Abstract
Therapeutic approaches are not well established in patients with myelodysplastic syndrome (MDS). We evaluated response to cyclosporin A (CyA) in 19 cases with MDS who were enrolled for the study [13 refractory anemia (RA), 5 refractory anemia with excess of blasts (RAEB), and 1 refractory anemia with ringed sideroblasts (RARS)]. Bone marrow was normocellular in ten, hypercellular in five, and hypocellular in four cases. Fifteen patients were transfusion dependent and the rest were not transfusion dependent but with a hemoglobin range of 6.4–8.8 g% with a mean of 7.4 g%. CyA was given at a dose of 3–5 mg/kg per day. A major response was observed in seven patients with RA, which was sustained on follow-up. Four cases of RA showed minor response and two cases of RA did not respond to CyA therapy. A minor response was also seen in one RAEB and one RARS case, while one RAEB case that initially showed a major response relapsed on therapy. The first effect of therapy was evident after a mean period of 2.5 months. A rise in platelets and leukocyte count was seen in three and two cases, respectively. One case developed renal failure on therapy and later died of septicemia. Response to CyA was independent of bone marrow cellularity. CyA could be an effective mode of therapy in patients with MDS especially those having RA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Myelodysplastic syndrome (MDS) is a clonal hematopoietic stem cell disorder characterized by anemia, neutropenia, or associated thrombocytopenia in various combinations with usually cellular marrow and a risk for leukemic transformation [1]. The therapeutic approach is not well established and the only curative therapy available is bone marrow transplantation with its inherent morbidity and limitations with regard to donor availability. Most of these patients receive supportive care only.
About 15% of MDS patients exhibit hypoplastic marrow [2, 3]. Immunosuppressive therapy such as cyclosporin A (CyA) and antithymocyte globulin (ATG) is being used successfully in aplastic anemia cases suggesting that immune dysregulation plays a role in the pathogenesis of cytopenia [4, 5]. Based on these observations, immunosuppressive therapy has been evaluated in a limited number of cases with MDS either alone [6–12] or in combination with cytokines [13]. We hereby present our experience with cyclosporin A (CyA) in 19 patients with MDS from this region.
Patients and methods
Nineteen consecutive cases of MDS (12 males and 7 females) attending the hematology clinic of All India Institute of Medical Sciences with normal renal and hepatic functions were selected for the study. Written informed consent was obtained before the enrollment. Patient characteristics are summarized in Table 1. The median time from the diagnosis of MDS to the start of therapy was 2 months (range: 1–12 months), and the mean age of the patients was 47.5 years (range: 20–77 years).
Diagnosis of MDS was based on clinical and hematological criteria including morphological examination of bone marrow. Pancytopenia was present in nine patients. Five patients had bicytopenia in the form of anemia and thrombocytopenia while five patients had anemia alone at the time of presentation. Bone marrow cellularity was assessed by examination of marrow biopsies taken from the posterior iliac crest. Patients were classified as per the French-American-British (FAB) criteria [12]. Thirteen patients had refractory anemia (RA), five patients refractory anemia with excess of blasts (RAEB), and one patient had refractory anemia with ringed sideroblasts (RARS). Bone marrow was normocellular in ten, hypercellular in five, and hypocellular in four patients.
Fifteen patients were transfusion dependent at presentation and four were transfusion independent with a hemoglobin level of less than 8 g%. One of these patients (patient 15) became transfusion dependent on follow-up. None of the patients had serious bleeding manifestations or autoimmune manifestations.
Cyclosporin A (Sandimmune neoral, Novartis, Cambridge, Mass., USA) was used in doses of 3–5 mg/kg per day in two to three divided doses. Frequent monitoring of blood levels of CyA was performed to maintain the levels between 100 and 300 mg/ml. Blood counts were performed at 2- to 3-weekly intervals. Renal and hepatic function studies were done at 4- to 6-week intervals.
Response to therapy was assessed as per the international working group’s criteria for hematological improvement in MDS [14]. Response was primarily assessed on the basis of erythroid response and interpreted as:
-
1.
Major response: rise in hemoglobin level of more than 2 g/dl for those patients who were transfusion independent while for cases who were transfusion dependent, transfusion independence with hemoglobin above 8 g%.
-
2.
Minor response: rise in hemoglobin between 1 and 2 g/dl for those who were transfusion independent and for patients who were transfusion dependent, 50% decrease in transfusion requirement.
Results
Thirteen patients were responders to CyA therapy. A major response was seen in eight patients (seven RA and one RAEB). All major responders with RA showed sustained response while the lone patient with RAEB showed a major response with transfusion independency for 5 months followed by a relapse to previous status despite continued therapy. Six patients (four RA, one RAEB, and one RARS) showed a minor response while five patients (two RA and three RAEB) were nonresponders. All of the responders were continued on CyA therapy. Two RAEB patients progressed to develop acute myeloid leukemia (AML) shortly after starting on therapy.
