Abstract
In 1992–1993, synergistic interaction of ribonucleotide reductase inhibitors (fludarabine, cladribine) and cytarabine (Ara-C) increasing Ara-CTP concentration in myeloblasts was proved. Based on these findings and encouraging results of the addition of cladribine to standard daunorubicin+Ara-C induction regimen (DAC) in acute myeloid leukemia (AML), the Polish Adult Leukemia Group (PALG) conducted a pilot study on the administration of cytarabine, daunorubicin, and fludarabine (DAF) as a reinduction treatment of AML to assess tolerance, toxicity, and early outcome. The DAF regimen consisted of daunorubicine 60 mg m−2 day−1 iv on days 1–3 and fludarabine 25 mg m−2 day−1 iv on days 1–5 given before cytarabine 200 mg m−2 day−1 in ci on days 1–7. Thirty-four AML patients with median age 39, 24% relapsed and 76% refractory, were included into the study between September 2003 and August 2004. Achieved response rate in the whole study population was 56%; n = 16 patients with complete remission (CR), and n = 3 patients with partial remission (PR). Fifteen of 16 patients achieved CR after the first course of therapy. Only 9% of total population died before the assessment of remission. All patients developed severe neutropenia. Serious infections were observed in 47% of the cases. Severe thrombocytopenia was observed in 72% of the patients. All patients required substitution of platelet concentrates (median 4), and PRBC (median 5). Severe alopecia, mucositis, vomiting were of low frequency. Liver, kidney, or circulatory failure, diarrhea, or polyneuropathy were not observed. The probability of overall survival (OS) for 1 year for the whole study population (34 patients) and the group of 16 patients in CR was: 44% (95% confidence interval [CI] 36–52%) and 69% (95% CI 55–83%), respectively. The probability of leukemia-free survival (LFS) for 1 year was 38% (95% CI 22–54%). Summarizing, DAF regimen used as the induction therapy in relapsed/refractory AML was well tolerated with acceptable toxicity and early efficacy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Results of acute myeloid leukemia (AML) patient’s therapy are still unsatisfactory. Limited effectiveness of induction chemotherapy regimens influence on decision to undertake clinical trials on fludarabine (FA) and cladribine (2-CdA) in the treatment of AML patients.
The first results of these medications in monotherapy of relapsed and refractory cases were not encouraging [1, 2].
However, in 1992–1993 synergistic interaction of ribonucleotide reductase inhibitors (fludarabine, cladribine) and cytarabine (Ara-C) resulting in increased Ara-CTP concentration in myeloblasts was proved [3–5]. The authors reported that infusion of fludarabine before infusion of an intermediate dose of Ara-C influence almost doubled the increase of Ara-CTP accumulation in circulating blasts collected from patients with AML [6]. Further experiments “in vitro” showed that this effect is related to leukemic cell lineage and is more frequently observed in myeloid than in lymphoid cells [7].
The above findings encouraged independent investigators to conduct trials on the combination of high-dose (HD) cytarabine and FA or 2-CdA as a second or succeeding line of induction chemotherapy in AML, which resulted in such regimens as: FLAG, FLAG-Ida, FLANG, FLAD, FLAI, CLAG, CLAG-M and did not confirm the preliminary concerns related to the escalation of toxicity [8–14].
In the previous studies, we proved that the addition of cladribine to standard 3+7 induction (DAC) and to consolidation chemotherapy with HD Ara-C is well tolerated and resulted in: (1) a higher rate of complete remission (CR) after the first induction course, and (2) in better survival in high risk AML patients in comparison with standard daunorubicin+AraC (DA) regimen [15, 16].
Based on above findings and continuing studies on using purine analogues in induction chemotherapy of AML patients, the Polish Adult Leukemia Group (PALG) carried out a two-phase clinical study to assess the safety, tolerance, and early outcome of the Daunorubicin, cytarabine and fludarabine (DAF) regimen (standard DA with the addition of fludarabine). Relapse or/and refractory adult acute myeloid leukemia patients were chosen as the study population. The aim of this report is to provide an assessment of this study.
This is the first analysis of an induction therapy of AML using the combination of Ara-C with FA and DNR in one regimen.
