Abstract
Purpose
Partial nephrectomy is gaining, nowadays, more interest in oncologic kidney surgery. This type of surgery requires the good knowledge of vascular renal anatomy to make it safe and to guarantee good functional and oncological outcomes. This paper exposes the clinical implication of the arterial renal anatomy in nephron-sparing surgery.
Methods
This is a cadaveric study of 71 human kidneys performed at Charles Nicolle mortuary. The right and left kidneys with surrounding tissues were removed en bloc with the adjacent part of the aorta and inferior vena cava, cleared and studied. Colored resin was injected in each artery, vein, and urinary ducts, with a specific color code for each structure. Corrosion technique was used to eliminate the surrounding tissue, leaving only the colored resin matrix. The Ternon anatomic classification of the inferior polar artery, based on its emergence point was used.
Results
Multiple renal arteries were noted in 9.85% of casts. Anterior and posterior division of main renal artery was found in 95.7% of cases. Posterior segmental artery crossed posteriorly the upper caliceal infundibulum and the renal pelvis in 93% of cases. The upper renal pole was vascularized by an apical segmental artery in 16.9% of cases and a superior polar artery in one case (1.4%). The mid pole of the kidney was supplied by a unique anterior branch and a single posterior branch in 40% of cases. Inferior polar artery was found in 52 casts (73.23%). Type I of Ternon was found in 6 casts (11.53%), Type II in 25 cases (48.07%), Type III in 19 cases (36.53%), Type IV in 2 cases (3.84%), and type V in 13 casts (25%).
Conclusion
Renal vascular anatomy presents large variations. Good knowledge of the segmental arterial anatomy of the kidney is a primordial to a safe partial nephrectomy. Good preoperative vascular mapping can be of great help for the surgeon.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Partial nephrectomy or nephron-sparing surgery for renal cells carcinoma has been stimulated by advances in renal imaging, improved surgical techniques and the increasing number of carcinomas discovered in low-stage of development [10, 13]. Per operative hemorrhage remains to fear in this type of surgery. Good knowledge of the vascular surgical anatomy of the kidney is primordial for the surgeon in carrying out successfully partial nephrectomy with good outcomes [10]. The purpose of the present work is to study the renal segmental arterial anatomy and its clinical implications to plan partial nephrectomy.
Materials and methods
The present study was conducted on 71 human kidneys obtained from the autopsy of fresh cadavers of both sexes, performed at Charles Nicolle Hospital’s mortuary in Tunis, Tunisia, after approval of the hospital’s research ethics committee.
We included all specimens of adult subjects dead in the previous 24 h in different circumstances (trauma, drowning, and sudden death). Cadavers with history of retroperitoneal or renal surgery and whose death occurred after an abdominal trauma where the lesions of organs and hemorrhagic suffusions could interfere with the identification of anatomical elements were not included. The dissection process, organ retrieval, corrosion technique, and measurements were all performed by the same author for all the specimens, aiming to neutralize any possible observational bias that might be inherent to multi-individual features.
All measurements were carried out in situ in the same way on both sides. The lengths and diameters were measured in millimeters using a Vernier caliper. The cadaveric specimens were placed in the supine position with their arms lying alongside. A rubber block was placed under the scapular points to allow better exposure of the retroperitoneal region. A mento-pubic incision was made.
The right and left kidneys with surrounding tissues were removed en bloc with the adjacent part of the aorta and inferior vena cava, cleared and studied. Kidneys along with their arteries and veins were explored and the morphological variations were noted. The corrosion cast methodology used has been conducted as reported in detail previously in many studies [17]. Transparent polyester resin was used. We added, a diluent, styrene, and methyl ethyl ketone peroxide as a catalyst. The injected polymer was tinted to improve the clarity of the final piece. A color code has been established to differentiate structures: red for arteries, blue for veins, and yellow for urinary ducts (calyx, renal pelvis, ureter).
