Abstract
Treatment options for patients with severe aplastic anemia (SAA) in developing countries are limited. A cohort of 261 patients with SAA received a novel immunosuppressive strategy of cyclosporine alternately combined with levamisole plus danazol (CSA&LMS-based regimen), which included 70 VSAA and 191 moderate SAA [initial absolute neutrophil count (ANC) >200/μL] cases. The CSA&LMS-based regimen was administrated orally with an initial dose of CSA 3 mg/kg in adults and 5 mg/kg in children every other day, LMS 150 mg in adults and 2.5 mg/kg in children every other day, and danazol (5.0–10.0) mg/kg daily, continued for 12 more months, followed by slow tapering. The 6-month response rates were 24.3 and 52.9 % for VSAA and moderate SAA (P < 0.001), respectively. Univariate and multivariate analyses demonstrated that younger age, higher pretreatment absolute reticulocyte count and ANC were favorable factors for achieving response at 6 months. The estimated 5-year overall survival rates were 33.8 % (95 % CI 20.6–47 %) and 80.5 % (95 % CI 69.7–91.3 %) for VSAA and moderate SAA, respectively (P < 0.001). To date, nine patients relapsed, and six patients evolved to clonal disorders. Thus, CSA&LMS-based regimen may represent a promising immunosuppressive strategy for moderate SAA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acquired severe aplastic anemia (SAA) is a life-threatening disorder characterized by pancytopenia and hypocellular bone marrow. Bone marrow transplantation (BMT) and immunosuppressive therapy (IST) with anti-thymocyte globulin (ATG) and cyclosporine A (CSA) have been the standard treatments for SAA over the last 20 years with continued improvements of outcomes [1–3]. SAA is a rare disease with a 2–3 times higher incidence in south-east Asia and poor countries than in Europe and North America [2]. Unfortunately, most of SAA patients in the developing countries have little opportunity to access these first-line treatments, which are associated with high costs and require hospitalization. As reasons of lacking fully HLA-matched siblings, or inability to locate fully HLA-matched unrelated donors, or age (>50 years), or lacking health insurance services, e.g., in one cohort of Chinese patients referred to our institutional hospital, more than 2/3 AA patients could only bear CSA [4]. Thus, it represents a medical challenge for both patients and physicians, as well as for the health authorities.
Unfortunately, the outcomes of SAA treated with CSA or danazol alone were not encouraging [5, 6]. As the addition of CsA to horse ATG (h-ATG) provided sufficient immunosuppression and improved 3- and 6-month response rates for SAA patients [3], it was possible to introduce a more appropriate immunomodulatory agent to replace h-ATG in SAA. Thus, a novel regimen retaining a marked immunosuppressive effect with the advantages of lower cost and ease of administration to circumvent SAA patients would be of benefit.
The time-honored agent levamisole (LMS), which had been originally designed for antihelminthic applications in 1971, has subsequently been reported to have a broad range of immunomodulatory effects [7–9]. Evidence is available that LMS may favor marrow restoration after chemotherapy [10]. Also, enhanced granulopoietic colony formation was observed when LMS was cocultured with human bone marrow stem cells in leukocyte-conditioned medium through promoting the release of colony-stimulating activity [11]. Previously, we reported the short-term effectiveness of CSA&LMS regimen in a small group of patients with refractory or relapsed SAA [12]. In addition, there were high response rates at 3 and 6 months and low progression rate to SAA in moderate AA (mAA) patients treated with the CSA&LMS regimen [13]. Thus, we postulated that the distinct complementary and synergistic effects between LMS and CSA would also lead to improved response rates and survival in SAA. We retrospectively analyzed the response, adverse events and survival of 261 SAA patients who received CSA&LMS-based regimen.