Patients and methods
Patient selection
Patients were considered eligible if they: (1) had diagnosed refractory or relapsed AML, (2) were in performance status ≤2 according to the WHO scale, and (3) had a life expectancy of ≥6 weeks.
Patients were excluded if they: (1) had acute promyelocytic leukemia, (2) had infection or organ insufficiency of grades III–IV according to the WHO criteria, or (3) refused to participate in the study.
Written informed consent was obtained from all patients before registration. The study was approved by a local ethics committee at each center.
Treatment protocol
The DAF induction regimen consisted of daunorubicine 60 mg m−2 day−1 iv on days 1–3 and fludarabine 25 mg m−2 day−1 in 1/2-h iv infusions on days 1–5 given before cytarabine 200 mg m−2 day−1 in ci on days 1–7. In the case of patients with partial remission (PR), the same induction regimen was repeated as soon as possible. In the event of non-remission (NR) after induction therapy, patients were treated alternatively.
After achieving CR, all patients were referred to high-dose consolidation chemotherapy followed by stem cells transplantation (SCT).
Consolidation treatment consisting of two consecutive courses was recommended for patients with no bone marrow donor: (1) HAM (AraC 1.5 g m−2 day−1 iv on days 1–3, mitoxantrone 10 mg m−2 day−1 on days 3–5) and 2) high-dose (HD) AraC (2 g m−2 iv every 12 h on days 1, 3, and 5.
The patients with suitable donor were referred to allogeneic hematopoietic stem cells transplantation (alloSCT) as soon as possible, whereas those without a donor were referred to autologous SCT (autoSCT) with stem cells collection after a second consolidation course.
The patients who were not eligible for SCT were receiving maintenance therapy for 2 years: DNR 45 mg m−2 iv on day 1 + AraC 100 mg m−2 sc every 12 h on days 1–5 alternately every second month with 6-thioguanin 100 mg m−2 day−1 po on days 1–5 + AraC 100 mg m−2 sc every 12 h on days 1–5. The maintenance treatment in AML is recommended by the Polish Adult Leukemia Group based on previous studies as a therapeutic strategy in patients who cannot be treated using SCT, or as a temporal therapeutic bridge in those cases who are waiting for unrelated alloSCT [15, 16].
Growth factors were not used during chemotherapy and to support hematopoietic recovery, either.
The study was approved by the local ethics committee at each center and was performed in accordance with the Helsinki Declaration of 1975.
Diagnosis, response criteria, and statistics
The efficacy of DAF therapy was established according to the criteria of Cheson et al. 2003 [17]: (1) complete remission (CR): ≤5% of blasts infiltration in bone marrow without Auer’s rodes, with ANC >1.0 g/l, and PLT >100 g/l; (2) partial remission (PR): 5–25% of blast infiltration or blast reduction by 50% in bone marrow or <5% of blasts in bone marrow, but with Auer’s rods, and with CR criteria for peripheral blood; (3) morphologic CR with incomplete blood count (CRi) when all of the criteria for CR are fulfilled except for residual neutropenia (<1.0 g/l) or thrombocytopenia (<100 g/l).
Treatment failure (NR) includes the patients who did not achieve CR, PR or died before the assessment of remission (ED).
Leukemia-free survival (LFS) was defined as the time from the documentation of CR to the date of (I) relapse or (II) death from any cause, or (III) the last survival and relapse-free date. The duration of overall survival (OS) was measured from the date of entry into the study to the date of death from any cause. Patients surviving at the time of the analysis were censored on the date they were last known to be alive. Survival curves of patients were prepared using the Kaplan–Meier method. Ninety-five percent confidence intervals were calculated for LFS and OS.
Adverse events were assessed using WHO scoring system.
Cytogenetic analysis
Cytogenetic risk categories were defined according to the Southwest Oncology Group (SWOG) criteria [18] (Table 1).
Results
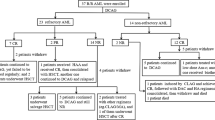
Thirty-four adult patients with AML, who gave their consent to this therapy: median age 39 (19–62) years; sex: female–12, male–22), were treated with DAF regimen in the period of 12 months from September 2003 to August 2004 in four PALG centers.
