Abstract
Secondary central nervous system (CNS) involvement in diffuse large B-cell lymphoma (DLBCL) includes an isolated CNS relapse or CNS involvement with systemic disease progression. This rare but fatal clinical problem still remains a therapeutic dilemma in the management of DLBCL. However, there are limited data about its treatment outcome. In this study, we gathered 73 cases with secondary CNS involvement of DLBCL from 11 hospitals in Korea. The data were retrospectively compared according to the status of systemic disease (isolated vs. combined CNS involvement) and the use of high-dose methotrexate treatment (HD MTX). Twenty-nine patients showed isolated CNS involvement while 44 had combined CNS involvement with systemic relapse or progression. Thirty-three cases (45.2%) occurred within 6 months from the initial diagnosis, and the majority of these were associated with systemic disease relapse or progression (n = 27). In isolated CNS involvement, HD MTX resulted in fewer treatment failures (3/11) than the other treatments such as other salvage chemotherapy and/or radiotherapy/intraventricular chemotherapy (14/15). However, neither HD MTX nor other treatments were effective at reducing the treatment failure rate in combined CNS involvement (8/10 and 23/30, respectively). Thus, isolated CNS involvement had a better survival than combined involvement (P = 0.008), but systemic disease progression was the main cause of death in combined as well as isolated CNS involvement. In conclusion, the prognosis of secondary CNS involvement was dismal even after intensive chemotherapy using HD MTX. Further research focusing on the development of an optimal treatment strategy is warranted.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Secondary central nervous system (CNS) involvement in patients with non-Hodgkin lymphoma (NHL) is a devastating complication that leads to death in most cases [1, 2]. The reported incidence is variable depending on various factors, such as the subtype of NHL, the study population, and associated risk factors [3–6]. CNS involvement can occur as an isolated event despite systemic remission, or might be combined with systemic disease relapse or progression [7]. In diffuse large B-cell lymphoma (DLBCL), secondary CNS involvement is uncommon, but does occur. A previous database study of 605 newly diagnosed DLBCL patients who had not undergone CNS prophylaxis showed that the probability of secondary CNS involvement at 1 year after diagnosis was 4.5% [4]. Therefore, CNS prophylaxis such as intrathecal prophylaxis with methotrexate (MTX) has been considered in DLBCL patients at high risk of CNS involvement. However, the use of CNS prophylaxis in DLBCL patients remains controversial [8, 9]. A recent report from the RICOVER-60 trial suggested that intrathecal prophylaxis has no role in preventing secondary CNS involvement for patients treated with R-CHOP-14 (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone) [10]. Thus, considering the unclear benefit of CNS prophylaxis and the poor prognosis, an effective treatment strategy is required for secondary CNS involvement. However, there are limited data on the treatment outcomes for patients with DLBCL with secondary CNS involvement. Thus, an isolated CNS involvement during follow-up or CNS involvement combined with systemic disease progression remains a therapeutic dilemma in DLBCL. A report from the International Primary CNS Lymphoma Collaborative Group suggested that systemic MTX might produce a favorable outcome in isolated CNS relapse involving brain parenchyma [11]. However, the efficacy of salvage treatments, including systemic MTX, remains uncertain in the case of CNS involvement combined with systemic disease progression. Thus, a comparison of treatment outcomes of isolated and combined CNS involvement might help to establish a treatment strategy for this rare but fatal complication of DLBCL. In this study, we analyzed the treatment outcomes of DLBCL patients with secondary CNS involvement by comparing cases of isolated and combined CNS involvement.