The second phase consisted on the elimination of all the surrounding tissue, leaving only the matrix that has already been injected with the resin.
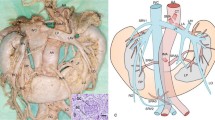
At that point, the casts are immerged carefully in sulphuric acid, which is a highly corrosive strong mineral acid, during 48–72 h, until total corrosion of the surrounding tissues around the cast (Fig. 1).
Historically, renal vasculature was first classified by Max Brödel, in 1901, into four to five branches, which distribution is such that three-fourths of the blood-supply is carried anteriorly, while one-fourth runs posteriorly.
According to the classical Graves classification in 1954 [8], the renal parenchyma can be subdivided into five segments (apical, upper, middle, lower, and posterior) each supplied by its own branch originated from the main renal artery.
Ternon [23] established a new classification based on the emergence and the origin of the inferior polar artery from the main renal artery into three branches: one anterior, another posterior, and an inferior polar artery. Five types of division were described (Fig. 2). We found that classification interesting to adopt in this study, since it was not based on the classic anatomical strict approach of anterior–posterior division of renal vasculature, which may offer a different surgical angle of view, with clinical and surgical implications.
Anatomic classification of Ternon [23]. Type I: inferior polar artery emerges from main renal artery, before the division point into anterior and posterior branches. Type II: inferior polar artery emerges from the division point of main renal artery. Type III: inferior polar artery emerges from the Terminus of anterior common division branch, after the rise of the posterior branch. Type IV: inferior polar artery originates from a posterior common renal trunk, after originating an anterior branch. Type V: absence of inferior polar artery
Results
The mean caliber of the renal artery was 4.67 mm (3–6 mm). The caliber of the right renal artery was 4.83 mm, whereas the left renal artery measured 4.70 mm. In casts with multiples renal arteries, the mean caliber was 3.40 mm.
A single main renal artery was clearly recognizable 64 corrosion casts (90.14%). Seven casts (9.85%) showed multiple main renal arteries (Table 1, Figs. 3, 4).
Posterior view of corrosion cast with triplicate right renal artery originating from the right lateral aspect of abdominal aorta (A: superior renal artery running in an anterior course towards the anterior face of right kidney. B: middle renal artery coursing along the posterior surface of vena cava and right renal vein supplying the posterior face of the kidney. C: inferior polar artery arising, as a separate branch, towards the inferior pole of the kidney)
Posterior view of corrosion cast with duplicate left renal artery originating from the left lateral aspect of abdominal aorta. Main renal artery (A) emerging directly from the aorta, with proximal emergence of a branch supplying the posterior part of the inferior pole of the kidney (B). (C) Inferior renal artery originating from aorta, as a single independent branch, running towards the anterior face of inferior renal pole
Sixty-eight casts (95.7%) showed the classic subdivision of the main renal artery into a posterior branch and an anterior branch (Fig. 5).
The posterior segmental artery crossed posteriorly the upper caliceal infundibulum in 93% of cases, and the posterior aspect of renal pelvis in 80% of cases.
With regard to renal segmental vascularization, the upper pole of the kidney was supplied in a small part by an apical segmental artery in 12 cases (16.9%) (Fig. 6). Its mean caliber was 2.13 mm (1–3.5 mm). Different sources of origin of this branch were noted (Table 2).
A superior polar artery was found in 1 cast (1.4%), arising directly as a separate branch from the abdominal aorta, supplying the upper pole of the kidney (Fig. 7).
We noted that branches supplying the superior pole of the kidney were organized in 90% of cases in anterior and posterior plexuses, crossing, anteriorly, the renal pelvis and the upper caliceal infundibulum in 67.6% of cases (Table 3).
The vascularization of the mid pole of the kidney is not systemized as the other poles. It was supplied by a unique anterior branch and a single posterior branch in 40% of cases. In the remaining casts, a single anterior branch and multiple posterior small branches were noted, arising from the vertical segment of the retropelvic artery. In one cast, a mid-polar artery arising directly from the aorta was revealed.
