Abstract
Anthracyclines are a major component in the therapy of non-Hodgkin's lymphoma. However, due to their cardiac toxicity potential, curative and palliative treatment is often limited in patients with preexisting cardiac dysfunction. Liposomal doxorubicin formulations have been described to be less cardiotoxic than conventional doxorubicin. In the current study, we analyzed the efficacy and toxicity of pegylated liposomal doxorubicin (PLD) as constituent of the cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) regimen replacing conventional doxorubicin in 21 patients with impaired cardiac left ventricular ejection fraction or preexisting cardiac risk factors and established diagnosis of diffuse large B cell lymphoma (n = 15), mantle cell lymphoma (n = 3), follicular lymphoma (n = 1), and T cell lymphoma (n = 2). Overall and complete response rate were 85% and 40%, respectively. Event-free survival and overall survival after 2 years were 58%. One lethal event of acute cardiac death occurred during the first cycle in a patient with transposition of the big arteries, atrial flutter, and mitral valve regurgitation. In the remaining 20 patients, no deterioration of myocardial function was observed in echocardiography performed before and after treatment. Seven cases of grade III–IV hematological toxicity were observed as well as four episodes of neutropenic fever leading to hospitalization. No infection-related death occurred. However, 25% of patients developed a hand–foot syndrome (HFS) leading to discontinuation of treatment. Importantly, the incidence of HFS increased considerably when PLD doses of 15 mg/m2/week were exceeded. We conclude that replacing conventional doxorubicin with PLD in polychemotherapy regimens such as CHOP is an efficient alternative in the treatment of patients with preexisting cardiac dysfunction. However, we recommend that PLD dose should not exceed 15 mg/m2/week. The rationale for the use of non-pegylated liposomal doxorubicin formulations should be evaluated in further studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Many standard treatment regimens for aggressive and indolent lymphoma such as cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) contain an anthracycline as a major component of the polychemotherapy protocol. Anthracyclines are potent anti-lymphoma agents, and the development of immunochemotherapy in the treatment of non-Hodgkin's lymphoma improved the prognosis of these patients impressively [7, 20]. Anthracyclines are counted among the antibiotic anticancer drugs and act as an inhibitor of topoisomerase IIa via DNA intercaliation. Doxorubicin may also inhibit polymerase activity, affect regulation of gene expression, and produce free radical damage to DNA [10]. Most common side effects are nausea and vomiting, fatigue, hematotoxicity, and mucositis. A particular challenge in treatment with anthracycline is early and late cardiotoxicity, which, in its pathogenesis, is not yet completely understood [25]. As far as it is elucidated, cardiotoxic events take place by increasing oxidative stress, suppression of gene expression, and induction of apoptosis on cardiac tissue [28] with clinical manifestations reaching from acute cardiac decompensation to chronic cardiac insufficience. Anthracycline cardiotoxicity is exponentially dose dependent with an incidence of up to 30% above 500 mg/m2 [15, 33]. In a retrospective analysis of 4,018 patient records, Von Hoff and co-workers explicated several risk factors for the development of cardiotoxicity as history of hypertension or heart disease, age at time of exposure (the very young and very old are most susceptible), high peak serum level of the drug, and concurrent chemo- and radiotherapy [31].