Methods
Patients
We reviewed the records of all newly diagnosed patients with SAA consecutively referred to Blood Disease Hospital of Chinese Academy of Medical Sciences (CAMS) and Peking Union Medical College (PUMC) between December 2008 and September 2013 and treated with the CSA&LMS-based regimen. Patients with a past history of myelodysplastic syndrome (MDS), overt paroxysmal nocturnal hemoglobinuria (PNH), and refractory or relapsed SAA, patients with severe infections with likely imminent death, congenital AA and children younger than 5 years old were excluded. All patients or their legal guardians signed written informed consent before CSA&LMS-based regimen. The CSA&LMS-based protocol described in this study was approved by the Ethics Committees of the Institute of Hematology, CAMS & PUMC according to the guidelines of the Declaration of Helsinki (Ethics number: NI2015004-EC-1).
Protocol details
The CSA&LMS regimen was designed to alternately administer each agent every other day. The initial dose of CSA was 3 mg/kg per day in adults or 5 mg/kg per day in children aged 6–18 years, with subsequent adjustment according to blood CSA, serum urea, creatinine and bilirubin levels to maintain trough blood CSA levels of 150–250 ng/mL. But the target blood CSA level was not achieved as CSA was administered every other day, so it was monitored only in the first 53 patients. Oral LMS was alternately administered at the same time with a dose of 150 mg per day in adults and 2.5 mg/kg per day in children (<40 kg) in three divided doses. Both CSA and LMS were continued for 12 more months, followed by a slow tapering rate of 25 % reduction in dose for every 3–4 months according to the response to reduce the risk of relapse.
All patients received oral danazol 5.0–10.0 mg/kg daily, folic acid 0.1–0.2 mg/kg daily, and vitamin B12 10 µg/kg, twice a week. All of these medicines were maintained for 12 months. RhuG-CSF was administered in all VSAA patients at a dose of 5–10 µg/kg/day, adjusted by white blood cell count (WBC) to keep it between 4000 and 10000/μL. In addition to this schedule, a short course of rhuG-CSF was administered if clinically indicated, usually for severe systemic infection. Red blood cells (RBCs) were transfused when the hemoglobin level was less than 6 g/dL, and platelets were transfused when the blood platelet count was less than 10 × 103/μL, or less than 20 × 103/μL in the presence of bleeding and/or fever.
Definitions
Severe aplastic anemia was defined as bone marrow cellularity of less than 30 % and satisfying two of the three following peripheral blood count criteria: (1) absolute neutrophil count (ANC) <500/μL; (2) absolute reticulocyte count (ARC) <60 × 103/μL; or (3) platelet count <20 × 103/μL [14]. Patients were classified as VSAA if they met the criteria for SAA and had an ANC less than 200/μL [15], and moderate SAA was defined as SAA with initial ANC higher than 200/μL. All patients were stratified for the severity of disease as indicated by ANC into three groups: group I <200/μL (VSAA), group II 200–500/μL, group III >500/μL. Hematological response was defined as no longer meeting the criteria for SAA without transfusion and rhuG-CSF. Complete response (CR) was defined as satisfying all three peripheral blood count criteria: (1) hemoglobin ≥11 g/dL; (2) ANC ≥1500/μL; (3) platelet count ≥80 × 103/μL. Partial response (PR) was defined as blood counts no longer meeting criteria for SAA without transfusions and rhuG-CSF, and no response (NR) was classified as still meeting criteria for SAA, continuous transfusion dependency or death within 6 months. Relapse was defined as meeting SAA criteria again for a responder who had previously achieved response and kept stable blood counts for at least 3 months. Evolution to myelodysplastic syndrome (MDS) was defined as the presence of characteristic dysplastic abnormalities in at least two bone marrow lineages with or without abnormal karyotype. The diagnosis of overt paroxysmal nocturnal hemoglobinuria (PNH) was established by more than two times positive Ham test or two series of more than 20 % glycosylphosphatidyl-anchored protein (GPI-AP)-deficient clone, with clinical and laboratory evidence of hemolysis. The PNH clone was determined by flow cytometric analysis. Detection of more than 5 % GPI-AP-deficient or more than 1 % abnormal clone by fluorescent aerolysin (FLAER) was considered positive. Early mortality was defined as death occurring within 3 months after the initial CSA&LMS-based regimen.
