Abstract
A 76-year-old male patient underwent magnetic resonance angiography of the head and neck vessels due to a recent incident of transitory ischemic attack. Cerebral angiogram uncovered the double proximal origin of the median unpaired pericallosal artery from the duplicated anterior communicating complex. The vessel curved around the corpus callosum and irrigated the paracentral lobule and the medial parietal cortical areas. The main trunks of the anterior cerebral arteries showed a branching pattern of the marginal callosal arteries, supplying orbital and frontal territories. The pre-communicating segment of the left anterior cerebral artery was identified as hypoplastic. The co-existence of the duplicated anterior communicating artery, with the medial pericallosal artery ascending from it, represents a potential danger for both open and endovascular surgery on the anterior circle of Willis as the deep half of this complex is obscured from the surgeon’s eyes. Thorough interpretation of preoperative radiographic images and understanding of the developmental mechanisms of such variability are vital. The described branching arrangement of the anterior communicative region and possible mechanisms of migration with following fusion of the pericallosal arteries are discussed in this paper.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The anterior cerebral artery (ACA) is a small terminal branch of the internal carotid artery that supplies the frontal poles and median surfaces of the cerebral hemispheres. Arising at the carotid bifurcation, it passes toward the midline over the optic chiasm to anastomose with its counterpart by means of the anterior communicating artery (Acom).
The following course of the ACA is closely related to the corpus callosum, and is conventionally divided into four segments [2]:
-
1.
The precommunicating segment (also known as A1) extends from the origin of the ACA to the connection with the Acom; the superior and inferior perforating branches arise from this part and irrigate the anterior portion of the hypothalamus, optic nerve, and optic chiasma.
-
2.
The infracallosal segment (A2) represents the part of the artery that travels in a vertical direction under the rostrum of the corpus callosum to its division into the pericallosal and callosomarginal arteries. It supplies the frontobasal region giving off two cortical branches: (a) the infraorbital artery which runs close to the midline in an anterior direction to the gyrus rectus, olfactory bulb, and medial aspect of the inferior frontal lobe; and (b) the frontopolar branch which travels anteriorly and superiorly towards the frontal pole.
-
3.
The precallosal segment (A3) is a short part of the ACA that bifurcates into the proper pericallosal and the callosomarginal terminal branches in front of the genu of the corpus callosum. The proper pericallosal artery bends backward and passes posteriorly over the corpus callosum giving off multiple short callosal branches, while the callosomarginal artery runs in a posterior direction within the cingulate sulcus feeding the medial cortical areas. Both terminal branches of ACA participate in giving off the anterior, medial and posterior internal frontal arteries to irrigate the superior frontal gyrus.
-
4.
The terminal supracallosal segment (A4) runs backward just above the body of corpus callosum giving numerous callosal branches and supplying the paracentral lobule and medial part of the parietal lobe. Some authors additionally subdivide the supracallosal segment into anterior A4 and posterior A5 parts by an imaginary line drawn behind the coronal suture.
Although the branching arrangement and territorial distribution of the ACA are highly variable, true developmental anomalies of the main arterial trunk are quite rare [8]. All variations of the ACA are summarized into three types: the unpaired or azygos ACA, where a single vessel feeds medial aspects of both hemispheres; the bi-hemispheric pattern, characterized by prominent dominance and branching of one of the arteries, while the other is underdeveloped; and the triplicated or accessory ACA, represented by an additional midline vessel supplying various pericallosal territory. The last variant was encountered by authors in 0.9–13% of studied cases and is widely known as a median artery of corpus callosum [3, 9, 10]. However, the nature and developmental mechanism of this vascular pattern is still one of the most controversial points of recent anatomic discussions [8].
Contrary to the scant variability of the ACA, the wide range of the Acom anatomical forms is described in literature [11]. Actually, the vessel is considered to be variable in 60% of studied cases demonstrating plexiform, dimple, fenestrated, duplicated, string, or fused forms, and only in 40% of cases it is presented by a single trunk [4]. The double Acom was encountered in 9.8–10% of brains in the cadaveric studies of Gunnal et al. [3] and Kardile et al. [6].