Based on these findings, the risk for an anthracycline-caused cardiomyopathy is exponentially elevated in elderly patients with cardiac predamage. Taking in consideration that aggressive non-Hodgkin's lymphoma (NHL) is a potentially curative disease, management of therapy-limiting adverse events becomes a major task. Concerning the successful action of anthracyclines as chemotherapeutic agents, several strategies have been tried to prevent/attenuate their side effects. Dexrazoxane, a Fe III chelator which decreases free radical development in myocardiac tissue, has shown to reduce anthracycline cardiotoxicity in clinical phase III studies, but negative effects on disease response rates cannot be excluded [26]. Carvedilol, a cardioselective beta blocker with antioxidant properties, seems to reduce cardiotoxic effects of anthracyclines in animal models [9]. In addition, in recent history, liposomal formulations of doxorubicin have been developed to reduce cardiac side effects of anthracycline chemotherapy by a pharmacokinetic approach. Pegylated non-liposomal (Myocet©) and pegylated liposomal (Caelyx©) doxorubicin formulations are designed to reduce cardiotoxicity by restraining in healthy vessels and extravasating in regions were cell–cell boundaries were disrupted because of tumor infiltration [27, 32]. Recent data from clinical trials point to a reduced cardiotoxicity and preserved anti-tumor efficacy of liposome-encapsulated formulations [12, 24]. Preexisting data from clinical trials concerning the use of PLD mostly derived from patients with breast cancer point to an improved cardiac tolerability and adequate response rates. In 2004 O' Brien and colleagues showed that PLD provides comparable efficacy to doxorubicin (PFS of 6.9 versus 7.8 months, OS of 21 versus 22 months), with significantly reduced cardiotoxicity (HR 3.16) in first-line treatment of women with metastatic breast cancer [18]. In a clinical phase I and II study, Anton and co-workers [1, 2] showed that a combination chemotherapy of trastuzumab, docetaxel, and liposomal non-pegylated doxorubicin is an effective neoadjuvant treatment with favorable cardiotoxicity profile in patients with stage II and IIIA Her2-overexpressing breast cancer. Data from clinical studies of patients with NHL and patients with preexisting cardiac morbidity treated with liposomal doxorubicin are rare. Zaja and colleagues published a study on 30 patients with a median age of 69 years and primary diagnosis of diffuse large-cell lymphoma treated with pegylated liposomal doxorubicin as component of rituximab + CHOP (R-CHOP) regimen and observed overall response and complete response rates of 76% and 59%. Projected 2-year event-free survival and overall survival in this study were 65.5% and 68.5% [34]. Besides hematologic and infectious adverse events, PLD formulations may result in the development of hand–foot syndrome (HFS). HFS is a potentially dose-limiting cutaneous toxicity of many chemotherapeutic agents, which manifests with palmar and plantar erythema, edema, and dysesthesia with varying degrees of pain, scaling, and vesiculation [17]. HFS has been reported as a side effect of several classical and modern therapeutic agents such as cytarabin, 5-FU, capecitabine, sorafenib, and sunitinib, with a time of onset varying from 24 h to 10 months after starting the causative medication [3]. A review published in 1991 by Baack and co-workers noted that the risk of HFS may be affected by four specific pharmacologic properties: dose, peak plasma level, total cumulative dose, and schedule of administration. At this time, the pathogenesis is incompletely understood, but clues from the histopathologic findings, risk factors, and clinical features have led to two primary theories to explain HFS; one contends that drugs cause the syndrome via a direct toxic effect, while the other invokes a “host-versus-altered host” response [4, 17]. In literature (Caelyx Product information, Schering–Plough Corporation, 2006), occurrence of therapy-limiting HFS is stated 4–7% during treatment with liposomal doxorubicin, and few is known about an effective treatment of this adverse event. As published earlier, reduction of dose intensity seems to be a successful approach to reduce the risk of HFS and has been used in patients with several different tumor types [8, 16]. Moreover, only symptomatic treatment and patient education seem to have a beneficial effect on development of HFS and patients' quality of life, but valid clinic data are lacking. In our study, we retrospectively analyzed data of patients with NHL treated with liposomal non-pegylated doxorubicin instead of conventional formulations because of high age and/or other preexisting risk factors for the development of anthracycline-dependent cardiomyopathy. We want to raise the question if such therapy is active against NHL and feasible by reasons of adverse events especially in older and cardiac-impaired patients.