Statistical analysis
Summary statistics, such as means, medians and percentiles were used to describe the baseline characteristics of the patients. Categorical data were summarized as frequency counts and proportions. To compare the differences between groups, the Chi square test or Fisher exact test was used for categorical variables, and the Mann–Whitney U test for continuous variables. Logistic regression was used to assess the predicted factors for response at 6 months in the multivariate analysis. Overall survival (OS) was determined from the initial CSA&LMS-based regimen to death or last follow-up (September 2014). Survival curves were calculated using the Kaplan–Meier method and compared using the log-rank test. All significance tests were 2-sided. All the analyses were performed using statistical package SPSS 16.0 (SPSS, Inc., Chicago, IL, USA).
Results
Patient characteristics
We retrospectively analyzed 315 SAA patients treated with CSA&LMS-based regimen between December 2008 and September 2013 in our hospital. 23 patients with severe infections, 2 patients without response at 3 months discontinuing this protocol and 29 patients who were lost to follow-up within 6 months were excluded. Thus, 261 patients were included for the response and survival. Of the 261 patients, 70 (26.8 %) cases were VSAA, including 7 hepatitis-associated AA patients. The median age for all cohort was 25 (range 5–77) years. All of these patients were transfusion dependent. RhuG-CSF was administered in 116 (44.4 %) patients. The median follow-up was 25 (range 0.2–70.5) months. Demographic data and clinical characteristics of these patients are outlined in Table 1.
Hematologic response
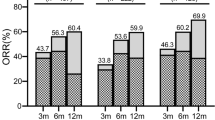
92 (35.2 %, 87 PR + 5 CR) and 118 (45.2 %, 102 PR + 16 CR) patients achieved response at 3 and 6 months, respectively. During the following days to 12 months, the blood count of the other 21 patients continuously improved and at least met the criteria of PR. Therefore, the response rate was 53.3 % (139/261, 102 PR + 37 CR) at 12 months. After stratification for pretreatment neutrophil counts (<200, 200–500, >500/μL), the response rates were 17.1 %, 36.8 % and 48.2 % at 3 months (P < 0.001) and 24.3 %, 46.2 % and 61.2 % at 6 months (P < 0.001), respectively. But when the deaths before 6 months were excluded, the difference among the three groups was not significant (45.9 vs 49.0 vs 62.7 % at 6 months, P = 0.11).
The patients with higher pretreatment ARC level had significantly higher response rates than those with lower ones at 3 (49.6 vs 20.8 %, P < 0.001) and 6 months (59.5 vs 30.8 %, P < 0.001). Interestingly, there was a better response in patients with long disease duration (>60 days); of them, 41.3 and 52.4 % reached PR or CR at 3 and 6 months, respectively, compared with 29.6 and 38.5 % of those with shorter disease duration (P = 0.05 and 0.03, respectively), In fact, most of the patients (89.7 %) with long disease duration were with moderate SAA in this series; thus, when patients were stratified by pretreatment neutrophil counts, these significances disappeared in both VSAA and moderate SAA (P = 0.72 and 0.51, respectively). The response rate of the patients older than 40 years was inferior to the younger ones, which were 19.7 % at 3 months and 28.2 % at 6 months, compared with 41.1 % (P = 0.001) and 51.6 % (P = 0.001), respectively. Univariate analysis revealed that the likelihood of 3- and 6-month response rates was not associated with sex, etiology, pretreatment platelet and PNH clone. Multivariate analysis further demonstrated that younger age of <40 years, pretreatment ARC >17.1 × 103/μL and higher initial ANC remained statistically significant favorable factors for achieving response at 6 months (Table 2).