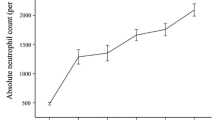
A rise in platelet count of more than 30×109/l was seen in three patients with thrombocytopenia, and two patients with neutropenia also showed a rise in total count of more than 1.5×109/l.
The effect of therapy was observed between 1 and 12 months with an average of 2.5 months. Interestingly, response to CyA therapy was independent of bone marrow cellularity and type of cytopenias in peripheral blood. Duration of therapy ranged from 4 to 26 months. Only one patient showed a significant side effect in the form of azotemia and later died of life-threatening infection. The rest of the patients tolerated the therapy well.
Discussion
Immune dysregulation has been proposed as the underlying mechanism in aplastic anemia (AA). Successful immunosuppressive treatment has been documented in AA [5]. Similarities in the profile of hypoplastic MDS and AA cases and occurrence of autoimmune manifestations in MDS cases prompted the use of immunosuppressive therapy in MDS [6]. Autoimmunity in MDS is considered to be a consequence of an abnormality of the immune system [17, 18]. It has been suggested that immune dysregulation may precede or predispose to the development of clonal hematological disorders and immune reaction against bone marrow stem cells may accompany or underline MDS [6]. Activated cytotoxic lymphocytes and abnormalities of T-cell function may play a role in development of MDS. Cytotoxic lymphocyte attack can induce aplastic as well as dysplastic marrow changes, can trigger apoptosis, and can induce chromosomal abnormalities as a consequence of DNA injury [15, 16].
The choice of CyA in our study was guided by its mechanism of action. CyA blocks synthesis and/or release of interleukin (IL)-2 from T-helper cells thereby inhibiting the expansion of unprimed T-helper cells, cytotoxic T-cell expansion, and T-cell-dependent B-cell activation [19]. CyA induces T-cell suppression, which may also affect production of several cytokines including interferons, tumor necrosis factors, and transforming growth factor-β that are capable of inhibiting hemopoiesis. Suppression of T-lymphocyte-facilitated antibody production by CyA may affect the potential pathogenic role of cytotoxic antibodies against hematopoietic precursors [20]. Moreover, CyA does not influence immunity mediated by polymorphonuclear cells and macrophages. The risk of secondary infections is lower than with other immunosuppressive drugs.
We observed a good response in cases of MDS with RA. A major response was seen in 7 of 13 cases (53.8%) while a minor response was present in 4 (30.7%) cases. Only one case of RA (patient 14) did not have any effect of CyA therapy. The response rate in RAEB was poor when compared to RA cases. Our observations are in conformity with other studies (Table 2).
Molldrem et al. [11] studied the response to single-dose ATG therapy in 25 MDS patients (14 RA, 6 RAEB, and 5 RARS cases). All of these cases were refractory to CyA, cyclophosphamide, and corticosteroids before ATG therapy. Eleven patients responded and became transfusion independent. Three cases (all RA) showed complete hematological recovery. Three responders relapsed later and again became transfusion dependent. The median response duration was 10 months (range: 3–38 months). Combination of ATG/antilymphocyte globulin (ALG) therapy with CyA may offer much more benefit than either drug alone and needs to be evaluated in larger studies.
Asano et al. [12] tried to correlate the response to immunosuppressive therapy (CyA and ATG) with HLA-DRB1*1501 and found a positive (but not statistically significant) association. Nand and Godwin [21] found a lower response rate in 11 patients with hypoplastic MDS to various immunosuppressive agents compared to normocellular and hypercellular MDS. In our study, however, the response was independent of bone marrow cellularity.
Both the present and the other studies did not show a significant benefit to patients with RAEB. The possibility that these patients may respond to a higher dose of CyA, either alone or in combination with ATG/ALG, needs to be explored.
In our series, one case suffered significant side effects requiring termination of therapy and later also died of serious infection. Other investigators [9–13] have also witnessed a similar side effect profile. The use of CyA in preleukemias raises the possibility of reducing the antitumor immunity thereby facilitating the transformation to acute leukemia. Two of our RAEB cases progressed to AML soon after starting CyA therapy, within 1 month, and thus CyA does not seem to have influenced the progression; however, the follow-up period was too short to address this issue convincingly.
We therefore believe that all patients with MDS-RA could be treated with CyA at least for 6 months and CyA should be continued in those patients who benefit from this therapy. However, there is need for a larger study so that response to CyA in various subtypes can be evaluated in an adequate number of cases.