Refractoriness or early relapse (before 6 months) was established in 76% (n = 26) and late relapse in 24% (n = 8) of the cases.
Clinical and laboratory characteristics of the patients in subgroups are presented in Table 2.
All the patients were in a good clinical condition (0–10 according to WHO criteria) before entry into the study.
Response to the DAF regimen
Response after DAF induction was achieved in 56% of treated patients: n = 16 patients with CR (47%); n = 3 patients with PR (9%; Table 3). We did not observe patients with CR with incomplete blood count recovery. In 15 cases (44%) the treatment failed to achieve a therapeutic success: n = 12 patients with NR (35%); n = 3 patients who died (9% ED).
The response in relapsed and in refractory/early relapsed AML patients, and in separate cytogenetic risk groups is given in Table 3. We did not find any difference in the therapy results between these groups (p = NS). Early deaths were observed during the agranulocytosis period after chemotherapy, because of bacterial sepsis (Table 4).
Toxicity
All the patients developed grade IV neutropenia according to WHO criteria after the DAF therapy, median 0.0 (0.0–0.44). Severe infections (grade III or IV) were observed in 47% (n = 16) of the cases: bacterial in 38% (n = 13), and fungal in 9% (n = 3). Median duration of an infection-related fever (>38°C), and intravenous antibiotic therapy was 6 and 18 days, respectively.
Severe alopecia was observed in 40% of the patients, severe mucositis in 18%, serious vomiting in 18%. Liver, kidney, or circulatory failure, diarrhea or polyneuropathy after DAF regimen were not observed. The patients required a hospital stay for a median of 32 (11–79) days during the therapy. Detailed data concerning posttherapeutic cytopenia and hemopoetic recovery are listed in Table 5.
Disease-free and overall survival
The patients were followed-up for about 761 days (range 529–993 days). The median OS for the whole patients population was 262 days (range 9–993 days) and for patients who achieved CR: 579 days (range 67–843 days). The probability of OS for 1 year for the whole study population (34 patients) and the group of 16 patients in CR was: 44% (95% CI 36–52%) and 69% (95% CI 55–83%), respectively (Fig. 1). Median time of LFS was 218 days (range 33–762). The probability of LFS for 1 year was 38% (95% CI 22–54%) (Fig. 2).
Postinduction treatment
All 16 patients in CR after DAF regimen entered the consolidation phase of treatment. Four of 16 patients in CR received SCT as postremission treatment; three alloSCT and one autoSCT. Eleven from remaining 12 patients relapsed during the following 6 months of therapy: eight during consolidation therapy, and remaining three after the first cycle of maintenance phase. Median LFS in transplanted patients was significantly longer compared with the group treated with chemotherapy alone, and amounted to 717 days and 81.5 days, respectively (p = 0.006). Median OS was 761 days in group 1 and 469 in group 2 (p = NS).
Discussion
Antileukemic action of active metabolite of fludarabine F-ara-A-(9-β-d-arabino-faranosyl-2-fluoroadenine) triphosphade is multidirectional. FA is incorporated into elongating nucleic acid chains, which results in the termination of DNA or RNA synthesis, and also inhibits several intracellular enzymes [19]. The first clinical use of fludarabine refers to lymphoproliferative disorders [20].
The first reports of FA used in AML concerned a single agent treatment in relapsed or refractory cases [21]. Next, the advantage of synergistic activity of FA and Ara-C was taken in AML therapy. The potential effect of fludarabine plus cytarabine on cells positive for multidrug resistance has also been shown [22]. First reports of this combination in relapsed and resistant AML cases (36% CR) and in newly diagnosed poor prognosis patients with AML (41% CR) was described in the MD Anderson Cancer Center [6, 23].
The next step in the studies of using FA and Ara-C regimens in AML was the addition of G-CSF as a rendering of previously dormant leukemic cells more sensitive to chemotherapy via mechanism of cycling, significantly enhance Ara-C incorporation into DNA, irrespective of the number of cells in s-phase [24]. This regimen was called FLAG by Estey et al. and described as well tolerated and effective in poor prognosis AML patients [25].
Next, antracyclines were added to FLAG because of the hypothesis that the addition of these drugs may result in further antileukemic synergism [9, 10, 26].