Patients and methods
In the current study, we retrospectively analyzed clinical data of 21 patients (15 diffuse large B cell lymphoma, 3 mantle cell lymphoma, 1 follicular lymphoma, 2 T cell lymphoma) who were treated with PLD as a component of R-CHOP regimen. Sixteen patients were at primary diagnosis, whereas five experienced a recurrent disease. Average age at diagnosis was 67.5 years (median 72 years) with a third of the patients over the age of 75. Patients were treated with PLD because of cardiac risk factors, poor general condition, and/or high age. The study was approved by the local ethics committee. Demographic data of the patients included are listed in Table 1. Response was evaluated after three and six cycles of chemotherapy via CT scan, laboratory testing, and clinical assessment using the International Working Group (IWG) response criteria for non-Hodgkin's lymphomas [6]. Before and after treatment, left ventricular ejection fraction was assessed by 2-D echocardiography and quantified as recommended by the American Society of Cardiology [14]. To objective side effects, NCI Common Terminology Criteria for Adverse Events were used (http://ctep.cancer.gov/).
Results
Cardiac risk factors
All patients were treated with liposomal pegylated instead of conventional doxorubicin because of high age and/or cardiac risk factors. Nine patients had a history of manifest myocardial ischemia and coronary heart disease, two patients had a known dilatative cardiomyopathy, one patient had a bilateral pulmonary embolism in the recent medical history, and seven patients showed an impairment of left ventricular ejection fraction under conventional doxorubicin or had already been treated with a cumulative threshold dose of anthracycline, respectively. Cardiac risk factors indicating the use of a liposomal doxorubicin formulation are specified in Table 2. The majority of patients showed cardiac impairment due to ischemic cardiomyopathy with a relevant number of the patients showing more than one cardiac risk factors.
Toxicity
On the whole, a number of 78 cycles were applied during which one lethal event of acute cardiac death occurred during the first cycle in a patient with transposition of the big arteries, atrial flutter, and mitral valve regurgitation. In echocardiography performed before and after treatment with liposomal doxorubicin, no deterioration of myocardial function was observed in the 20 patients evaluated. For a detailed listing of left ventricular ejection fraction, see Table 3. An average number of 3.7 cycles were applied (median, 4 cycles). Severe adverse events leading to therapy termination were hematotoxicity grade III–IV (19%), HFS (25%), and infections (28.5%). The incidence of HFS seems to correlate with the applied peak dose as no case of HFS was observed in patients when less than 15 mg/m2/week of PLD was applied (Fisher test, p = 0.052; see Table 4).
Remission status
Remission was evaluated using the IWG response criteria for lymphoma at the end of chemotherapy. Furthermore, remission was assessed with clinical and laboratory examinations every 3 months. Complete remission was present in 8/20 (40%), and partial remission was observed in 9/20 (45%) leading to an overall response rate of 85%. One patient showed a stable disease, whereas two patients with T cell lymphoma had a progressive disease.
Event-free survival and overall survival
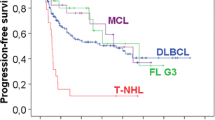
Twenty of the 21 patients could be analyzed for event-free survival and overall survival. In the study, an event was defined as death by any cause as well as tumor progression and newly developed cardiac disease. Data were analyzed using the Kaplan–Maier method. The median overall survival and event-free survival were not reached. Two-year overall survival and event-free survival were 58% (Fig. 1). Interestingly, no difference in median event-free survival was observed between the two patient groups who received PLD ≤15 mg/m2/week vs. PLD >15 mg/m2/week (Fig. 2). As expected, patients with low or intermediate–low international prognostic index (IPI) had a superior outcome compared to intermediate–high or high-risk patients (Fig. 3).