Patients and methods
Data collection and eligibility criteria
Patients were diagnosed with DLBCL according to the Revised European-American Lymphoma classification or the World Health Organization classification. Among patients diagnosed as DLBCL between 1994 and 2008, those with isolated CNS relapse or CNS involvement combined with systemic disease progression or relapse were selected for this retrospective analysis. All patients should be initially treated with chemotherapy such as CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) or rituximab plus CHOP or CHOP-like regimens as a curative intent. Patients who initially had CNS involvement were excluded. Patients at high risk of secondary CNS involvement such as testicular DLBCL were also excluded because intrathecal chemoprophylaxis and cerebrospinal fluid (CSF) analysis were repeated in these patients, and this might affect the outcome of secondary CNS involvement. The investigators affiliated with the Consortium for Improving Survival of Lymphoma, a Korean lymphoma study group, reviewed the medical records of patients with DLBCL and filled out data collection forms for information regarding secondary CNS involvement. Requested information included patient demographics and clinical characteristics at the initial presentation, the type of first-line treatment and its response, neurologic symptoms associated with the secondary CNS involvement, the date of diagnosis of secondary CNS involvement and systemic relapse or progression, treatment modalities for secondary CNS involvement, systemic lymphoma, treatment response, and survival status. This study was approved by the Institutional Review Board of each institute for the release of case information, which was rendered anonymous. Informed consent was exempted because this was a retrospective study.
Diagnosis of secondary CNS involvement
Secondary CNS involvement was defined as an isolated CNS involvement despite systemic remission (isolated CNS involvement) or CNS involvement at the time of systemic relapse or progression (combined CNS involvement). In order to diagnose isolated CNS involvement, the systemic evaluations including chest and abdomen–pelvic computed tomography (CT) scan and/or 18F-fluorodeoxyglucose positron emission tomography/CT scan at the time of CNS involvement should demonstrate no evidence of systemic disease progression or relapse. The time points of secondary CNS involvement were categorized according to the time from diagnosis of DLBCL as follows: (1) <6 months, (2) 6–12 months, and (3) >12 months. The patterns of CNS involvement were parenchymal, leptomeningeal, and combined involvement. Parenchymal involvement was diagnosed with a brain CT scan or MRI. Leptomeningeal involvement was diagnosed using one of the following three criteria: (1) presence of lymphoma cells in the CSF, (2) presence of suspicious cells with increased protein levels in the CSF, or (3) presence of suspicious cells with radiographic findings.
Treatment response
Treatment response was assessed according to the International Working Group criteria published in 1999 [12]. A complete response (CR) was defined as the disappearance of all previously measurable lesions and the absence of any new tumor lesions. Unconfirmed complete response was defined as ≥75% reduction of lesions on CT scan with no radiologic or clinical evidence of new lesions. Partial response was defined as a decrease of at least 50% in the product of the two perpendicular diameters of each measurable lesion. Stable disease was defined as less than a partial response but not progressive disease, and progressive disease was defined as a ≥50% increase in the product of the two diameters of at least one tumor, or the presence of a newly developed lesion. In cases with leptomeningeal involvement, negative CSF cytology and no evidence of leptomeningeal enhancement were defined as CR. Disease progression of leptomeningeal involvement was diagnosed if the CSF cytologic examination continued to show persistent malignant or suspicious cells, additional new lesions were found in brain imaging studies, or neurologic manifestations persisted.
Statistical analysis
The relationship of secondary CNS involvement with clinical characteristics was evaluated using the chi-square test. The length of time to secondary CNS involvement was defined as the time from the beginning of therapy to the diagnosis of secondary CNS involvement, and the overall survival (OS) after the secondary CNS involvement was defined as the time from the date of CNS involvement to the time of death from any cause. These survival curves were estimated using Kaplan–Meier methods. The follow-up duration was calculated by the method of Kaplan–Meier estimate of potential follow-up as previously reported [13]. A two-sided P value of less than 0.05 was considered significant.