It must be stressed that our report provides the first observation of regimen consisting of standard induction 3 + 7 DNR plus Ara-C with the addition of FA (DAF) in AML patients. This regimen was composed by analogy to previously described DAC (DNR, Ara-C, 2-CdA) protocol, which was well tolerated, and had better results in comparison with standard regimen in high-risk AML patients [15, 16]. Our observations were encouraging, so PALG decided to begin studies with the second widely used purine analogue fludarabine in AML.
Cytarabine dose 200 mg daily was chosen because the tendency for improved survival in patients aged <60 years was found for AraC 200 mg compared with AraC 100 mg [27]. Daunorubicin was given in dose 60 mg m−2 per day based on sequential studies by the SWOG (Southwest Oncology Group), and the ECOG (Eastern Cooperative Oncology Group), which showed a better response rate for patients receiving DNR 60–70 mg m−2 compared to DNR 45 mg m−2. Fludarabine was given as a standard and well tolerated in other regimens a 25 mg m−2 day−1 dose.
We compared this II phase study with DAF regimen results with II phase study with DAC regimen in previously untreated AML patients [15]. In both studies, all the patients developed severe IV grade agranulocytosis with influence on the frequency of severe infections (36% in DAC, 47% in DAF regimen). Death complications were noted more frequently in DAC than in DAF regimen (18 vs. 9%, respectively). A hospital stay with median 32 days and with hematological recovery time after DAC and the DAF therapy was similar.
A complete remission rate after the DAF therapy equaled 47% (16/34) of the patients. In other protocols describing alternative induction with a high dose of cytarabine with purine analogues used in relapsed/refractory AML patients, CR equaled 49–82% [8–13] (Table 6).
Survival and especially LFS were better after DAF induction therapy in group of transplanted patients.
In conclusion, it must be underlined that DAF is a well-tolerated induction regimen with acceptable toxicity. This regimen with a standard dose of Ara-C was an effective induction therapy in poor risk-relapsed/refractory AML patients in comparison with other alternative reinduction regimens with high-dose cytarabine, and should be recommended especially in cases with bone marrow donor, and with the perspective of alloSCT.
These results encouraged us to design an original, randomized, multicenter trial comparing DAF with DAC, and with the standard daunorubicin+cytarabine (3 + 7) induction program. This project is currently ongoing.
References
Gandhi V (1993) Fludarabine for treatment of adult acute myelogenous leukemia. Leuk Lymphoma 11:7–13
Santana VM, Hurvitz CA, Blakley RL, Crom WR, Luo X, Roberts WM, Ribeiro R, Mahmoud H, Krance RA (1994) Complete hematologic remissions induced by 2-chlorodeioxyadenosine in children with newly diagnosed acute myeloid leukemia. Blood 84:1237–1242
Gandhi V, Plunkett W (1992) Cell cycle-specific metabolism of arabinosyl nucleosides in K 562 human leukemia cells. Cancer Chemother Pharmacol 31:11–17
Gandhi V, Estey E, Keating MJ, Chucrallah A, Plunkett W (1996) Chlorodeoxyadenosine and arabinosylcytosine in patients with acute myelogenous leukemia: pharmacokinetic, pharmacodynamic, and molecular interactions. Blood 87:256–264
Kornblau SM, Gandhi V, Andreeff HM, Beran M, Kantarjian HM, Koller CA, O’Brien S, Plunkett W, Estey E (1996) Clinical and laboratory srudies of 2-chlorodeoxyadenosine ± cytosine arabinoside for relapsed or refractory acute myelogenous leukemia in adults. Leukemia 24:1563–1569