Inferior renal polar artery was observed in 52 cases (73.23%). Its origin showed variability in different casts basing on Ternon’s anatomic classification (Table 4, Figs. 8, 9, 10, 11).
It runs in an oblique, inferior and external pathway. It crosses the anterior face of the uretero-pelvic junction in 36 cases (69%). It courses the renal vein anteriorly in 15 casts (29.57%), and posteriorly in the remaining 21 casts (39.43%).
In 60% of cases, the inferior polar renal artery divided after crossing the uretero-pelvic junction. Three division patterns were observed:
It divided into an anterior branch and a posterior branch in 34 cases (65.21%), three branches: anterior, posterior, and inferior branches in 6 cases (11.59%) and a single anterior trunk in the remaining 12 cases (23.18%).
Whenever the division point of the renal inferior polar artery sited after crossing the uretero-pelvic junction, the posterior branch skirts the inferior edge of renal pelvis to pass posteriorly.
Discussion
Precise knowledge of renal vascularization is of great importance to perform partial nephrectomy safely, obtaining oncological radicality and preserving the healthy parenchyma.
Selective clamping of the pertinent segmental artery may reduce the extent and duration of warm ischemic time and minimize blood loss, with better functional outcomes comparing to the clamping of the main renal artery [10].
Inferior partial nephrectomy is easier to realize regarding the vasculature of the lower renal pole. This segment of kidney is a well-defined arterial unit due to the inferior renal polar artery, which was found in our series in 73.23% of cases, all five types of Ternon included, originating mainly from the division point of the renal artery (Type II of Ternon) (48%). Most series identified the inferior polar artery as the separate branch emerging before the division point, which matches Type 1 of Ternon classification. Our results matched those reported in literature (Table 5).
In 65% of cases of the present study, we observed that the inferior polar artery was the only branch supplying the lower renal pole, with a division into an anterior branch and posterior branch. In this case, simple clamping of the trunk of the inferior renal pole artery identifies easily the demarcation line and allows a safe inferior partial nephrectomy. Failure to obtain complete ischemia of the tumor area by selective clamping, could be attributable to the multiplicity of segmental branches supplying a renal pole or segment. Furthermore, overlapping arterial branches may be detached from a segmental artery supplying a different segment [3]. Bjurlin et al. [2] suggested, in a recent multicenter study, the use of infrared-fluorescence imaging technology before tumoral resection to assess the efficiency of selective clamping. It showed that 35% of cases required main renal artery clamping because of continued tumor perfusion [2].
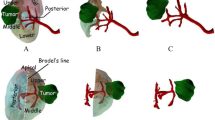
The segmental apical artery, supplying only the small uppermost part of the superior pole, was isolated in 17% of cases. Superior partial nephrectomy should begin with isolation and ligation of the segmental apical artery, often of proximal origin. Given its small caliber and its small vascular territory, its clamping will lead to ischemia of a small parenchymal territory and it cannot control all bleeding [19].
In cases where a superior polar artery exists, we should start with its clamping which will show the ischemic demarcation line. If the outlined ischemic area contains the tumor with healthy tissue margin, we can stop at this level without dissecting the posterior segmental artery, keeping in mind that the risk of cutting the latter is important since it crosses the upper stem in 80% of cases. Therefore, during superior partial nephrectomy, the surgeon should carefully identify, skeletonize, and safeguard the posterior division of renal artery due to its close relationship to the upper pole [20]. In our series, we isolated the superior polar artery in one case only (1.3%). In the literature, the presence of this artery was noted in 1.8–22.2% of cases (Table 6).
It is necessary to have accurate analysis of renal and tumor vascular mapping before partial nephrectomy whether performed by open, laparoscopic or robotic surgery. So far, nephrectomy scores [6, 11] have always helped surgeons to assess the complexity of partial nephrectomy. Actually, three dimensional (3D)-printed renal modeling produced from preoperative computed tomography images is an innovative tool that helps in establishing the preoperative planning for partial nephrectomy. First described by Von Rundstedt et al. [24], it showed high concordance with patient’s anatomy, the tumor exact location and its anatomical relationships within the kidney.