Discussion
In the current study, we addressed the question if substitution of conventional doxorubicin by a liposomal formulation is a feasible and effective therapeutic option in patients with contraindication for anthracycline-based treatment. We retrospectively analyzed data of 21 patients treated with liposomal pegylated doxorubicin as a component of R-CHOP regimen. PLD was indicated in patients with preexisting cardiac impairment, high age, and/or impaired general condition. In the 21 patients analyzed, a number of 78 cycles have been administered, with a median number of four cycles per patient. Complete remission was present in 40%; partial remission was observed in 45% leading to an overall response rate of 85%. From randomized clinical trials, complete remission rates for aggressive lymphoma treated with R-CHOP-14 regimen are ranging from 55% [23] to 78% [21] with 3-year event-free survival rates of 66.5% [21]. Data from clinical trials of NHL patients treated with liposomal doxorubicin are rare. Zaja and colleagues published a study on 30 patients with a median age of 69 years and primary diagnosis of diffuse large-cell lymphoma treated with pegylated liposomal doxorubicin as a component of R-CHOP regimen and observed overall response and complete response rates of 76% and 59%, respectively. Projected 2-year event-free survival and overall survival in this study were 65.5% and 68.5% [34]. A Greek and an Austrian research group obtained similar results using a liposomal non-pegylated doxorubicin formulation as R-CHOP component in patients with aggressive NHL with complete response rates of 52% with a median time to progression of 26 months [29] and 75% CR rates with 73% of the patients alive after a median observation time of 14 months [13]. From the studies mentioned above, only Heintel and colleagues included elderly patients with cardiac comorbidity as well as patients with relapsed disease and T cell lymphoma so that we conclude that this might be the reason for the inferiority of our remission rates compared to the published data of Zaja and Tsavaris. These findings are consistent with a recent study published by Gimeno et al. in which 35 frail, elderly patients were treated with intermediate-dose non-pegylated liposomal doxorubicin (30 mg/m2) in R-CHOP-like regimen [11]. In this study, overall and complete response rates of 86% and 69%, respectively, with 2-year overall survival rates of 70% were reported. Major toxicities observed in our study were infections (28.5%), hand–foot syndrome (25%), and neutropenia (19%). Published data from studies assessing liposomal doxorubicin in lymphoma treatment show similar results [13, 29, 34]. Previously published data from randomized clinical trials assessing the R-CHOP-14 regimen suggest rates of common toxicity criteria (CTC) grade 3/4—infections between 16% [23] and 29% [21] as well as neutropenia CTC grade 3/4 between 19% [5] and 48% [21], respectively. Therefore, we conclude that replacement of conventional doxorubicin seems to have no negative effect on incidence and severity of infectious complications and hematotoxicity. In our study, 25% of the patients developed a HFS, which was one of the major reasons for preterm therapy termination. In our patients, development of HFS seems to correlate positively with the dose intensity as published before [8]. The company recommends a dose of 10 mg/m2 Caelyx/week as several studies indicate a significant increase of HFS with higher dosage [22]. Unfortunately, randomized studies assessing efficacy of R-CHOP regimen with this dosage are lacking. In our collective, dose escalation seems not to have a positive effect on event-free survival as no difference was found between average event-free survival in the subgroups treated with <15 mg/m2 and ≥15 mg/m2, respectively (see Table 4 and Fig. 1). The reason for this observation could be the increasing number of therapy interruptions when doses >15 mg/m2/week were administered. Clinical studies using liposomal pegylated or non-pegylated doxorubicin instead of conventional doxorubicin in polychemotherapy regimens for several tumor entities show that rates of cardiotoxic events of non-pegylated liposomal formulations were in between pegylated liposomal at one end and conventional doxorubicin on the other end [2, 26, 30]. Previous reports provide evidence that PEG-modified liposomes may be of little advantage in terms of maximizing drug accumulation in tumor sites compared to other liposomal formulations [19]. In the study published by Heintel et al., non-pegylated liposomal doxorubicin was included in the R-CHOP-14 schedule showing complete remission rates of 75% with 73% of the patients alive after a median observation time of 14 months. No case of HFS and no cardiotoxic event were observed [13]. In our study, during the 78 cycles applied, only one event of acute cardiotoxicity was recorded. This 70-year-old patient with serious cardiac predamage died from acute cardiac decompensation. In echocardiography performed before and after treatment, no significant change was found among the remaining 20 patients analyzed, and to date, no cardiac event pointing to a chronic cardiac toxicity was recorded.
