Abstract
Introduction
The elbow joint is particularly exposed to soft tissue injuries associated with fractures and dislocations. Different coverage options within the past decades for recovering loss of soft tissue defects around the elbow region have been proposed based on anatomical research. Our aim was to make an updated focus on the anatomical basis of different techniques of coverage of loss of tissues around the elbow.
Materials and methods
The main procedures of flaps were defined: local random, axial fasciocutaneous, local muscle pedicle, propeller and free microvascular flaps. A systematic literature review on anatomical basis on these different flaps options was conducted searching on PubMed databases and the selection process was undergone according to the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guideline. Inclusion criteria were: review and original articles, including anatomical basis of the procedures, in English and French languages.
Results
The final analysis included 37 relevant articles out of 1499 published references. 640 flaps were referenced, for covering 302 elbows. Local random flaps provide a good quality skin for small tissue defects in posterior elbow and periolecranon area, and depend on dermal and subdermal blood supply. Axial fasciocutaneous flaps have well-defined blood supplies and are designed as peninsular, island, or microvascular free flaps, as the radial forearm, lateral arm, ulnar artery, antecubital fasciocutaneous, and posterior interosseous flaps. Muscular flaps have advantages as strength, capacity to contrast local infection and to avoid empty spaces, and can be used as pedicle or as free transfers. Propeller flaps can be rotated up to 180° around an axis corresponding to the perforator vessel and do not require the sacrifice of a major artery or functional muscle. The concept of perforasome is evoked. Free microsurgical transfers can be proposed to cover any defect around the elbow.
Discussion and conclusion
The anatomical basis of the flap’s harvesting and the possibilities of elbow coverage are discussed through the selected articles. The different indications according to the areas of soft tissues defects are considered.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Soft tissue defects in the elbow region are numerous and range from those caused by tumor excision, burns, trauma, infections and exposed prostheses [17]. The elbow is particularly prone to trauma due to its position and high mobility [38]. It is a difficult region to reconstruct from a functional point of view.
Elbow wounds can have extremely disabling effects [45], compromising the flexion–extension of the upper limb and thus, affecting patient’s quality of life. The healing of soft tissue defects at the elbow level (closure by first or second intention, skin grafts), is hindered by the intimate anatomical relationship between skin and bone, most of all in the dorsal area. Supplying a good quality tissue is, therefore, primordial to not compromise joint function.
Defects of soft tissues surrounding the elbow area require a reconstruction with flexible and adaptable tissue, allowing repetitive motion of flexion and extension and authorizing an early mobilization to limit stiffness and contracture risks.
There are numerous flap options to cover soft tissue defects of elbow. These include:
-
Local random flaps, based on dermal and subdermal vascular network. They include cutaneous tissue rearrangements as Z-plasty, rhomboids flaps, advancement/transposition/rotational flaps [22, 28].
-
Axial fasciocutaneous flaps, based on a known axial blood supply. They can be designed as peninsular, island, or microvascular free flaps. The radial forearm flap was first described by Yang et al. in 1981 [53], rapidly followed by the lateral arm flap, the antecubital, the ulnar artery (UA) flap and then by the posterior interosseous flap.
-
Local muscle pedicle flaps They include the anconeus, the brachioradialis, the flexor carpi ulnaris, the extensor carpi radialis longus and the latissimus dorsi flaps [16, 21, 25, 27, 35].
-
Propeller flaps, based on a single vascular pedicle, a perforator that supplies a fasciocutaneous island of the skin. The term “Propeller” is related to the translation by rotation of the flap. This island can be turned helically until vascular tolerance of the flap. Propeller flaps have been well defined by Blondeel et al. in 2003 [3]. For elbow coverage, radial collateral artery perforator (RCAP) or posterior ulnar recurrent artery perforator (PURAP) flaps are usually used.