References
Koeffler HP (1996) Myelodysplastic syndromes. Semin Hematol 33:87–94
Kouides PA, Bennet JM (1996) Morphology and classification of myelodysplastic syndrome and their pathological variants. Semin Hematol 33:95–110
Tuzuner N, Cox C, Rowe JM, Watrous D, Bennet JM (1995) Hypocellular myelodysplastic syndrome: new proposals. Br J Haematol 91:612–617
Fohlmeister I, Fischer R, Modder B, Rister M, Shaeffer HE (1985) Aplastic anemia and hypoplastic myelodysplastic syndrome: histomorphological, diagnostic and prognostic features. J Clin Pathol 38:1218–1224
Bacigalupo A, Broccia G, Corda D, Arcese W, Carotenuto M, Gallamini A, Locatelli F, Mori PG, Saracco P, Todeschini G et al (1995) Antithymocyte globulin, cyclosporine and granulocyte colony stimulating factor in patients with acquired aplastic anemia (SAA): a pilot study of EBMT SAA working party. Blood 85:1348–1353
Miescher PA, Favre H, Beris P (1991) Autoimmune myelodysplasias. Semin Hematol 28:322–330
Muller EW, De Wolfe JT, Vallenga E (1993) Successful immunosuppressive treatment after failure of erythropoietic therapy in two subjects with refractory anemia. Br J Hematol 83:171–172
Biesma DH, van den Tweel JG, Verdonck IF (1997) Immunosuppressive therapy for hypoplastic myelodysplastic syndrome. Cancer 79:1548–1551
Jonasova A, Neuwirtova R, Cermak J, Vozobulova V, Mocikova K, Siskova M, Hochova I (1998) Cyclosporin A therapy in hypoplastic MDS patients and certain refractory anaemias without hypoplastic bone marrow. Br J Haematol 100:304–309
Shimamoto T, Iguchi T, Ando K, Katagiri T, Tauchi T, Ito Y, Yaguchi M, Kimura Y, Masuda M, Mizoguchi H, Ohyashiki K (2001) Successful treatment with cyclosporin A for myelodysplastic syndrome with erythroid hypoplasia associated with T-cell receptor gene rearrangements. Br J Haematol 114:358–361
Molldrem JJ, Caples M, Mavroudis D, Plante M, Young NS, Barrett AJ (1997) Antithymocyte globulin for patients with myelodysplastic syndrome. Br J Haematol 99:699–705
Asano Y, Maeda M, Uchida N, Yokoyama T, Osaki K, Shimoda K, Gondo H, Okamura T, Okamura S, Niho Y (2001) Immunosuppressive therapy for patients with refractory anemia. Ann Hematol 80:634–638
Imai Y, Fukuoka T, Nakatani A, Ohsaka A, Takahashi A (1996) Sustained trilineage recovery and disappearance of abnormal chromosome clone in a patient with myelodysplastic syndrome following combination therapy with cytokines (granulocyte colony-stimulating factor and erythropoietin) and high-dose methylprednisolone. Br J Haematol 93:146–150
Cheson BD, Bennet JM, Kantarjian H, Pinto A, Schiffer CA, Nimer SD, Lowenberg B, Beran M, de Witte TM, Stone RM, Mittelman M, Sanz GF, Wijermans PW, Gore S, Greenberg PL (2000) Report of an international working group to standardize response criteria for myelodysplastic syndrome. Blood 96:3671–3674
Enright H, Jacob SH, Vercellotti G, Howe R, Belzer M, Miller W (1995) Paraneoplastic autoimmune phenomenon in patients with myelodysplastic syndrome: response to immunosuppressive therapy. Br J Haematol 91:403–408
Young NS (1992) Problem of clonality in aplastic anemia. Blood 79:1385–1392
Hamblin TJ (1996) Immunological abnormalities in myelodysplastic syndromes. Semin Hematol 33:150–162
Mufti GJ, Figes A, Hamblin TJ, Oscier DG, Copplestone JA (1986) Immunological abnormalities in myelodysplastic syndromes. Br J Haematol 63:143–147
Editorial (1985) Cyclosporine in autoimmune disease. Lancet i:211–909
Trucco M, Rovera G, Ferrero D (1984) A novel human lymphokine that inhibits hematopoietic progenitor cell proliferation. Nature 309:166–168
Nand S, Godwin JE (1988) Hypoplastic myelodysplastic syndrome. Cancer 62:958–962
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dixit, A., Chatterjee, T., Mishra, P. et al. Cyclosporin A in myelodysplastic syndrome: a preliminary report. Ann Hematol 84, 565–568 (2005). https://doi.org/10.1007/s00277-005-1016-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-005-1016-6