Results
Characteristics of patients and secondary CNS involvement
Seventy-three patients from 11 hospitals in Korea were analyzed; of these, 29 patients showed isolated CNS involvement and 44 had combined CNS involvement. The median follow-up duration of these 73 patients from the initial diagnosis of DLBCL was 33.93 months (95% confidence interval (CI) 20.17–47.69 months). Baseline characteristics of patients at diagnosis were not significantly different between these two groups (Table 1). However, patients with combined CNS involvement were more likely to have an elevated level of serum lactate dehydrogenase (LDH) (75%, 33/44) compared with those with isolated CNS involvement (51.7%, 15/29). Thirty-three cases (33/73, 45.2%) with secondary CNS involvement occurred within 6 months from the initial diagnosis of DLBCL, and the majority of these were associated with systemic disease relapse or progression (Table 2). The median time to secondary CNS involvement was shorter in cases with combined CNS involvement (5.63 months, 95% CI 4.58–6.68 months) than in those with isolated CNS involvement (10.20 months, 95% CI 5.38–15.02 months). The most common neurologic manifestations were cranial nerve-associated symptoms such as facial palsy and diplopia (Table 2).
Treatments and outcomes of secondary CNS involvement
Sixty-six patients received high-dose MTX-based treatment (HD MTX, n = 21) or other treatments (n = 45) while seven patients did not receive any treatment due to follow-up loss. In the HD MTX group, MTX was used alone (n = 5) or in combination with other drugs such as procarbazine and vincristine (n = 16). In 12 patients, 3.5 g/m2 MTX was infused over 6 h every 2 weeks, while in the other patients the dose of MTX was reduced to 1.5 g/m2 (n = 5) or 3.0 g/m2 (n = 4). Twenty-seven patients received localized CNS-directed therapy (Local Tx) alone: intrathecal chemotherapy (n = 11), whole brain radiotherapy (n = 6), or both (n = 10). The other 18 patients received systemic chemotherapy other than HD MTX with the addition of Local Tx after the occurrence of secondary CNS involvement (CTx/Local Tx).
Among 66 patients who received any treatment as a curative intent, 26 patients had isolated CNS involvement and 40 patients had combined CNS involvement. Twenty-six patient with isolated CNS involvement received treatment as follows (Table 3): HD MTX (n = 11), Local Tx (n = 13), and CTx/Local Tx (n = 2). HD MTX was predominantly used for patients with parenchymal involvement (n = 8) while Local Tx was mainly used for leptomeningeal involvement (n = 9). The overall response of CNS lesions was 80.7% (21/26), but 12 patients died due to CNS or systemic disease progression and 5 were lost to follow-up, corresponding to a treatment failure rate of 65.3% (17/26) (Table 3). The median OS of patients treated with HD MTX (13.93 months, 95% CI 0.33–27.53 months) was better than that of other treatments (5.63 months, 95% CI 0.01–11.56 months) although this difference was not statistically significant (P = 0.093, Fig. 1a).
a Overall survival (OS) after isolated CNS involvement. Patients treated with high-dose methotrexate-based chemotherapy (HD MTX) showed a better survival trend than patients who received other treatments (P = 0.093). b We found no significant differences among patients with combined CNS involvement according to the treatment modalities (P = 0.699)
Among 40 patients treated for combined CNS involvement, 16 patients received a more intensive salvage chemotherapy regimen with localized CNS-directed therapy (Table 3). Another 10 patients were treated with HD MTX, and the other 14 patients were primarily treated with localized CNS-directed therapy (Table 3). The overall response rate of CNS lesions was 50.0% (22/44); however, 50% of the patients subsequently showed systemic disease progression. The outcome of parenchymal involvement combined with systemic disease progression was poor; thus, the response to HD MTX was worse than the response of those with isolated parenchymal involvement (Table 3). Although leptomeningeal involvement was well controlled by the addition of local therapy (11 CR among 12 patients treated with CTx/Local Tx, 91.7%), the median survival of patients treated with CT/Local Tx (5.77 months, 95% CI 4.27–7.27 months) was not different from that of patients treated with HD MTX (4.33 months, 95% CI 2.74–5.92 months, Fig. 1b).