-
Free microvascular transfers that can include muscle-only, myocutaneous transfers, fasciocutaneous or composite transfers. Free transfers options used for elbow reconstruction include the anterolateral thigh (ALT), scapular, and parascapular, gracilis muscle, latissimus dorsi muscle, or fibular osteocutaneous flaps [24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54].
The various coverage options within the past decades for recovering loss of substances in this particular anatomical region have increased, and are related to anatomical basis. This supports part of the great interest of plastic and trauma surgeons for early recovering, and the elbow joint is a special area that justifies in surgical practice skills for managing with soft tissue defects around a mobile joint. This article aimed to provide an anatomic overview on different flap options for elbow coverage through a systematic review.
Materials and methods
We undertook this review in March 2018 in accordance with the Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) statement [31].
Eligibility criteria
The inclusion criteria were as follows: published report about the anatomical basis of flaps for elbow coverage including original articles and case series. We excluded: case reports, articles with no clear anatomical basis techniques, and all studies in any language other than English or French.
Search strategy
Eligible studies were identified from the PubMed database using the following keywords: “elbow coverage” OR “elbow coverage anatomical basis” OR “elbow coverage flaps” OR “anatomical basis elbow flaps” OR “flaps for elbow” OR “flaps for elbow coverage” OR “soft-tissue coverage of elbow” OR “soft-tissue reconstruction for elbow” OR “reconstruction of soft-tissue for elbow” OR “soft-tissue coverage options for elbow” OR “soft-tissue coverage flaps for elbow”.
Reference lists of selected articles were also examined to identify additional potentially eligible articles.
Data collection and analysis
Data were extracted independently by two researchers.
Data were collected on authors, publication date, country, type of study and level of evidence, number of elbows covered, localization of the loss of substance, dimension of the loss of substance, type of flap, source vessel supplying the flap.
A descriptive analysis of all data was carried out.
Results
Among the 1499 articles initially identified by the search, 37 were finally selected (Fig. 1). Articles were published between 1985 and 2018. They included clinical application (n = 25) and cadaveric studies (n = 12). In total 640 flaps were performed to cover 302 elbows.
Table 1 shows the presentation of the included articles with their geographic distribution, study design, level of evidence, number of flaps undergone and number of elbows covered.
Table 2 shows the characteristics of the studied flaps.
Indications and anatomy
Local random flaps provide a good quality skin for small tissue defects in posterior elbow and periolecranon area [36]. They depend on dermal and sub-dermal blood supply. Bunkis et al. [4], through an anatomic study, demonstrated the presence of a rich fascial vascular plexus in the forearm originated from multiple muscular perforator vessels that provided enhanced blood flow to the overlying skin. Carriquiry et al. [6] showed that this fascial vascular plexus is supplied by multiple branches of the brachial artery (BA) reaching the skin through the medial intermuscular septum of the arm.
Axial fasciocutaneous flaps have well-defined blood supplies and can be designed as peninsular, island, or microvascular free flaps. The most common examples of axial fasciocutaneous flaps for elbow coverage are the radial forearm, lateral arm, ulnar artery, antecubital fasciocutaneous, and the posterior interosseous flaps. It is a single-stage procedure not requiring the sacrifice of any dominant vessel or functional muscle. Davalbhakta and Niranjan [12] in their case series have used the medial arm flap based on vessels from the inferior ulnar collateral artery that would appear to be more useful than the lateral arm flap because it retains the skin innervation by the medial cutaneous nerve of the forearm. However, according to Prantl et al. [40] the vessel supplying the flap runs directly behind the medial epicondyle and posterior to the ulnar nerve within the medial intermuscular septum, so in case of harvesting this flap, great care should be taken on the ulnar nerve. Hayashi et al. [20] described the axial adipo-fasciocutaneous flap based on branches of the ulnar recurrent artery (URA). In their opinion subcutaneous fat should be left attached to the skin flaps to incorporate majorly the subdermal plexus and to relatively reduce donor site scar. Reverse lateral arm flap has been described in the series reported by Davalbhakta and Niranjan [12], Prantl et al. [40], Coessens et al. [11], Tung et al. [49], Morrison et al. [32], and Turegun et al. [50]. This flap provides stable soft tissue coverage for soft tissue defect of the posterior and lateral areas of the elbow. This fasciocutaneous flap is based on the posterior radial collateral artery (PRCA), which forms a vascular arcade in the lateral intermuscular septum with the middle collateral artery. Penteado et al. [39] and Mazzer et al. [30] described the anatomical basis of the posterior interosseous flap in cadaveric studies. In 82 specimens, they verified the constant presence of the posterior interosseous artery (PIA) which runs on a line drawn from the lateral epicondyle of the humerus to the head of the ulna, in the septum between the extensor carpi ulnaris and extensor digiti minimi muscles, giving off 7–14 cutaneous branches from elbow to the wrist. They found that the PIA anastomoses with the anterior interosseous artery and the dorsal carpal network in 98.6% of their cases. This posterior interosseous flap is reliable for small tissue defects in the olecranon zone [17].