During the following days up to 35 months, the blood count of the other 9 patients improved and met the criteria of PR; thus, the overall response rate was 57.1 % at the last follow-up. The median level of hemoglobin, ANC and platelet at the last follow-up were 13.5 (6.6–17.3)g/dL, 1880 (490–6770)/μL and 72 (10–306) × 103/μL, respectively. The magnitude and timeline of hematologic improvement of the responders are presented in Fig. 1. It was interesting that the median level of hemoglobin and ANC were comparable at 3, 6 and 12 months between the responders of group I and those with pretreatment ANC >200/μL, but the median platelet count of VSAA was higher than those with moderate SAA at 6 (54 × 103/μL vs 32 × 103/μL) and 12 (88.5 × 103/μL vs 39 × 103/μL) months, although the difference was not significant (P = 0.09 and 0.07, respectively). When the responders were divided by disease duration (60 days), patients with duration <60 days had higher median hemoglobin than those with duration ≥60 days at 3 (10.8 g/dL vs 8.8 g/dL, P = 0.01) and 6 (13.3 g/dL vs 9.3 g/dL, P = 0.002) months, but the difference disappeared at 12 months (13.1 g/dL vs 12.3 g/dL, P = 0.10). The median ANC (1855/μL vs 1490/μL) and platelet (79.5 × 103/μL vs 36 × 103/μL) counts of patients with duration <60 days were higher than those of patients with duration ≥60 days (P = 0.04 and 0.001, respectively).
Blood CSA levels
CSA concentrations were measured at 2 weeks after initiation of the CSA&LMS regimen in the first 53 patients. The trough blood CSA levels (which were measured 36 h after last take of CSA) of all these patients were less than 150 ng/mL, with a median level of 20.90 (range 2.00–131.50) ng/mL. The median peak level of blood CSA was 315.48 (range 70.60–786.50) ng/mL. When these patients were divided into two groups according to the response at 6 months, the median trough blood CSA levels were comparable between the two groups (17.18 vs 23.70 ng/mL, P = 0.42), but the median peak level of blood CSA of the response group (363.65 ng/mL) was significantly higher than that of the non-response group (258.70 ng/mL, P = 0.05).
Adverse effects
Levamisole and danazol-associated adverse events were scored according to the NCI-CTC Version 2.0 criteria. LMS-associated toxicities recorded were mild and only occurred in 3 (1.15 %) patients, including grade 1 rash, grade 2 rash and grade 2 nausea in each patient, which required adjusting the dose of LMS in the latter two patients. Danazol-associated toxicities were more frequent and required intervention: 18 (6.90 %) patients had mild/moderate acne and 2 (0.77 %) of them had grade 2; 32 (12.26 %) patients had impairment of liver function with transaminase values up to 670 U/L, including grade 3, grade 2 and grade 1 in 7 (2.68 %), 12 (4.60 %) and 13 patients (4.98 %), respectively; another patient had grade 2 gynecomastia. CSA-associated toxicities were modest: 7 patients experienced gingival hyperplasia; other adverse effects included hypertrichosis (6 patients), impairment of renal function with creatinine values up to 164.3 μmol/L (2 patients), involuntary movement (1 patient) and laboratory evidence of hepatotoxicity (5 patients). All of these toxicities were mild except for the relatively high creatinine value in 1 patient, who had the dose of CSA further adjusted (Table 3).
Survival
In this cohort, 62/261 (23.75 %) patients died within a median interval of 4.5 (0.2–46) months after the CSA&LMS regimen. Most (82.26 %, 51/62) of these episodes occurred in the first year. All of them were non-responders at 6 months. Of these deaths, 41 resulted from infections and 15 hemorrhagic episodes, 2 occurred after MDS/leukemia transformation and the other 4 from unknown causes. Early mortality occurred in 23 patients, 19 of whom died from infection complications.