Altogether, we conclude that replacement of conventional doxorubicin by its liposomal pegylated formulation is a feasible therapeutic option, which is effective in patients with cardiac impairment, high age, and reduced general condition. Major therapy-limiting events were infections (28.5%), the development of a HFS in 25% of subjects. The incidence of HFS increases considerably when doses of 15 mg/m2/week were exceeded. As no prophylaxis or curative treatment option for HFS is known, we recommend that the applied doses of PLD should not exceed 15 mg/m2/week, or alternatively, a non-pegylated liposomal doxorubicin formulation should be evaluated. However, these results are based on a small retrospective analysis and should be confirmed in a prospective randomized trial.
References
Antón A, Ruiz A, Seguí MA, Calvo L, Muñoz M, Lao J, Sancho F, Fernández L (2009) Phase I clinical trial of liposomal-encapsulated doxorubicin citrate and docetaxel, associated with trastuzumab, as neo-adjuvant treatment in stages II and IIIA, HER2-overexpressing breast cancer patients. GEICAM 2003–03 study. Ann Oncol 20:454–459, Epub 2008 Dec 11
Antón A, Ruiz A, Plazaola A, Calvo L, Seguí MA, Santaballa A, Muñoz M, Sánchez P, Miguel A, Carrasco E, Lao J, Camps J, Alfaro J, Antolín S, Cámara MC (2010) Phase II clinical trial of liposomal-encapsulated doxorubicin citrate and docetaxel, associated with trastuzumab, as neoadjuvant treatment in stages II and IIIA HER2-overexpressing breast cancer patients. GEICAM 2003–03 study. Ann Oncol 22:74–79
Baack BR, Burgdorf WH (1991) Chemotherapy-induced acral erythema. J Am Acad Dermatol 24:457–461
Beard JS, Smith KJ, Skelton HG (1993) Combination chemotherapy with 5-fluorouracil, folinic acid, and alpha-interferon producing histologic features of graft-versus-host disease. J Am Acad Dermatol 29:325–330
Brusamolino E, Rusconi C, Montalbetti L, Gargantini L, Uziel L, Pinotti G, Fava S, Rigacci L, Pagnucco G, Pascutto C, Morra E, Lazzarino M, 4 (2006) Dose-dense R-CHOP-14 supported by pegfilgrastim in patients with diffuse large B-cell lymphoma: a phase II study of feasibility and toxicity. Haematologica 91:496–502, Epub 2006 Mar 15
Cheson BD, Horning SJ, Coiffier B, Shipp MA, Fisher RI, Connors JM, Lister TA, Vose J, Grillo-López A, Hagenbeek A, Cabanillas F, Klippensten D, Hiddemann W, Castellino R, Harris NL, Armitage JO, Carter W, Hoppe R, Canellos GP (1999) Report of an international workshop to standardize response criteria for non-Hodgkin's lymphomas. NCI Sponsored International Working Group. J Clin Oncol 17(4):1244
Coiffier B, Lepage E, Briere J et al (2002) CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med 346:235–242
Coleman RE, Biganzoli L, Canney P et al (2006) A randomised phase II study of two different schedules of pegylated liposomal doxorubicin in metastatic breast cancer (EORTC-10993). Eur J Cancer 42:882–887
de Nigris F, Rienzo M, Schiano C, Fiorito C, Casamassimi A, Napoli C (2008) Prominent cardioprotective effects of third generation beta blocker nebivolol against anthracycline-induced cardiotoxicity using the model of isolated perfused rat heart. Eur J Cancer 44(3):334–340
Gewirtz DA (1999) A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochem Pharmacol 57(7):727–741