Among axial fasciocutaneous flaps, antecubital flap finds its use for small-to-medium loss of substance in periolecranon area. In the study by Duteillel et al. [14], the predominant vessel of antecubital flap originated about 1 cm after the origin of the radial artery (RA) (the most proximal located at 0.85 cm and the most distal at 1.15 cm). This collateral branch immediately crossed the fascia before the brachialis muscle to join the subcutaneous plan. Conversely, in the series of Van Landuyt et al. [51], the major vessel supplying the flap corresponded to the inferior part of the ulnar artery, segment of the ulnar artery distal to the origin of the common interosseous artery. In all cases, the lateral cutaneous nerve of the forearm could be harvested with the flap to ensure its sensory function. According to Jones et al. [23], the radial forearm flap, based on the radial artery, because of its thin, supple skin and long pedicle, is the optimal flap for coverage of medium size defects in any elbow zone. However, a preliminary Allen test for clinical testing of the presence and patency of the ulnar artery, or a preoperative angiogram should be performed.
Muscular flaps are commonly preferred to fasciocutaneous flaps for their strength, their capacity to contrast local infection and to avoid empty spaces. They can be used as pedicle or as free transfer. Elhassan et al. [15], Fleager et al. [16] and Schmidt et al. [43] described the anconeus flap to cover small-to-medium defects in the posterior and lateral aspects of the elbow. This muscle is supplied by the middle collateral artery (MCA), the recurrent interosseous artery (RIA), and posterior branch of the radial collateral artery (PBRCA). In the cadaveric study by Schmidt et al. [43], RPIA and MCA were present in all specimens, while PBRCA was found only in 58.3% of the specimens. In the same study, RPIA, that originated from either the ulnar artery or the posterior interosseous artery, had an average diameter of 1.1 ± 0.4 mm, and the MCA that originated from the profunda brachii artery had an average diameter of 0.8 ± 0.1 mm; in contrast, according to Fleager et al. [16], MCA was the main pedicle.
Several studies [1, 2, 37, 46, 52] explored the utility and the vascular network of the flexor carpi ulnaris flap. In all these studies, this flap was supplied by both the ulnar artery and the posterior ulnar recurrent artery, with the ulnar artery providing the dominant blood supply. This flap provides a predictable coverage for small tissue defects (2–4 cm) in the posterior elbow. Brachioradialis flap is a pedicle muscular flap used to cover small defects in posterior elbow, one-third anterior forearm, the antecubital fossa, and the posterolateral elbow region [1, 26, 41]. It is supplied by branches from the radial recurrent artery (RRA), the radial artery (RA), and the brachial artery (BA). In a dissection study of 53 upper extremities, Rohrich et al. [41] reported that the major vascular pedicles to the brachioradialis muscle arose from the RRA in 58%, the BA in 24%, and from the RA in 17% of specimens. The dominant pedicle was located between 4.2 cm proximal and 4.7 cm distal to the elbow fold. Similar results have been reported by Leversedge [26], who showed that the RRA perfused an average of 41% of the length of the muscle and 65% of the entire muscle volume.