Patterns of treatment failure
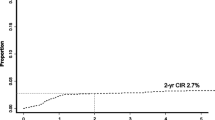
If we consider that the patients lost during follow-up might have experienced disease progression, both death and loss to follow-up could be regarded as treatment failure. In cases of isolated CNS involvement, high-dose MTX chemotherapy showed a lower number of treatment failures compared with other treatments (3/11 versus 14/15, Table 3). However, neither high-dose MTX nor other treatments were effective in reducing the number of treatment failures in cases of combined CNS involvement. Thus, the majorities of patients either died or were lost during follow-up, with the main cause of death being systemic disease progression (Table 3). Therefore, when the OS of all patients with secondary CNS involvement (n = 73) was compared according to the type of involvement pattern regardless of treatment, patients with isolated CNS involvement (n = 29) showed a better survival than those with combined CNS involvement (n = 44, P = 0.008, Fig. 2).
Discussion
Many risk factors for secondary CNS involvement have been suggested, but there is still no clear consensus about which patients are at risk of secondary CNS involvement and what the CNS prophylaxis indications are in DLBCL patients [14]. Although the goal of our study was not to identify risk factors for secondary CNS involvement of DLBCL, we did observe that the proportion of patients with bone marrow involvement, advanced disease stage, elevated serum LDH, and increased International Prognostic Index was high in our series (Table 1). These baseline characteristics might represent a patient group at increased risk of secondary CNS involvement and are similar to those reported previously [11]. There were no significant differences between the clinical features of isolated and combined CNS involvement except that serum LDH was more frequently elevated in combined CNS involvement (Table 2).
The results of our study showed that most cases (51/73, 69.9%) of secondary CNS involvement occurred early, either during or after the first-line of treatment (median time to CNS involvement—5.80 months), a finding that is consistent with previous studies reporting similar median times from 5.4 to 6.0 months [4, 15, 16]. These data suggest that most cases of secondary CNS involvement have subclinical CNS involvement at diagnosis. Although a series of recent published reports suggested that the addition of rituximab to CHOP could reduce secondary CNS involvement in DLBCL, this reduction was mainly in the incidence of CNS relapse after 1 year [10, 17, 18]. Thus, considering that a large proportion of secondary CNS involvement occurred early in the disease course, the protective effect of rituximab against CNS involvement might be limited in the presence of occult CNS disease at diagnosis. Thus, an active evaluation to identify the involvement of CNS at diagnosis should be performed in DLBCL patients when a risk of secondary CNS involvement is clinically suspected.
The prognosis of patients experiencing secondary CNS involvement, especially during progression or after relapse of systemic lymphoma is extremely poor [4, 6, 19]. We observed many deaths due to systemic disease progression, especially in cases of combined CNS involvement. Thus, combined CNS involvement can be regarded as an expression of end-stage lymphoma refractory to most therapeutic strategies. However, active treatment with high-dose MTX was reported to prolong the OS of patients with isolated brain parenchyma involvement [11]. High-dose MTX-based chemotherapy also appeared to be effective for isolated parenchymal involvement in our study because six patients were alive at the time of the final analysis. The outcome of localized CNS-directed therapy with or without systemic chemotherapy was worse than that of high-dose MTX-based chemotherapy (Table 3). In cases of isolated leptomeningeal CNS involvement, localized therapy produced CR rates that were comparable to those of high-dose MTX-based chemotherapy, but their survival outcomes were poor and death due to systemic disease progression or follow-up loss was frequently observed. Thus, the main cause of treatment failure in isolated CNS involvement was systemic disease progression rather than CNS progression (Table 3). Considering the relatively lower incidence of CNS progression in patients treated with high-dose MTX-based chemotherapy, systemic administration of high-dose MTX might be helpful in controlling the CNS lesions. However, its role in controlling systemic disease progression is still not clear.