Gimeno E, Sánchez-González B, Alvarez-Larrán A, Pedro C, Abella E, Comín J, Saumell S, García-Pallarols F, Gómez M, Besses C, Salar A (2011) Intermediate dose of nonpegylated liposomal doxorubicin combination (R-CMyOP) as first line chemotherapy for frail elderly patients with aggressive lymphoma. Leuk Res 35:358–362
Harris L, Batist G, Belt R, Rovira D, Navari R, Azarnia N, Welles L, Winer E, TLC D-99 Study Group (2002) Liposome-encapsulated doxorubicin compared with conventional doxorubicin in a randomized multicenter trial as first-line therapy of metastatic breast carcinoma. Cancer 94(1):25–36
Heintel D, Skrabs C, Hauswirth A, Eigenberger K, Einberger C, Raderer M, Sperr WR, Knöbl P, Müllauer L, Uffmann M, Dieckmann K, Gaiger A, Jäger U (2010) Nonpegylated liposomal doxorubicin is highly active in patients with B and T/NK cell lymphomas with cardiac comorbidity or higher age. Ann Hematol 89(2):163–169
Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise J, Solomon S, Spencer KT, St John Sutton M, Stewart W, 2 (2006) American Society of Echocardiography's Nomenclature and Standards Committee; Task Force on Chamber Quantification; American College of Cardiology Echocardiography Committee; American Heart Association; European Association of Echocardiography, European Society of Cardiology. Recommendations for chamber quantification. Eur J Echocardiogr 7:79–108
Lefrak EA, Pitha J, Rosenheim S, Gottlieb JA (1973) A clinicopathologic analysis of Adriamycin cardiotoxicity. Cancer 32:302–314
Lorusso D, Naldini A, Testa A, D'Agostino G, Scambia G, Ferrandina G (2004) Phase II study of pegylated liposomal doxorubicin in heavily pretreated epithelial ovarian cancer patients. May a new treatment schedule improve toxicity profile? Oncology 67:243–249
Nagore E, Insa A, Sanmartin O (2000) Antineoplastic therapy-induced palmar plantar erythrodysesthesia (‘hand-foot’) syndrome. Incidence, recognition and management. Am J Clin Dermatol 1:225–234
O'Brien ME, Wigler N, Inbar M, Rosso R, Grischke E, Santoro A, Catane R, Kieback DG, Tomczak P, Ackland SP, Orlandi F, Mellars L, Alland L, Tendler C (2004) CAELYX Breast Cancer Study Group. Reduced cardiotoxicity and comparable efficacy in a phase III trial of pegylated liposomal doxorubicin HCl (CAELYX/Doxil) versus conventional doxorubicin for first-line treatment of metastatic breast cancer. Ann Oncol 15(3):440–449
Parr MJ, Masin D, Cullis PR, Bally MB (1997) Accumulation of liposomal lipid and encapsulated doxorubicin in murine Lewis lung carcinoma: the lack of beneficial effects by coating liposomes with poly(ethylene glycol). J Pharmacol Exp Ther 280(3):1319–1327
Pfreundschuh M, Trümper L, Osterborg A, Pettengell R, Trneny M, Imrie K, Ma D, Gill D, Walewski J, Zinzani PL, Stahel R, Kvaloy S, Shpilberg O, Jaeger U, Hansen M, Lehtinen T, López-Guillermo A, Corrado C, Scheliga A, Milpied N, Mendila M, Rashford M, Kuhnt E, Loeffler M, MabThera International Trial Group (2006) CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lymphoma: a randomised controlled trial by the MabThera International Trial (MInT) Group. Lancet Oncol 7:379–391
Pfreundschuh M, Schubert J, Ziepert M, Schmits R, Mohren M, Lengfelder E, Reiser M, Nickenig C, Clemens M, Peter N, Bokemeyer C, Eimermacher H, Ho A, Hoffmann M, Mertelsmann R, Trümper L, Balleisen L, Liersch R, Metzner B, Hartmann F, Glass B, Poeschel V, Schmitz N, Ruebe C, Feller AC, Loeffler M, German High-Grade Non-Hodgkin Lymphoma Study Group (DSHNHL) (2008) Six versus eight cycles of bi-weekly CHOP-14 with or without rituximab in elderly patients with aggressive CD20+ B-cell lymphomas: a randomised controlled trial (RICOVER-60). Lancet Oncol 9(2):105–116