Latissimus dorsi flaps can be used as a pedicle or as a free flap. It is an interesting option to cover large tissue defects in any elbow zone. The vascular pedicle is constant (thoracodorsal artery, TA), and the implication within the flap of the thoracodorsal nerve could be useful to restore flexion–extension [7, 19, 46]. Delgove et al. [13] described a new local muscular flap for elbow coverage: the medial triceps brachii flap. It can provide coverage for small-to-medium-sized defects in olecranon and medial elbow areas and it is supplied by branches from the middle collateral artery and the arterial peri-articular circle.
Propeller flaps, defined as cutaneous or fasciocutaneous islands based on perforator vessels, can be rotated up to 180°, around an axis corresponding to the perforator vessel. They do not require the sacrifice of a major artery or functional muscle [5, 10, 29, 33, 34, 47]. The most used propeller flaps to cover elbow zone are the radial collateral artery perforator (RCAP)-based and the ulnar recurrent artery perforator (PURAP)-based propeller flap. They can repair small-to-medium defects around cubital fossa, medial dorsal, olecranon for the PURAP propeller flap and around the Cubital fossa, lateral and dorsal areas, for the RCAP-based propeller flap, respectively. Based on the anatomical study of Tiengo et al. [47], a mean of 20 perforator arteries raised up from the radial artery, and the most proximal perforator branch of the radial artery was located at a mean distance 2.4 cm from its origin. The same study confirmed that in the proximal forearm, the collateral branches are less numerous, but larger in diameter than in the distal forearm. Recently, Cil et al. [10] described in a cadaveric study a new perforator flap based on the main perforator of the distal brachial artery (DBAMP). In all specimens, the main perforator was found 11.5 cm above and 1.3 cm medial to the medial epicondyle of the humerus. The mean length and diameter of the DBAMP were 3.3 cm and 0.95 mm, respectively. The mean skin territory was 10.7 cm × 5.6 cm allowing to cover the posterior elbow. Another propeller flap option has been proposed by Camuzard [10]; the inferior cubital artery perforator flap, based on the inferior cubital artery perforator (ICA), that can be used to cover small and medium loss of substance in posterior, lateral and medial elbow. The ICA originated from the radial artery in 62.5% of specimens, from the radial recurrent artery in 17.5%, from the brachial artery in 7.5%, from the brachial artery bifurcation in 2.5%.
Free microsurgical transfers can be utilized as composite to cover any defect around the elbow. The flap is designed on a precise vascular pedicle and a suitable recipient vessel has to exist for the microvascular anastomosis. According to Chui et al. [9], the free anterolateral thigh flap (ATL) offers good quality skin and subcutaneous tissue, a large skin paddle (about 35 × 25 cm), a vastus lateralis muscle that can be used to obliterate dead space and contra local infections, and access to fascia lata if a triceps tendon repair is necessary. The vascularization of this ATL-flap is based on the lateral circumflex femoral artery descending branch (LCFA).
Discussion
We provided a systematic review focusing specifically on the anatomical basis of flaps used for elbow coverage.
Preoperative considerations before reconstruction of soft tissue defects in elbow region are important and careful, and are related to the wound size and complexity. Wounds with exposed muscle and subcutaneous tissue are accessible to healing by secondary intention or skin grafting. When structural integrity is not preserved (joint, bone, neurovascular structure exposure, or tendon without paratendon), coverage requires an appropriate flap.
The main objectives of the elbow area soft tissues reconstruction are:
-
Anterior part: thin cutaneous tissue to avoid limitation during the elbow flexion, and cutaneous flexibility to avoid retractile skin scar during elbow extension.
-
Posterior part: thin, and flexible skin too, but also “cutaneous reserve” allowing 140° of elbow flexion.
Local random flaps based on dermal and subdermal vascular plexus are only used to cover small defects to avoid excessive tension compared to primary closure.