Gandhi V, Estey E, Keating MJ, Plunkett W (1993) Fludarabine potentiates metabolism of cytarabine in patients with myelogenous leukemia during therapy. J Clin Oncol 11:116–124
Ahlmann M, Lanvers C, Lumkemann K, Rossig C, Freund A, Baumann M, Boos J (2001) Modulation of ara-CTP levels by fludarabine and hydroxyurea in leukemic cells. Leukemia 15:69–73
Clavio M, Venturino C, Pierri I, Garrone A, Miglino M, Canepa L, Balleari E, Balocco M, Michelis GL, Ballerini F, Gobbi M (2004) Combination of liposomal daunorubicin (DaunoXome), fludarabine, and cytarabine (FLAD) in patients with poor-risk acute leukemia. Ann Hematol 83:696–703
Russo D, Pricolo G, Michieli M, Michelutti A, Raspadori D, Bertone A, Marin L, Pierri I, Bucalossi A, Zuffa E, De Vivo A, Mazza P, Gobbi M, Lauria F, Zaccaria A, Baccarani M (2001) Fludarabine, arabinosyl cytosine and idarubicin (FLAI) for remission induction in poor-risk acute myeloid leukemia. Leuk Lymphoma 40:335–443
Clavio M, Carrara P, Miglino M, Pierri I, Canepa L, Balleari E, Gatti AM, Cerri R, Celesti L, Vallebella E, Sessarego M, Patrone F, Ghio R, Damasio E, Gobbi M (1996) High efficacy of fludarabine-containing therapy (FLAG-FLANG) in poor risk acute myeloid leukemia. Haematologica 81:513–520
Visani G, Tosi P, Zinzani PL, Manfroi S, Ottaviani E, Testoni N, Clavio M, Cenacchi A, Gamberi B, Carrara P et al (1994) FLAG (fludarabine + high-dose cytarabine + G-CSF): an effective and tolerable protocol for the treatment of ‘poor risk’ acute myeloid leukemias. Leukemia 8:1842–1846
Wrzesien-Kus A, Robak T, Lech-Maranda E, Wierzbowska A, Dmoszynska A, Kowal M, Holowiecki J, Kyrcz-Krzemien S, Grosicki S, Maj S, Hellmann A, Skotnicki A, Jedrzejczak W, Kuliczkowski K; Polish Adult Leukemia Group (2003) A multicenter, open, non-comparative, phase II study of the combination of cladribine (2-chlorodeoxyadenosine), cytarabine, and G-CSF as induction therapy in refractory acute myeloid leukemia—a report of the Polish Adult Leukemia Group (PALG). Eur J Haematol 71:155–162
Robak T, Wrzesien-Kus A, Lech-Maranda E, Kowal M, Dmoszynska A (2000) Combination regimen of cladribine (2-chlorodeoxyadenosine), cytarabine and G-CSF (CLAG) as induction therapy for patients with relapsed or refractory acute myeloid leukemia. Leuk Lymphoma 39:121–129
Wrzesien-Kus A, Robak T, Wierzbowska A, Lech-Maranda E, Pluta A, Wawrzyniak E, Krawczynska A, Kuliczkowski K, Mazur G, Kiebinski M, Dmoszynska A, Wach M, Hellmann A, Baran W, Holowiecki J, Kyrcz-Krzemien S, Grosicki S; Polish Adult Leukemia Group (2005) A multicenter, open, noncomparative, phase II study of the combination of cladribine (2-chlorodeoxyadenosine), cytarabine, granulocyte colony-stimulating factor and mitoxantrone as induction therapy in refractory acute myeloid leukemia: a report of the Polish Adult Leukemia Group. Ann Hematol 84:557–564
Holowiecki J, Robak T, Kyrcz-Krzemien S, Grosicki S, Hellmann A, Skotnicki A, Jedrzejczak W, Konopka L, Giebel S, Kuliczkowski K, Zdziarska B, Dmoszynska A, Marianska B, Pluta A, Zawilska K, Komarnicki M, Kloczko J (2002) Daunorubicin, cytarabine, and 2-CdA (DAC-7) for remission induction in “de novo” adult acute myeloid leukaemia patients. Acta Haematol Pol 33:839–847
Holowiecki J, Grosicki S, Robak T, Kyrcz-Krzemien S, Giebel S, Hellmann A, Skotnicki A, Jedrzejczak WW, Konopka L, Kuliczkowski K, Zdziarska B, Dmoszynska A, Marianska B, Pluta A, Zawilska K, Komarnicki M, Kloczko J, Sulek K, Haus O, Stella-Holowiecka B, Baran W, Jakubas B, Paluszewska M, Wierzbowska A, Kielbinski M, Jagoda K (2004) Addition of cladribine to daunorubicin and cytarabine increases complete remission rate after a single course of induction treatment in acute myeloid leukemia. Multicenter, phase III study. Leukemia 18:989–997