Rose PG (2005) Pegylated liposomal doxorubicin: optimizing the dosing schedule in ovarian cancer. Oncologist 10(3):205–214
Rueda A, Sabin P, Rifá J, Llanos M, Gómez-Codina J, Lobo F, García R, Herrero J, Provencio M, Jara C (2008) Grupo Oncológico para el Tratamiento y Estudio de los Linfomas (GOTEL) R-CHOP-14 in patients with diffuse large B-cell lymphoma younger than 70 years: a multicentre, prospective study. Hematol Oncol 26(1):27–32
Safra T (2003) Cardiac safety of liposomal anthracyclines. Oncologist 8(Suppl 2):17–24
Simůnek T, Stérba M, Popelová O, Adamcová M, Hrdina R, Gersl V, 1 (2009) Anthracycline-induced cardiotoxicity: overview of studies examining the roles of oxidative stress and free cellular iron. Pharmacol Rep 61:154–171
Swain SM, Whaley FS, Gerber MC, Weisberg S, York M, Spicer D, Jones SE, Wadler S, Desai A, Vogel C, Speyer J, Mittelman A, Reddy S, Pendergrass K, Velez-Garcia E, Ewer MS, Bianchine JR, Gams RA (1997) Cardioprotection with dexrazoxane for doxorubicin-containing therapy in advanced breast cancer. J Clin Oncol 15(4):1318–1332
Symon Z, Peyser A, Tzemach D, Lyass E, Sucher E, Shezen E, Gabizon A (1999) Selective delivery of doxorubicin to patients with breast carcinoma metastases by stealth liposomes. Cancer 86(1):72–78
Takemura G, Fujiwara H, 5 (2007) Doxorubicin-induced cardiomyopathy from the cardiotoxic mechanisms to management. Prog Cardiovasc Dis 49:330–352
Tsavaris N, Kosmas C, Vadiaka M, Giannouli S, Siakantaris MP, Vassilakopoulos T, Pangalis GA, 3 (2002) Pegylated liposomal doxorubicin in the CHOP regimen for older patients with aggressive (stages III/IV) non-Hodgkin's lymphoma. Anticancer Res 22:1845–1848
van Dalen EC, Michiels EM, Caron HN, Kremer LC (2010) Different anthracycline derivates for reducing cardiotoxicity in cancer patients. Cochrane Database Syst Rev 5:CD005006
Von Hoff DD, Layard MW, Basa P, Davis HL Jr, Von Hoff AL, Rozencweig M, Muggia FM (1979) Risk factors for doxorubicin-induced congestive heart failure. Ann Intern Med 91(5):710–717
Waterhouse DN, Tardi PG, Mayer LD, Bally MB (2001) A comparison of liposomal formulations of doxorubicin with drug administered in free form. Drug Saf 24(12):903–920
Yeh ET, Tong AT, Lenihan DJ, Yusuf SW, Swafford J, Champion C, Durand JB, Gibbs H, Zafarmand AA, Ewer MS (2004) Cardiovascular complications of cancer therapy: diagnosis, pathogenesis, and management. Circulation 109(25):3122–3131
Zaja F, Tomadini V, Zaccaria A, Lenoci M, Battista M, Molinari AL, Fabbri A, Battista R, Cabras MG, Gallamini A, Fanin R (2006) CHOP-rituximab with pegylated liposomal doxorubicin for the treatment of elderly patients with diffuse large B-cell lymphoma. Leuk Lymphoma 47(10):2174–2180
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schmitt, C.J., Dietrich, S., Ho, A.D. et al. Replacement of conventional doxorubicin by pegylated liposomal doxorubicin is a safe and effective alternative in the treatment of non-Hodgkin's lymphoma patients with cardiac risk factors. Ann Hematol 91, 391–397 (2012). https://doi.org/10.1007/s00277-011-1308-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-011-1308-y