Axial fasciocutaneous flaps have the advantage of including a known and constant axial blood supply compared to the random local flaps. The radial forearm flap was first described by Yang et al. in 1981 [53], followed by the lateral arm flap, the ulnar artery flap and then by the posterior interosseous flap.
These flaps provide good soft tissues coverage, a smooth surface for tendon sliding, and minor functional defects in donor sites. Among these flaps, the reverse lateral arm flap was the most reported in our review [11, 12, 32, 40, 49]; its vascular pedicle is consistent, the donor site can be closed primarily, it does not require sacrifice of a major artery or functional muscle and allows an early mobilization.
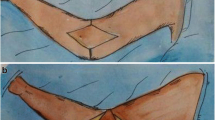
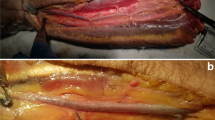
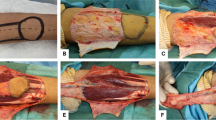
Pedicle muscular flaps include the brachioradialis, the extensor carpi radialis longus, the anconeus, and the flexor carpi ulnaris, the latissimus dorsi flap, and recently the medial triceps brachii flap [13]. They are used as rotation flaps to cover small and medium defects. All these muscular flaps can be harvested without measurable functional deficit. A special attention should be given when harvesting the flexor carpi radialis; due to a relatively short muscle length, only 5 cm of muscle distal to the pedicle from the radial artery has a reliable perfusion [1]. This pattern should be taken into consideration for lateral epicondyle coverage to minimize functional morbidity or any situation which could require an alternative to the brachioradialis or the flexor carpi ulnaris flaps. The latissimus dorsi flap is really useful for achieving a complete coverage of a large posterior elbow defect, as shown in a recent case (Figs. 1, 2a, b, 3). The covering possibility is associated to a functional effect; partial, but useful when the triceps brachii has been lost (Figs. 4, 5, 6). Moreover, bone healing is also an important goal, and the early coverage contributes to decrease the risk of non-union (Fig. 7).
a Patient 72-year-old, bicycle accident (cyclist overturned by a truck), internal repair of a complex open supra- and intercondylar fracture of the distal humerus by plates and screws, and olecranotomy fixed by pins and cerclage. b The initial skin closure has been complicated by a large necrosis of the dorsal surface of the left elbow
Propeller flaps are cutaneous or fasciocutaneous flaps based on perforator vessels that can be rotated up to 180°, around their vascular axis [8]. Perforator vessels originating from deep source pedicles supply a given “perforasome” through fascia or septum (septocutaneous perforators) or through muscles (musculocutaneous perforators) [42]. Based on perforator vessels, the realization of this flap does not sacrifice a major vascular axis and does not compromise the realization of a conventional flap (axial fasciocutaneous, pedicle muscular or free transfer) in case of failure. The most common propeller flaps used for elbow coverage are the RCAP-based propeller flap and the PURAP-based propeller flap.
Free microvascular transfers can be used as composite when a tendon reconstruction or a vascularized bone graft is required and for large defects, when no other possibilities of coverage are admitted. Authors have found that the anterior–lateral thigh flap provides a good versatility for the coverage of soft tissue defects around the elbow. The ATL can be harvested with a portion of the tensor fascia lata, vastus lateralis muscle, or femoral cutaneous nerve [9].
It is interesting to remark that in our review, we did not find suitable articles about anatomical basis of free transfers for elbow reconstruction, but most of the selected articles focused on fasciocutaneous flaps and on propeller flaps. In the last decades, the concept of reparation has changed, and became less and less invasive in the respect of the economy of the donor site and the different researches within the anatomical study clearly highlighted this recent phenomenon.
Conclusion
This review of the literature contributed to emphasize the place of anatomical research in the development of the different soft tissues coverage procedures in elbow reconstruction. The constant increase of knowledge about skin cartography, muscle’s blood supply, perforators and perforasomes, leads to an important relevance in plastic and reconstructive techniques.
