancy.
There were 1093 subjects with term deliveries (GAB≥37weeks). There was a significant increase in the prevalence of tLBW (8.5, 10.4 and 16.8%) from the first to the third tertile of supplemental iron intake (P=0.001) (Table 2). The increased risk of tLBW in the highest tertile of iron intake was confirmed in the adjusted log binomial model (adjusted risk ratio of 1.89; 95% CI: 1.26, 2.83) adjusting for maternal age, BMI at recruitment, maternal height, parity, education and type of delivery. Preterm delivery and SGA were not associated with tertiles of supplemental iron intake (Table 2). The confounding effect of total supplemental folic acid intake was also examined. Adjusting for these intakes in the regression model did not significantly modify the association of iron intake with all the outcomes, thus demonstrating the independent effect of iron.
Birth weight and GAB were also significantly associated with supplemental iron intake. In linear regression models adjusted for the same confounding variables noted in Table 2, birth weight was 71.9 g lower (95% CI: −126.0, −17.8) in the highest tertile compared with the lowest tertile and GAB was 0.6 weeks lower (95% CI: −0.8, −0.4). Figure 2 depicts the means of birth weight and GAB within tertiles of supplemental iron intake.
Mean GAB and birth weight within tertiles of iron intake. Values are mean±s.e.; Solid line: GAB (n=1196); Dotted line: birth weight (n=1093). Values for supplemental iron intake: Tertile 1=⩽36.4 mg/day (mean: 33.6 mg/day; corresponds to ⩽81% of prescribed doses; mean dietary iron intake: 15.0 mg/day), Tertile 2=36.5–39.1 mg/day (mean: 37.8 mg/day; corresponds to 81–87% of prescribed doses; mean dietary iron intake: 14.7 mg/day), Tertile 3=>39.2 mg/day (mean: 41.5 mg/day; corresponds to >87% of prescribed doses; mean dietary iron intake: 14.5 mg/day); *significantly different between groups by analysis of variance and post hoc Bonferroni adjustment.
Discussion
Iron deficiency anaemia is a common problem during pregnancy and can have adverse effects on the mother and the baby, manifesting as pre-eclampsia, LBW, preterm delivery, SGA, intrauterine growth restriction, as well as maternal and infant mortality in severe cases. However, current global practice guidelines are not based on a woman’s initial Hb or iron status. Therefore, the effect of prophylactic oral iron supplementation is likely to be highly variable, depending on compliance to the medication, as well as the woman’s initial iron status. In our analysis of non-anaemic women, we observed that as compliance improved (examined as the proportion of recommended supplemental iron actually consumed), the risk of tLBW increased and the mean birth weight decreased by approximately 72 g. This study being a cohort had a variation in the dose of iron consumed depending on the compliance to the iron supplement, which enabled us to make observations on the effect of iron on outcomes such as LBW, preterm delivery and SGA. This is similar to other studies in which a high iron intake tended to have a higher proportion of LBW compared with moderate intake.12 Our findings are also particularly interesting, as they are seen in women whose supplemental iron intake was 45 mg/day, which is much lower compared with the current recommendation of 100 mg/day.2 Earlier analysis in the same cohort has shown that subjects with low B12 intake in the presence of high folate intake had a higher risk of SGA.14 Although in the cohort, iron was supplemented as a combined tablet with folic acid, we observed a poor correlation between total iron and total folic acid supplement intake during pregnancy. This may be because the latter was initiated without iron in the first trimester, with a dose 10 times higher compared with the combined iron and folic acid tablet in the second trimester. The quantum of folic acid intake in first trimester greatly depended on the gestational age at recruitment.