Cheson BD, Bennett JM, Kopecky KJ, Buchner T, Willman CL, Estey EH, Schiffer CA, Doehner H, Tallman MS, Lister TA, Lo-Coco F, Willemze R, Biondi A, Hiddemann W, Larson RA, Lowenberg B, Sanz MA, Head DR, Ohno R, Bloomfield CD, LoCocco F; International Working Group for Diagnosis, Standardization of Response Criteria (2003) Treatment outcomes, and reporting standards for therapeutic trials in Acute Myeloid Leukemia. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol 21:4642–4649
Slovak ML, Kopecky KJ, Cassileth PA, Harrington DH, Theil KS, Mohamed A, Paietta E, Willman CL, Head DR, Rowe JM, Forman SJ, Appelbaum FR (2000) Karyotypic analysis predicts outcome of preremission and postremission therapy in adult acute myeloid leukemia: a Southwest Oncology Group/ Eastern Cooperative Oncology Group study. Blood 96:4075–4083
Adkins JC, Peters DH, Markham A (1997) Fludarabine. An update of its pharmacology and use in the treatment of haematological malignancies. Drugs 53:1005–1037
Kantarjian HM, Alexanian R, Koller CA, Kurzrock R, Keating MJ (1990) Fludarabine therapy in macroglobulinemic lymphoma. Blood 75:1928–1931
Warrell RP Jr, Berman E (1986) Phase I and II study of fludarabine phosphate in leukemia: therapeutic efficacy with delayed central nervous system toxicity. J Clin Oncol 4:74–79
Michelutti A, Michieli M, Damiani D, Melli C, Ermacora A, Grimaz S, Candoni A, Russo D, Fanin R, Baccarani M (1997) Effect of fludarabine and arabinosylcytosine on multidrug resistant cells. Haematologica 82:143–147
Estey E, Plunkett W, Gandhi V, Rios MB, Kantarjian H, Keating MJ (1993) Fludarabine and arabinosylcytosine therapy of refractory and relapsed acute myelogenous leukemia. Leuk Lymphoma 9:343–350
te Boekhorst PA, Lowenberg B, Sonneveld P (1994) Hematopoietic growth factor stimulation and cytarabine cytotoxicity in vitro: effects in untreated and relapsed or primary refractory acute myeloid leukemia cells. Leukemia 8:1480–1486
Estey E, Thall P, Andreeff M, Beran M, Kantarjian H, O’Brien S, Escudier S, Robertson LE, Koller C, Kornblau S et al (1994) Use of granulocyte colony-stimulating factor before, during, and after fludarabine plus cytarabine induction therapy of newly diagnosed acute myelogenous leukemia or myelodysplastic syndromes: comparison with fludarabine plus cytarabine without granulocyte colony-stimulating factor. J Clin Oncol 12:671–678
Dillman RO, Davis RB, Green M, Weiss RB, Gottlieb AJ, Caplan S, Kopel S, Preisler H, McIntyre OR, Schiffer C (1991) A comparative study of two different doses of cytarabin for acute myeloid leukaemia: a phase III trial of Cancer and Leukemia Group B. Blood 78:2520–2526
Weick JK, Kopecky TJ, Appelbaum FR, Head DR, Kingsbury LL, Balcerzak SP, Bickers JN, Hynes HE, Welborn JL, Simon SR, Grever M (1996) A randomized investigation of high-dose versus standard-dose cytosine arabinoside with daunorubicin in patients with previosly untreated acute myeloid leukemia: a Southwest Oncology Group study. Blood 88:2841–2951
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hołowiecki, J., Grosicki, S., Kyrcz-Krzemien, S. et al. Daunorubicin, cytarabine and fludarabine (DAF) for remission induction in relapsed or refractory acute myeloid leukemia. Evaluation of safety, tolerance and early outcome—Polish Adult Leukemia Group (PALG) pilot study. Ann Hematol 87, 361–367 (2008). https://doi.org/10.1007/s00277-007-0421-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-007-0421-4