References
Avashia YJ, Shammas RL, Poveromo LP, Dekker TJ, Brubacher JW, Richard MJ, Ruch DS, Mithani SK (2018) Forearm based turnover muscle flaps for elbow soft tissue reconstruction: a comparison of regional coverage based on distal flap perfusion. Plast Reconstr Surg. https://doi.org/10.1097/PRS.0000000000004472
Bayne CO, Slikker W III, Ma J, Ruch DS, Leversedge FJ, Cohen MS, Wysocki RW (2015) Clinical outcomes of the flexor carpi ulnaris turnover flap for posterior elbow soft tissue defects. J Hand Surg Am 40(12):2358–2363
Blondeel PN, Van Landuyt KH, Monstrey SJ, Hamdi M, Matton GE, Allen RJ, Dupin C, Feller AM, Koshima I, Kostakoglu N, Wei FC (2003) The “Gent” consensus on perforator flap terminology: preliminary definitions. Plast Reconstr Surg 112(5):1378–1383
Bunkis J, Ryu RK, Walton RL, Epstein LI, Vasconez LO (1998) Fasciocutaneous flap coverage for periolecranon defects. Ann Plast Surg 14(4):361–370
Camuzard O, Foissac R, Clerico C, Fernandez J, Balaguer T, Ihrai T, de Peretti F, Baqué P, Boileau P, Georgiou C, Bronsard N (2016) Inferior cubital artery perforator flap for soft-tissue coverage of the elbow: anatomical study and clinical application. J Bone Jt Surg Am 98(6):457–465
Carriquiry CE (1990) Versatile fasciocutaneous flaps based on medial septal cutaneous vessels of the arm. Plast Reconstr Surg 86(1):103–109
Chang LD, Goldberg NH, Chang B, Spence R (1994) Elbow defect coverage with a one-staged, tunneled latissimus dorsi transposition flap. Ann Plast Surg 32(5):496–502
Chaput B, Gandolfi S, Ho Quoc C, Chavoin JP, Garrido I, Grolleau JL (2014) Reconstruction of cubital fossa skin necrosis with radial collateral artery perforator-based propeller flap (RCAP). Ann Chir Plast Esthet 59(1):65–69
Chui CH, Wong CH, Chew WY, Low MH, Tan BK (2012) Use of the fix and flap approach to complex open elbow injury: the role of the free anterolateral thigh flap. Arch Plast Surg 39:130–136
Cil Y, Kocabiyik N, Ozturk S, Isik S, Ozan H (2010) A new perforator flap from distal medial arm: a cadaveric study. Eplasty 10:541–548
Coessens B, Vico P, De Mey A (1993) Clinical experience with the reverse lateral arm flap in soft-tissue coverage of the elbow. Plast Reconstr Surg 92(6):1133–1136
Davalbhakta AV, Niranjan NS (1999) Fasciocutaneous flaps based on fascial feeding vessels for defects in the periolecranon area. Br J Plast Surg 52:60–63
Delgove A, Weigert R, Casoli V (2018) A new local muscle flap for elbow coverage—the medial triceps brachii flap: anatomy, surgical technique, and preliminary outcomes. J Shoulder Elbow Surg 27:4:733–738
Duteillel F, Rocchi L, Dautell G, Merle M (2001) Le lambeau antécubital: intérêt dans les pertes de substance du coude. Etude anatomique et expérience de cinq cas cliniques. Ann Chir Plast Esthet 46(1):18–22
Elhassan B, Karabekmez F, Hsu CC, Steinmann S, Moran S (2011) Outcome of local anconeus flap transfer to cover soft tissue defects over the posterior aspect of the elbow. J Shoulder Elbow Surg 20:807–812
Fleager KE, Cheung EV (2011) The “anconeus slide”: rotation flap for management of posterior wound complications about the elbow. J Shoulder Elbow Surg 20:1310–1316
Giele H (2012) Soft tissue coverage around the elbow. In: Stanley D, Trail I (eds) Operative elbow surgery: expert consult. Churchill Livingstone, London, p 719