Many authors [1, 15, 24] concluded that this new means of simulation was revealed to significantly enhance the prediction of the tumor complexity, the difficulties that the surgeon may encounter, for instance the risk of cutting an aberrant vessel or the failure of selective clamping. Shorter operating time and warm ischemic duration were also reported. Michiels et al. [15] underlined particular benefit of 3D modeling and printing in management of bilateral complex renal tumors, with excellent results and a touchable benefit in minimizing intra-operative blood loss and optimize the functional outcome of the remainder healthy parenchyma (Fig. 12).
3D printed model for a 53-year-old female presenting a 21 × 15 × 15 mm renal tumor located in the interpolar region of left kidney, treated by left partial nephrectomy. Comparative views of the CT scan (a axial, b coronal, and c sagittal planes) and corresponding views of the physical model (d superior view, e median view, and f median view). The cubes show the 3D printed model orientation in space (I = inferior face, A = anterior face, L = lateral side, S = superior face, P = posterior face, and M = median side). Reprinted with permission from Bernhard et al. [1]
Many authors [1, 15, 22] have concluded the usefulness of 3D models, comparing to enhanced CT, in preoperative education among patients and their families prior to partial nephrectomy. It was especially beneficial for elderly patients. Teishima et al. [22], reported that understanding about the anatomy of kidneys and tumors improved significantly using a 3D model in both patients and families. 3D model was beneficial for them to understand fundamental information on their own diseases, thus, improve the postoperative observance and reduce possible complications, as described by other previous studies [7, 22].
There were some limitations in our study. First, the sample size was small, resulting in low number of variations. Second, the Ternon classification adopted was innovative, and not classically reported in literature, which resulted in small number of studies used in discussing our results.
Conclusion
The knowledge of the variations of the renal artery is of great importance in partial nephrectomy. The awareness about the presence of such variations is important from the academic, surgical, and radiological point of view, to facilitate the surgery and avoid per operative complications.
Novel technology of three-dimensional modeling helps to take consideration of vascular anatomic variability. It can be used for accurate vascular mapping before renal partial surgery, but also improves patient’s information about his disease and the surgical procedure. Further research, studies, and practical applications are needed to help for better understanding and safer surgery.
Availability of data and material
Data are available on demand.
References
Bernhard JC, Isotani S, Matsugasumi T et al (2016) Personalized 3D printed model of kidney and tumor anatomy: a useful tool for patient education. World J Urol 34:337–345
Bjurlin MA, Gan M, McClintock TR, Volpe A, Borofsky MS, Mottrie A, Stifelman MD (2014) Near infrared fluorescence imaging: emerging applications in robotic upper urinary tract surgery. Eur Urol 65:793–801
Budhiraja V, Rastogi R, Asthana AK (2010) Renal artery variations: embryological basis and surgical correlation. Rom J Morphol Embryol 51:533–536
Chauhan P, Khima D, Pandya A, Rathod S (2016) A cadaveric study of renal artery variation in Rajkot. JMSCR 4:10066–10069
Eisendrath DN (1920) The relation of variations in the renal vessels to pyelotomy and nephrectomy. Am J Surg 71:726–743
Ficarra V, Novara G, Secco S et al (2009) Preoperative aspects and dimensions used for an anatomical (PADUA) classification of renal tumours in patients who are candidates for nephron-sparing surgery. Eur Urol 56:786–793
Fink C, Diener MK, Bruckner T et al (2013) Impact of preoperative patient education on prevention of postoperative complications after major visceral surgery: study protocol for a randomized controlled trial (PEDUCAT trial). Trials 14:271