There has been a rising interest in the deleterious effect of iron supplementation.13, 16 Ziaei et al.13 examined the effects of iron supplementation in non-anaemic pregnant women, with Hb ≥13.5 g/dl, and found that those who were supplemented with a dose of 50 mg/day were at a higher risk of being SGA at birth. They also found that the prevalence of preterm deliveries was higher, although not significant. The Cochrane review included studies that assessed the effect of high and low doses of iron on birth weight, preterm delivery and LBW comparing the group receiving the supplement with those on a placebo.16 This review, however, did not include studies specifically assessing the effect of different doses of iron supplements in non-anaemic women. The current study on the other hand set out to explicitly evaluate the effect of relatively low daily doses of iron supplements in non-anaemic women. There are several possible mechanisms to explain the adverse effects. Until recently, the body’s mechanism to regulate the intestinal absorption of iron by changing the rate of its absorption was considered protective,17 leading to the belief that there was no upper limit for the dose of iron prescribed. More recent studies suggest that iron in high doses can cause oxidative stress through the increase in circulating NTBI, irrespective of basal iron status. In case of iron insufficiency as in iron-deficient anaemic women, the NTBI fraction, although potentially higher in concentration,18 may not be harmful because this iron can be rapidly used for erythropoiesis or can be added to the exhausted iron stores. However, in cases of iron sufficiency, the NTBI has the potential to be more toxic.19 With daily oral doses of supplemental iron, the transferrin in the circulation gets saturated, leading to an increase in the circulating NTBI levels. Studies have shown that the NTBI levels in the plasma following a high dose of oral iron are similar to that found in iron overload conditions, irrespective of the transferrin saturation.9 The NTBI is part of the free iron pool, which causes the release of oxygen-free radicals such as the hydroxyl ion (OH•) via the Fenton reaction. These hydroxyl radicals could cause damage to the placental cells by lipid peroxidation and damage to the DNA.8 This could in turn reduce the amount of nutrition reaching the foetus, thus impacting growth.20
In addition, there is an influx of oxygenated blood in the placenta due to the physiological opening of the spiral arteries, which occurs around the 10th to 12th week of gestation. This increases the oxidative stress at the placental level by two to three times.20 It is worth noting that the iron supplementation is also started around the same time during pregnancy and may contribute to the increased oxidative stress in the placenta. In summary, through oxidative stress, studies have shown that high doses of iron can reduce the growth of the baby, resulting in LBW infants.8, 13, 20 Failure of plasma volume expansion is shown to be associated with a higher incidence of LBW.21
An additional finding in this study was a small but significant effect of supplemental iron on GAB. Iron may reduce gestational age by a variety of mechanisms, one of them being the increased Hb, which combined with the failure of plasma volume expansion increases the viscosity of maternal blood, thus compromising the placental blood flow,22 although a concrete mechanism for the effect of iron on gestational age has not been postulated or proved yet. However, in the present study, the effect was small with a decrease in gestational age by half a week, and this could account for a smaller birth weight of 35 g assuming a foetal growth rate of 70 g/week in term deliveries.15 In effect, this could partially explain the difference in birth weight (72 g) between the first and third tertiles of iron intake.
Nonetheless, even if there was an increased risk of LBW with high iron supplementation, it is not advisable to change the present policy, in view of the risks and benefits. There is no doubt that in a resource-poor situation, individualizing prophylactic care is operationally difficult. The benefit of iron supplementation to anaemic pregnant women in terms of a decrease in LBW is offset by the risk of increased LBW in non-anaemic women, given that the percentage difference in both scenarios is 8% in this cohort of pregnant women. However, there is sufficient evidence on the additional benefits of supplementation on haematological outcomes, as well as on associated factors such as maternal morbidity and mortality, given that about 60% of women are likely to be anaemic.2 Therefore, the overall benefit of iron supplementation in anaemic women outweighs the risk of LBW accruing to iron supplemented non-anaemic women, and the operational difficulties only complement this reasoning. All the same, there is a need for further studies looking at the association of oral iron supplementation with birth outcomes in a larger, more diverse population in settings where women are prescribed various doses of iron including the ideal 100 mg elemental iron as per the current guidelines.2 In addition to the routine antenatal investigations, serum ferritin should also be measured (especially for non-anaemic women), as it is a more sensitive marker of iron status and will strengthen the findings.
The strengths of the study are its relatively large sample size, along with adequate variations in supplemental iron intake. The limitations of the study are the lack of biomarkers of iron status and its urban setting in a tertiary hospital, which precludes generalization beyond the sample.
In conclusion, it appears that a high iron intake during pregnancy is potentially adverse to women who started their pregnancy with a normal Hb, by increasing the risk of tLBW. However, the benefits of universal supplementation will continue to outweigh the risks in operationally difficult and resource-poor conditions. As the burden of anaemia falls in the population, considerations of targeted supplementation may become necessary.
References
World Health Organization Worldwide prevalence of anaemia 1993-2005: WHO global database on anaemia.. Geneva WHO, 2008, http://www.who.int/vmnis.
Ministry of Health and Family Welfare, Government of India: Guidelines for the control of iron deficiency anaemia: A national iron plus initiative. Ministry of Health and Family Welfare: Adolescent division 2013.
World Health Organization Guideline: Daily iron and folic acid supplementation in pregnant women. WHO Geneva, 2012.
Chang SC, O’Brien KO, Nathanson MS, Mancini J, Witter FR . Haemoglobin concentrations influence birth outcomes in pregnant African-American adolescents. J Nutr 2003; 133: 2348–2355.
Milman N . Oral iron prophylaxis in pregnancy: not too little and not too much!. J Pregnancy 2012; 2012: 514345.