Gupta A, Yenna Z (2014) Soft tissue coverage of the elbow. Hand Clin 30:479–485
Harvey EJ, Aponte R, Levin LS (1999) The application of the island pedicle latissimus dorsi flap for soft tissue coverage of the elbow. Can J Plast Surg 7(1):23–26
Hayashi Y, Maruyama M, Saze E, Okada (2004) Ulnar recurrent adipofascial flap for reconstruction of massive defects around the elbow and forearm. Br J Plast Surg 57:632–637
Janevicius RV, Greager JA (1992) The extensor carpi radialis longus muscle flap for anterior elbow coverage. J Hand Surg 17(1):102–106
Jensen M, Moran SL (2008) Soft tissue coverage of the elbow: a reconstructive algorithm. Orthop Clin N Am 39(2):251–264 (vii)
Jones NF, Jarrahy R, Kaufman MR (2008) Pedicled and free radial forearm flaps for reconstruction of the elbow, wrist, and hand. Plast Reconstr Surg 121:887–898
Kremer T, Bickert B, Germann G et al (2006) Outcome assessment after reconstruction of complex defects of the forearm and hand with osteocutaneous free flaps. Plast Reconstr Surg 118(2):443–454
Lalikos JF, Fudem GM (1997) Brachioradialis musculocutaneous flap closure of the elbow utilizing a distal skin island: a case report. Ann Plast Surg 39(2):201–204
Leversedge FJ, Casey PJ, Payne SH, Seiler GJ III (2001) Vascular anatomy of the brachioradialis rotational musculocutaneous flap. J Hand Surg 26A:711–721
Lingaraj K, Lim AY, Puhaindran ME et al (2007) Case report: the split flexor carpi ulnaris as a local muscle flap. Clin Orthop Relat Res 455:262–266
Lister GD, Gibson T (1972) Closure of rhomboid skin defects: the flaps of Limberg and Dufourmentel. Br J Plast Surg 25(3):300–314
Mateev MA, Trunov L, Hyakusoku H, Ogawa R (2009) Analysis of 22 posterior ulnar recurrent artery perforator flaps: a type of proximal ulnar perforator flap. Eplasty 10:e2
Mazzer N, Barbieri CH, Cortez M (1996) The posterior interosseous forearm island flap for skin defects in the hand and elbow a prospective study of 51 cases. J Hand Surg (Br Eur Vol) 21B(2):237–243
Moher D, Liberati A, Tetzlaff J, Altman DJ, PRISMA Group (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 62(10):1006–1012
Morrison CS, Sullivan SR, Bhatt RA, Chang JT, Taylor HO (2013) The pedicled reverse-flow lateral arm flap for coverage of complex traumatic elbow injuries. Ann Plast Surg 71:37–39
Murakami M, Ono S, Ishii N, Hyakusoku H (2012) Reconstruction of elbow region defects using radial collateral artery perforator (RCAP)-based propeller flaps. J Plast Reconstr Aesthet Surg 65(10):1418–1421
Nakao J, Umezawa H, Ogawa R, Mateev MA (2017) Reconstruction of elbow skin and soft tissue defects using perforator-pedicled propeller flaps. Microsurgery 37:1–6
Ohtsuka H, Imagawa S (1985) Reconstruction of a posterior defect of the elbow joint using an extensor carpi radialis longus myocutaneous flap: case report. Br J Plast Surg 38(2):238–240
Orgill DP, Pribaz JJ, Morris DJ (1994) Local fasciocutaneous flaps for olecranon coverage. Ann Plast Surg 32(1):27–31
Payne DES, Kaufman AM, Wysocki RW, Richard MJ, Ruch DS, Leversedge FJ (2011) Vascular perfusion of a flexor carpi ulnaris muscle turnover pedicle flap for posterior elbow soft tissue reconstruction: a cadaveric study. J Hand Surg Am 36A:246–251