Graves FT (1956) The anatomy of the intrarenal arteries in health and disease. Br J Surg 43(182):605–616
Khamanarong K, Prachaney P, Utraravichien A et al (2004) Anatomy of renal arterial supply. Clin Anat 17:334–336
Klatte T, Ficarra V, Gratzke C et al (2015) A literature review of renal surgical anatomy and surgical strategies for partial nephrectomy. Eur Urol 68:980–992
Kutikov A, Uzzo RG (2009) The R.E.N.A.L. nephrometry score: a comprehensive standardized system for quantitating renal tumor size, location and depth. J Urol 182:844–853
Lloyd LW (1935) The renal artery in Whites and American Negroes. Am J Phys Anthropol 20:153–163
Macchi V, Crestani A, Porzionato A et al (2017) Anatomical study of renal arterial vasculature and its potential impact on partial nephrectomy. BJU Int 120:83–91
Merklin RJ, Michels NA (1958) The variant renal and suprarenal blood supply with data on the inferior phrenic, ureteral and gonadal arteries: a statistical analysis based on 185 dissections review of the literature. J Int Coll Surg 29:41–76
Michiels C, Jambon E, Bernhard JC (2019) Measurement of the accuracy of 3D-printed medical models to be used for robot-assisted partial nephrectomy. AJR Am J Roentgenol 213:626–631
Palmieri BJ, Petroianu A, Silva LC et al (2011) Study of arterial pattern of 200 renal pedicle through angiotomography. Rev Col Bras Cir 38:116–121
Rueda-Esteban R et al (2017) Corrosion casting, a known technique for the study and teaching of vascular and duct structure in anatomy. Int J Morphol 35:1147–1153
Saldarriaga B, Perez AF, Ballesteros LE (2008) A direct anatomical study of additional renal arteries in a Colombian mestizo population. Folia Morphol 67:129–134
Sampaio FJ (1992) Anatomical background for nephron-sparing surgery in renal cell carcinoma. J Urol 147:999–1005
Sampaio FJ, Passos M (1992) Renal arteries: anatomic study for surgical and radiological practice. Surg Radiol Anat 14:113–117
Talovic E et al (2007) Review of the supernumerary renal arteries by dissection method. Acta Med Acad 36:59–69
Teishima J, Takayama Y, Iwaguro S et al (2018) Usefulness of personalized three-dimensional printed model on the satisfaction of preoperative education for patients undergoing robot-assisted partial nephrectomy and their families. Int Urol Nephrol 50:1061–1066
Ternon Y (1959) Recherches sur l'anatomie chirurgicale de l'artère rénale. Bases d'une segmentation artérielle du rein. Introduction anatomique des artériographies rénales. Thèse. Médecine, Paris
Von Rundstedt FC, Scovell JM, Agrawal S et al (2017) Utility of patient-specific silicone renal models for planning and rehearsal of complex tumour resections prior to robot-assisted laparoscopic partial nephrectomy. BJU Int 119:598–604
Funding
No funding was received.
Author information
Authors and Affiliations
Contributions
Dr AB: project development, cadaveric dissection, corrosion process, measurements, data collection, manuscript writing, and results discussion. Dr AS: project development, results discussion, and manuscript writing. Dr AH: project development, results discussion, manuscript writing, and figures design. Dr MC: project development, results discussion, and manuscript writing. Dr NB: project development and data collection. Dr MA: project development and data collection. Dr MH: project development, data collection, and manuscript correction. Dr MMM: project development, data collection, and manuscript correction. Dr MC: project supervision, results discussion, and manuscript correction.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
The approval of the current study has been granted by the medical committee of research ethics of Charles Nicole Hospital. A copy of the approval is available for review by the Editor-in-Chief of this journal on request. Reference number is not available.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bouzouita, A., Saadi, A., Hermi, A. et al. Cadaveric study of arterial renal anatomy and its surgical implications in partial nephrectomy. Surg Radiol Anat 43, 1449–1459 (2021). https://doi.org/10.1007/s00276-021-02769-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00276-021-02769-8