The 5-year OS of all patients was 67.9 % (95 % CI 59.4–76.4 %) (Fig. 2a). The inferiority of 5-year OS at 33.8 % (95 % CI 20.6–47 %) in VSAA patients was observed when compared with 80.5 % (95 % CI 69.7–91.3 %) in moderate SAA patients (P < 0.001), as estimated by the Kaplan–Meier method (Fig. 2b). The comparable 5-year OS between groups II and III (80.7 vs 78.6 %, P = 0.08) was also observed. Sex, etiology, age, disease duration, PNH clone, ARC and PLT counts were not correlated with survival by univariate analysis.
Relapse and clonal evolution
A total of 9 (3.45 %, 7PR + 2CR) patients relapsed at a median interval of 24.0 months (range 9–42 months) after an initial response. The relapse in 2 patients occurred when the drugs were tapered, and both of them met the criteria for PR again when the CSA&LMS-based regimen was re-administered. 2 patients died 13 and 15 months after their relapses. The other 5 patients were still transfusion dependent.
In this cohort, 6/261 patients (2.30 %) evolved to clonal disorders, including 2 overt PNH, 3 MDS and 1 acute myeloid leukemia (AML). Cytogenetic abnormalities predominantly involving chromosome 7 occurred in the 3 MDS patients. Two patients (1 NR, 1 CR) developed clonal cytogenetic abnormalities in the bone marrow cell without the distinctive morphologic features of MDS, which were 9q+ and 6p+, respectively, and neither of them evolved to MDS/AML.
Discussion
We have previously demonstrated the unexpectedly encouraging efficacy of CSA&LMS regimen in mAA [13] and refractory or relapsed severe AA (rSAA) [12] with much less costs and acceptable side effects, which resulted in 91.5 % overall response rate in mAA and 50 % in rSAA. Thus, we conducted a single arm clinical research cautiously to further explore its application to SAA patients from 2008, who were not eligible for ATG therapy and BMT. On account of the potential interactions between danazol and CSA as reported in the literature [16, 17], danazol, as a synthetic anabolic steroid, was added to CSA&LMS-based regimen to achieve adequate immunosuppressive intensity. Before ATG + CSA was introduced for the treatment of AA, androgens were the usual therapy in the early 1960s [18]. The mechanism for the action of androgens was unknown until later they were found to have a positive effect on hematopoiesis and could also up-regulate telomerase activity [19, 20]. In the majority of clinical trials in the 1970s–1990s, androgens were routinely used alone or in combination with ALG/ATG [21–24]. Due to the highly variable responses, hepatotoxicity and virilization of these drugs, the application of androgens has been limited in recent years. But it may be useful in a combined regimen for patients in whom the standard immunosuppressive treatment is unavailable.
Although the use of CSA&LMS-based regimen as primary therapy for SAA remains preliminary, the treatment options for those patients ineligible for ATG and BMT are limited. The response rates of CSA alone for mAA were disappointing [25–27], and the outcome was even more discouraging for SAA patients [5, 28]. In the only published randomized multicenter study for SAA by Gluckman E, the response rate was only 11.6 % in the CSA group at 3 months [5]. CSA-associated toxicities were frequent when long-term and full dosage were used, including nephrotoxicity (in 42–56 % patients) [5, 26], hypertrichosis (38 % patients) [29], gingival hyperplasia, hypertension, hepatotoxicity, edema and muscle cramps, etc. [3, 5, 29]. Danazol was used in AA only in the recent years, and few studies with unremarkable results have been reported, with no more than 40 % overall response rate in all types of AA treated with danazol alone [6, 30]. The results of this series of SAA patients treated with this combined regimen confirmed the efficacy and acceptable side effects of this strategy. 35.2, 45.2 and 57.1 % of SAA patients achieved response at 3 and 6 months and the last follow-up. No virilization was observed, and CSA-associated toxicities were rare and modest. More importantly, all of those adverse events were reversible, and only 8 patients had to have a further adjusted dose of CSA and danazol in this study.