Patel KM, Higgin JP (2013) Posterior elbow wounds: soft tissue coverage options and techniques. Orthop Clin N Am 44:409–417
Penteado CV, Masquelet AC, Chevrel JP (1986) The anatomic basis of the fascio-cutaneous flap of the posterior interosseous artery. Surg Radiol Anat 8:209–215
Prantl L, Schreml S, Schwarze H, Eisenmann-Klein M, Nerlich M, Angele P, Jung P, Fuchtmeier B (2008) A safe and simple technique using the distal pedicled reversed upper arm flap to cover large elbow defects. J Plast Reconstr Aesthet Sur 61(5):546–551
Rohrich RJ, Ingram AE Jr (1995) Brachioradialis muscle flap: clinical anatomy and use in soft-tissue reconstruction of the elbow. Ann Plast Surg 351:70–76
Saint-Cyr M, Wong C, Schaverien M, Mojallal A, Rohrich RJ (2009) The perforasome theory: vascular anatomy and clinical implications. Plast Reconstr Surg 124(5):1529–1544
Schmidt CC, Kohut NG, Greenberg JA, Kann SE, Idler RS, Kiefhaber TR (1999) The anconeus muscle flap: its anatomy and clinical application. J Hand Sur Am 4(2):359–369
Sharpe F, Barry P, Lin SD, Stevanovic M (2014) Anatomic study of the flexor carpi ulnaris muscle and its application to soft tissue coverage of the elbow with clinical correlation. J Shoulder Elbow Surg 23:82–90
Sharpe F, Stevanovic M, Itamura JM (2004) Soft tissue coverage of the elbow. In: Mirzayan R, Itamura JM (eds) Shoulder and elbow trauma. Thieme, New York, p 89
Stevanovic M, Sharpe F, Thommen VD, Itamura JM, Schnall SB (1999) Latissimus dorsi pedicle flap for coverage of soft tissue defects about the elbow. J Shoulder Elbow Surg 8(6):634–643
Tiengo C, Macchi V, Porzionato A, Stecco C, Parenti A, Bassetto F, De Caro R (2007) The proximal radial artery perforator Xap (PRAP-Xap): an anatomical study for its use in elbow reconstruction. Surg Radiol Anat 29:245–251
Tripathy S, Khan AH, Sharma S (2010) Clinical study of the recurrent flaps of the arm for resurfacing of elbow defects. Eur J Plast Surg 33:23–28
Tung TC, Wang KC, Fang CM, Lee CM (1997) Reverse pedicled lateral arm flap for reconstruction of posterior soft-tissue defects of the elbow. Ann Plast Surg 38:6:635–641
Turegun M, Nisanci M, Duman H, Aksu M, Sengezer M (2005) Versatility of the reverse lateral arm flap in the treatment of post-burn antecubital contractures. Burns 31:212–216
Van Landuyt K, De Cordier BC, Monstrey SJ, Blondeel PN, Tonnard P, Verpaele A, Matton G (1998) The antecubital fasciocutaneous island flap for elbow coverage. Ann Plast Surg 41:3:252–257
Wysocki RW, Gray RL, Fernandez JJ, Cohen MS (2008) Posterior elbow coverage using whole and split flexor carpi ulnaris flaps: a cadaveric study. J Hand Surg 33A:1807–1812
Yang GF, Chen PJ, Gao YZ et al (1981) Forearm free skin flap transplantation. Chin Med J 61:139–141
Yildirim S, Taylan G, Eker G et al (2006) Free flap choice for soft tissue reconstruction of the severely damaged upper extremity. J Reconstr Microsurg 22:8:599–609
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gandolfi, S., Auquit-Auckbur, I., Poirot, Y. et al. Focus on anatomical aspects of soft tissue coverage options in elbow reconstruction: an updating review. Surg Radiol Anat 40, 943–954 (2018). https://doi.org/10.1007/s00276-018-2066-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00276-018-2066-5