Regardless of the treatment modality or the pattern of CNS involvement, the outcomes of patients with combined CNS involvement were worse than those with isolated CNS involvement (Fig. 2). Systemic disease progression accounted for the majority of deaths in cases with combined CNS involvement (Table 3); thus, high-dose MTX was not effective in controlling systemic disease in this condition. Furthermore, the strategy of systemic chemotherapy against systemic disease progression and intrathecal chemotherapy against leptomeningeal CNS involvement also failed to improve outcomes for patients with combined CNS involvement because of the frequent progression of systemic disease. Although this study summarized the outcomes of a relatively large number of patients, it was a retrospective analysis with several weak points. For example, the treatments were heterogeneous because participating centers used their own treatment regimens or strategies. Thus, the dosage of MTX varied from 1.5 to 3.5 g/m2—a variation that might affect the outcomes of patients treated with high-dose MTX-based chemotherapy since the dosage of MTX has been reported to be a factor influencing the outcome of primary CNS lymphoma [20]. Therefore, comparison of the efficacies of those treatments could not be conclusive although high-dose MTX-based chemotherapy appeared to be beneficial for patients with isolated CNS involvement, especially brain parenchymal involvement.
In summary, the prognoses of patients with secondary CNS involvement remain dismal. Therefore, a prospective study with a uniform treatment strategy comprising CNS-penetrating agents and intensive systemic therapy to control systemic disease is warranted to establish a more effective treatment strategy for patients with isolated and combined CNS involvement.
References
Haioun C, Besson C, Lepage E, Thieblemont C, Simon D, Rose C, Tilly H, Sonet A, Lederlin P, Attal M, Briere J, Reyes F (2000) Incidence and risk factors of central nervous system relapse in histologically aggressive non-Hodgkin’s lymphoma uniformly treated and receiving intrathecal central nervous system prophylaxis: a GELA study on 974 patients. Groupe d’Etudes des Lymphomes de l’Adulte. Ann Oncol 11:685–690
Hollender A, Kvaloy S, Nome O, Skovlund E, Lote K, Holte H (2002) Central nervous system involvement following diagnosis of non-Hodgkin’s lymphoma: a risk model. Ann Oncol 13:1099–1107
Hegde U, Filie A, Little RF, Janik JE, Grant N, Steinberg SM, Dunleavy K, Jaffe ES, Abati A, Stetler-Stevenson M, Wilson WH (2005) High incidence of occult leptomeningeal disease detected by flow cytometry in newly diagnosed aggressive B-cell lymphomas at risk for central nervous system involvement: the role of flow cytometry versus cytology. Blood 105:496–502
van Besien K, Ha CS, Murphy S, McLaughlin P, Rodriguez A, Amin K, Forman A, Romaguera J, Hagemeister F, Younes A, Bachier C, Sarris A, Sobocinski KS, Cox JD, Cabanillas F (1998) Risk factors, treatment, and outcome of central nervous system recurrence in adults with intermediate-grade and immunoblastic lymphoma. Blood 91:1178–1184
MacKintosh FR, Colby TV, Podolsky WJ, Burke JS, Hoppe RT, Rosenfelt FP, Rosenberg SA, Kaplan HS (1982) Central nervous system involvement in non-Hodgkin’s lymphoma: an analysis of 105 cases. Cancer 49:586–595
Bierman P, Giglio P (2005) Diagnosis and treatment of central nervous system involvement in non-Hodgkin’s lymphoma. Hematol Oncol Clin North Am 19:597–609, v
Tomita N, Kodama F, Kanamori H, Motomura S, Ishigatsubo Y (2006) Secondary central nervous system lymphoma. Int J Hematol 84:128–135