One may argue that there was a high long-term survival in this trial, as the 5-year OS was comparable with that of SAA patients treated with ATG in our previous study [31]. Here, we underscored the surprisingly remarkable response rate (41.9 % at 3 months and 52.9 % at 6 months, respectively) and 5-year OS (80.5 %) in patients with pretreatment ANC >200/uL, and certainly most (73.2 %) of the patients were with moderate SAA in this series. Because of the high early mortality of VSAA, this combined regimen may be more suitable for patients whose initial ANC level was relatively higher with better quality of life and long-term survival. Interestingly, in the responders, the median level of hemoglobin and ANC of VSAA at 3, 6 and 12 months was comparable with those seen in moderate SAA, and the median platelet count was unexpectedly higher in VSAA patients than that seen in groups II and III at 6 (54 × 103/μL vs 32 × 103/μL) and 12 (88.5 × 103/μL vs 39 × 103/μL) months. It partially demonstrated that some VSAA patients could also respond to CSA&LMS-based regimen with more remarkable blood counts than moderate SAA patients, which certainly needs verification of more long-term follow-up.
In the present study, multivariate analysis showed that younger age, higher pretreatment ARC and initial ANC were favorable factors for achieving response at 6 months, and initial ANC was also the predictor of survival. However, in our previous clinical research, the likelihood of overall response rate in mAA treated with CSA&LMS regimen was not associated with any of these factors. This may suggest some different pathophysiology between SAA and mAA. SAA patients with short disease duration had higher median ANC and platelet counts at 12 months, which was confirmed in mAA patients whose disease duration <6 months had a significantly superior CR rate in our early study [13]. In many previous studies, the CSA levels were used to restrict drug toxicity and also predict responses [2, 14, 32, 33]. In our research, the trough blood CSA levels were all less than 150 ng/mL when alternately administered with LMS. Therefore, it was monitored only in a small part of patients in this study. Despite this, it was demonstrated that the peak level had some correlation with the response (P = 0.05) in our study. The lower frequency of CSA-associated toxicities and the unexpectedly encouraging results of this novel regimen gave us the indication that the optimal time point and appropriate monitoring of CSA levels need further exploration.
In conclusion, CSA&LMS-based regimen might represent a promising strategy for SAA patients with several practical merits of desirable effectiveness, much less costs, more convenience and acceptable side effects, especially for patients with moderate SAA who are ineligible for ATG and BMT in developing countries. Thus, this pilot approach implicated a new concept of treatment for SAA and given the rarity of SAA, international collaborative efforts are needed to explore the long-term outcome of these patients treated with this combined regimen in the near future.
References
Young NS, Calado RT, Scheinberg P. Current concepts in the pathophysiology and treatment of aplastic anemia. Blood. 2006;108:2509–19.
Marsh JC, Ball SE, Cavenagh J, Darbyshire P, Dokal I, Gordon-Smith EC, et al. Guidelines for the diagnosis and management of aplastic anaemia. Br J Haematol. 2009;147(1):43–70.
Frickhofen N, Kaltwasser JP, Schrezenmeier H, Raghavachar A, Vogt HG, Herrmann F, et al. Treatment of aplastic anemia with antilymphocyte globulin and methylprednisolone with or without cyclosporine. N Engl J Med. 1991;324:1297–304.
Li Y, Li X, Ge M, Shi J, Qian L, Zheng Y, et al. Long-term follow-up of clonal evolutions in 802 aplastic anemia patients: a single-center experience. Ann Hematol. 2011;90:529–37.
Gluckman E, Esperou-Bourdeau H, Baruchel A, Boogaerts M, Briere J, Donadio D, et al. Multicenter randomized study comparing cyclosporine-A alone and antithymocyte globulin with prednisone for treatment of severe aplastic anemia. Blood. 1992;79:2540–6.