Sharma JB, Jain S, Mallika V, Singh T, Kumar A, Arora R et al. A prospective, partially randomized study of pregnancy outcomes and hematologic responses to oral and intramuscular iron treatment in moderately anaemic pregnant women. Am J Clin Nutr 2004; 79: 116–122.
Chaturvedi S, Ranadive B . Are we really making motherhood safe? A study of provision of iron supplements and emergency obstetric care in rural Maharashtra. Natl Med J India 2007; 20: 294.
Casanueva E, Viteri FE . Iron and oxidative stress in pregnancy. J Nutr 2003; 133: 1700 S–1708 S.
Dresow B, Petersen D, Fischer R, Nielsen P . Non-transferrin-bound iron in plasma following administration of oral iron drugs. Biometals 2008; 21: 273–276.
Bhatla N, Kaul N, Lal N, Kriplani A, Agarwal N, Saxena R et al. Comparison of effect of daily versus weekly iron supplementation during pregnancy on lipid peroxidation. J Obstet Gynaecol Res 2009; 35: 438–445.
Beard JL . Effectiveness and strategies of iron supplementation during pregnancy. Am J Clin Nutr 2000; 71: 1288 s–1294 s.
Ribot B, Aranda N, Giralt M, Romeu M, Balaguer A, Arija V . Effect of different doses of iron supplementation during pregnancy on maternal and infant health. Ann Hematol 2013; 92: 221–229.
Ziaei S, Norrozi M, Faghihzadeh S, Jafarbegloo E . A randomised placebo-controlled trial to determine the effect of iron supplementation on pregnancy outcome in pregnant women with haemoglobin ≥13.2 g/dl. Br J Obstet Gynaecol 2007; 114: 684–688.
Dwarkanath P, Barzilay JR, Thomas T, Thomas A, Bhat S, Kurpad AV . High folate and low vitamin B-12 intakes during pregnancy are associated with small-for-gestational age infants in South Indian women: a prospective observational cohort study. Am J Clin Nutr 2013; 98: 1450–1458.
World Health Organization Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Committee. World Health Organ Tech Rep Ser 1995; 854: 1–452.
Peña-Rosas JP, De-Regil LM, Dowswell T, Viteri FE . Daily oral iron supplementation during pregnancy (review). Cochrane Database Syst Rev 2012; ((12)): CD004736.
Hall JE, Guyton AC . Textbook of medical physiology, 12th edn, Elsevier Saunders Inc.: Philadelphia, USA, 2011.
Brittenham GM, Andersson M, Egli I, Foman JT, Zeder C, Westerman ME et al. Circulating non–transferrin-bound iron after oral administration of supplemental and fortification doses of iron to healthy women: a randomized study. Am J Clin Nutr 2014; 100: 813–820.
Halliwell B, Gutteridge JMC . Oxygen, free radicals and iron in relation to biology and medicine: some problems and concepts. Arch Biochem Biophys 1986; 246: 501–514.
Burton GJ, Jauniaux E . Oxidative stress. Best Pract Res Clin Obstet Gynaecol 2011; 25: 287–299.
Aranda N, Ribot B, Viteri F, Cavalle P, Arija V . Predictors of hemoconcentration at delivery: association with low birth weight. Eur J Nutr 2012; 52: 1631–1639.
Steer P, Alam MA, Wadsworth J, Welch A . Relation between maternal haemoglobin concentration and birth weight in different ethnic groups. BMJ 1995; 310: 489–491.
Acknowledgements
We greatly appreciate the assistance of Nancy Nanditha M in the collection and entry of data. We thank the women and their infants who participated in this study and the doctors and nurses who made this study possible. LS, PEM, PD, TT and AVK took part in conceptualizing the study, analyzing data and writing the manuscript. PD and AT were involved in data collection. CD, RB and CMM were involved in analyzing data and writing the manuscript. AVK stands as guarantor of the study.
Registered at Clinical Trials Registry-India (www.ctri.nic.in) REF/2013/07/005342.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Shastri, L., Mishra, P., Dwarkanath, P. et al. Association of oral iron supplementation with birth outcomes in non-anaemic South Indian pregnant women. Eur J Clin Nutr 69, 609–613 (2015). https://doi.org/10.1038/ejcn.2014.248
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ejcn.2014.248
- Springer Nature Limited
This article is cited by
-
The relationship between markers of antenatal iron stores and birth outcomes differs by malaria prevention regimen—a prospective cohort study
BMC Medicine (2021)
-
Iron supplementation during pregnancy – a cross-sectional study undertaken in four German states
BMC Pregnancy and Childbirth (2018)
-
Review of the Plausibility of Iron Deficiency Hypothesis of Autism
Review Journal of Autism and Developmental Disorders (2017)