Dietrich PY, Dietrich PY (2009) Intrathecal MTX for DLBCL: from an inappropriate prophylactic tradition to a medical error? Blood 114:1999, author reply 1999
Schmitz N, Zeynalova S, Loeffler M, Pfreundschuh M (2009) Response: intrathecal methotrexate and central nervous system events. Blood 114:1999–2000
Boehme V, Schmitz N, Zeynalova S, Loeffler M, Pfreundschuh M (2009) CNS events in elderly patients with aggressive lymphoma treated with modern chemotherapy (CHOP-14) with or without rituximab: an analysis of patients treated in the RICOVER-60 trial of the German High-Grade Non-Hodgkin Lymphoma Study Group (DSHNHL). Blood 113:3896–3902
Doolittle ND, Abrey LE, Shenkier TN, Tali S, Bromberg JE, Neuwelt EA, Soussain C, Jahnke K, Johnston P, Illerhaus G, Schiff D, Batchelor T, Montoto S, Kraemer DF, Zucca E (2008) Brain parenchyma involvement as isolated central nervous system relapse of systemic non-Hodgkin lymphoma: an International Primary CNS Lymphoma Collaborative Group report. Blood 111:1085–1093
Cheson BD, Horning SJ, Coiffier B, Shipp MA, Fisher RI, Connors JM, Lister TA, Vose J, Grillo-Lopez A, Hagenbeek A, Cabanillas F, Klippensten D, Hiddemann W, Castellino R, Harris NL, Armitage JO, Carter W, Hoppe R, Canellos GP (1999) Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas. NCI Sponsored International Working Group. J Clin Oncol 17:1244
Schemper M, Smith TL (1996) A note on quantifying follow-up in studies of failure time. Control Clin Trials 17:343–346
van Besien K, Gisselbrecht C, Pfreundschuh M, Zucca E (2008) Secondary lymphomas of the central nervous system: risk, prophylaxis and treatment. Leuk Lymphoma 49(Suppl 1):52–58
Bernstein SH, Unger JM, Leblanc M, Friedberg J, Miller TP, Fisher RI (2009) Natural history of CNS relapse in patients with aggressive non-Hodgkin’s lymphoma: a 20-year follow-up analysis of SWOG 8516—the Southwest Oncology Group. J Clin Oncol 27:114–119
Tilly H, Lepage E, Coiffier B, Blanc M, Herbrecht R, Bosly A, Attal M, Fillet G, Guettier C, Molina TJ, Gisselbrecht C, Reyes F (2003) Intensive conventional chemotherapy (ACVBP regimen) compared with standard CHOP for poor-prognosis aggressive non-Hodgkin lymphoma. Blood 102:4284–4289
Villa D, Connors JM, Shenkier TN, Gascoyne RD, Sehn LH, Savage KJ (2009) Incidence and risk factors for central nervous system relapse in patients with diffuse large B-cell lymphoma: the impact of the addition of rituximab to CHOP chemotherapy. Ann Oncol 21:1046–1052
Shimazu Y, Notohara K, Ueda Y (2009) Diffuse large B-cell lymphoma with central nervous system relapse: prognosis and risk factors according to retrospective analysis from a single-center experience. Int J Hematol 89:577–583
Feugier P, Virion JM, Tilly H, Haioun C, Marit G, Macro M, Bordessoule D, Recher C, Blanc M, Molina T, Lederlin P, Coiffier B (2004) Incidence and risk factors for central nervous system occurrence in elderly patients with diffuse large-B-cell lymphoma: influence of rituximab. Ann Oncol 15:129–133
Uhm JE, Kim KH, Yi SY, Chang MH, Park KW, Kong DS, Lee JI, do Nam H, Park W, do Lim H, Kim SJ, Kim K, Ko YH, Kim WS (2009) A retrospective study to compare two methotrexate-based regimens for primary central nervous system lymphoma. Leuk Lymphoma 50:1110–1118
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Kim, S.J., Oh, S.Y., Kim, J.S. et al. Secondary central nervous system (CNS) involvement in patients with diffuse large B-cell lymphoma: a therapeutic dilemma. Ann Hematol 90, 539–546 (2011). https://doi.org/10.1007/s00277-010-1104-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-010-1104-0