Jaime-Pérez JC, Colunga-Pedraza PR, Gómez-Ramírez CD, Gutiérrez-Aguirre CH, Cantú-Rodríguez OG, Tarín-Arzaga LC, et al. Danazol as first-line therapy for aplastic anemia. Ann Hematol. 2011;90:523–7.
Stevenson HC, Green I, Hamilton JM, Calabro BA, Parkinson DR. Levamisole: known effects on the immune system, clinical results, and future applications to the treatment of cancer. J Clin Oncol. 1991;9:2052–66.
Ramot B, Biniaminov M, Shoham C, Rosenthal E. Effect of levamisole on E-rosette-forming cells in vivo and in vitro in Hodgkin’s disease. N Engl J Med. 1976;294:809–11.
Hersey P, Ho K, Werkmeister J, Abele U. Inhibition of suppressor T cells in pokeweed mitogen-stimulated cultures of T and B cells by levamisole in vitro and in vivo. Clin Exp Immunol. 1981;46:340–9.
Lods JC, Dujardin P, Halpern GM. Levamisole and bone-marrow restoration after chemotherapy. Lancet. 1976;1:548.
Senn JS, Lai CC, Price GB. Levamisole: evidence for activity on human haemopoietic progenitor cells. Br J Cancer. 1980;41:40–6.
Shao Y, Li X, Shi J, Ge M, Huang J, Huang Z, et al. Cyclosporin combined with levamisole for refractory or relapsed severe aplastic anaemia. Br J Haematol. 2013;162:552–5.
Li X, Shao Y, Ge M, Shi J, Huang J, Huang Z, et al. A promising immunosuppressive strategy of cyclosporine alternately combined with levamisole is highly effective for moderate aplastic anemia. Ann Hematol. 2013;92:1239–47.
Rosenfeld S, Follmann D, Nunez O, Young NS. Antithymocyte globulin and cyclosporine for severe aplastic anemia: association between hematologic response and long-term outcome. JAMA. 2003;289(9):1130–5.
Bacigalupo A, Hows J, Gluckman E, Nissen C, Marsh J, Van Lint MT, et al. Bone marrow transplantation (BMT) versus immunosuppression for the treatment of severe aplastic anemia (SAA): a report of the EBMT SAA working party. Br J Haematol. 1988;70(2):177–82.
Borrás-Blasco J, Rosique-Robles JD, Peris-Marti J, Navarro-Ruiz J, Gonzalez-Delgado M, Conesa-Garcia V. Possible cyclosporin-danazol interaction in a patient with aplastic anaemia. Am J Hematol. 1999;62(1):63–4.
Blatt J, Howrie D, Orlando S, Burckart G. Interaction between cyclosporine and danazol in a pediatric patient. J Pediatr Hematol Oncol. 1996;18(1):95.
Shahidi NT, Diamond LK. Testosterone-induced remission in aplastic anemia of both acquired and congenital types. Further observations in 24 cases. N Engl J Med. 1961;11(264):953–67.
Shahidi NT. Androgens and erythropoiesis. N Engl J Med. 1973;289:72–80.
Calado RT, Yewdell WT, Wilkerson KL, Regal JA, Kajigaya S, Stratakis CA, et al. Sex hormones, acting on the TERT gene, increase telomerase activity in human primary hematopoietic cells. Blood. 2009;114:2236–43.
Camitta BM, Thomas ED, Nathan DG, Santos G, Gordon-Smith EC, Gale RP, et al. Severe aplastic anemia: a prospective study of the effect of early marrow transplantation on acute mortality. Blood. 1976;48(1):63–70.
Champlin RE, Ho WG, Feig SA, Winston DJ, Lenarsky C, Gale RP. Do androgens enhance the response to antithymocyte globulin in patients with aplastic anemia? A prospective randomized trial. Blood. 1985;66:184–8.
Doney K, Pepe M, Storb R, Bryant E, Anasetti C, Appelbaum FR, et al. Immunosuppressive therapy of aplastic anemia: results of a prospective, randomizedtrial of antithymocyte globulin (ATG), methylprednisolone, and oxymetholone to ATG, very high-dose methylprednisolone, and oxymetholone. Blood. 1992;79(10):2566–71.
Kojima S, Hibi S, Kosaka Y, Yamamoto M, Tsuchida M, Mugishima H, et al. Immunosuppressive therapy using antithymocyte globulin, cyclosporine, and danazol with or without human granulocyte colony-stimulating factor in children with acquired aplastic anemia. Blood. 2000;96:2049–54.
Yamazaki H, Sugimori C, Chuhjo T, Nakao S. Cyclosporine therapy for acquired aplastic anemia: predictive factors for the response and long-term prognosis. Int J Hematol. 2007;85:186–90.
Maschan A, Bogatcheva N, Kryjanovskii O, Shneider M, Litvinov D, Mitiushkina T, et al. Results at a single centre of immunosuppression with cyclosporine A in 66 children with aplastic anaemia. Br J Haematol. 1999;106:967–70.
Marsh J, Schrezenmeier H, Marin P, Ilhan O, Ljungman P, McCann S, et al. Prospective randomized multicenter study comparing cyclosporin alone versus the combination of antithymocyte globulin and cyclosporin for treatment of patients with nonsevere aplastic anemia: a report from the European Blood and Marrow Transplant (EBMT) Severe Aplastic Anaemia Working Party. Blood. 1999;93:2191–5.
Leonard EM, Raefsky E, Griffith P, Kimball J, Nienhuis AW, Young NS. Cyclosporine therapy of aplastic anaemia, congenital and acquired red cell aplasia. Br J Haematol. 1989;72:278–84.
Frickhofen N, Heimpel H, Kaltwasser JP, Schrezenmeier H. Antithymocyte globulin with or without cyclosporin A: 11-year follow-up of a randomized trial comparing treatments of aplastic anemia. Blood. 2003;101:1236–42.
Chuhjo T, Yamazaki H, Omine M, Nakao S. Danazol therapy for aplastic anemia refractory to immunosuppressive therapy. Am J Hematol. 2008;83(5):387–9.
Li X, Shi J, Ge M, Shao Y, Huang J, Huang Z, et al. Outcomes of optimized over standard protocol of rabbit antithymocyte globulin for severe aplastic anemia: a single-center experience. PLoS One. 2013;8(3):e56648.
Kosaka Y, Yagasaki H, Sano K, Kobayashi R, Ayukawa H, Kaneko T, et al. Prospective multicenter trial comparing repeated immunosuppressive therapy with stem-cell transplantation from an alternative donor as second-line treatment for children with severe and very severe aplastic anemia. Blood. 2008;111:1054–9.
Song MK, Chung JS, Joo YD, Kim YS, Kim SH, Seol YM, et al. Is the early cyclosporine A level predictive of the outcome of immunosuppressive therapy in severe aplastic anemia? Eur J Haematol. 2009;83:72–8.
Acknowledgments
The authors would like to thank Prof. Seiji Kojima (Nagoya University Graduate School of Medicine) for critical revision of the manuscript for important intellectual content and interesting scientific discussions. This study was supported by a grant from the National Natural Science Foundation of China (81470289). We thank the nursing staff of Severe Aplastic Anemia Studying Program and our physician colleagues for the excellent care of patients.
Conflict of interest
The authors declared that they have no commercial, proprietary or financial interest in the products or companies described in this manuscript.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Wang, M., Li, X., Shi, J. et al. Outcome of a novel immunosuppressive strategy of cyclosporine, levamisole and danazol for severe aplastic anemia. Int J Hematol 102, 149–156 (2015). https://doi.org/10.1007/s12185-015-1818-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-015-1818-9