In the present case report, the triplicated pattern of the ACA with double origin of the accessory vessel from a duplicated Acom complex is discussed. The accessory ACA is interpreted as an unpaired or median pericallosal artery subject to its branching arrangement, while the main ACA trunks occupying the ‘callosomarginal’ territories are considered as the callosal marginal arteries.
The duplication of the Acom artery cohered with the double origin of the median pericallosal artery represents a potential danger for both open and endovascular surgery on the anterior circle of Willis, as the deep limb and distant Acom are secluded from the surgeon and hardly reachable for manipulations. Careful interpretation of preoperative radiographic images and thorough understanding of the developmental mechanisms of such variability are crucial in avoiding iatrogenic mistakes.
Case report
A 76-year-old patient underwent magnetic resonance angiography (MRA) of the neck and head due to a recent transitory ischemic attack. Following the intravenous introduction of 20 ml of di-meglumine gadopentate (Magnevist 469 mg/ml), the Flash 3D sequence scan from head to aortic arch was carried on automatically with slice thickness 1.4 mm, repetition time 3.2 ms, echo time 1.2 ms and field of view 34 cm. The obtained images were analyzed in a RadiAnt DICOM viewer (version 3.4.2.), and 3D volume rendering (VR) was performed.
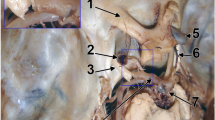
The angiograms of the head and neck demonstrated a normal distribution of the aortic arch vessels. The hypoplastic right vertebral artery showed distal voiding of filling, and the torturous trunk of the basilar artery had major inflow from the left one. On the obtained cerebral MRA images, the complex anatomy of the anterior communicating region was disclosed. The duplicated Acom served as a source of origin of an atypical vessel identified as a median pericallosal artery. Respectively, the median pericallosal artery possessed two limbs at origin, which fused immediately above the Acom complex (Figs. 1, 2). The precommunicating segment of the left anterior cerebral artery (A1) was hypoplastic with the inner diameter up to 1.18 mm, while the dominant right A1 (up to 5 mm in width) fed the both ACAs (Figs. 1, 2). The distal segments (A2, A3) of the ACA continued forward and upward following the course of the callosal marginal artery and gave off the infraorbital, frontopolar, and internal frontal branches on both sides. The atypical median pericallosal artery bent over the genu of the corpus callosum, following the course of the supracallosal segments of the ACA (A4, A5), and branched out the paracentral and paired parietal branches.
a Cerebral MRA, 3D volume rendering (posterior-superior view) and b schematic diagram demonstrating complex structure of the anterior circle of Willis: the precommunicating segment of the anterior cerebral artery is hypoplastic on the left (single white arrow), the duplicated anterior communicating artery (double white arrow), two typical infracallosal segments of the anterior cerebral artery (gray arrowheads) with atypical median pericallosal artery (white arrowhead) arising from the anterior communicating artery complex. Medial cerebral artery (black arrow), posterior cerebral artery (black arrowhead)
Cerebral MRA, 3D volume rendering (lateral view) showing proximal and distal limbs (white arrows) of the median pericallosal artery (white arrowhead). Precommunicating segment of the anterior cerebral artery (black arrow), infracallosal segment of the anterior cerebral artery (black arrowhead), medial cerebral artery (gray arrow), posterior cerebral artery (gray arrowhead)
Discussion
The medial pericallosal artery is one of the rarest (0.9–13%) and the most controversial anatomical variation of the anterior circulation in human [3, 8, 9]. On the one hand, the atypical vessel is closely related to the frontal and medial cortical zones and is supposed to derive from the developing ACA. On the other, being the most ancient phylogenetic vessel and the direct cranial continuation of the primordial carotid artery in the human embryo, the ACA does not provide any prerequisites for such development.
The term ‘median artery of the corpus callosum’—frequently employed by authors to describe the additional midline vessel, takes its origin in the early works of Padgett (1948). The author described the temporal vessel passing along the midline of the telencephalon in the human embryo during the 9th week of development, which normally underwent progressive regression [10]. Actually, this is the only fact that underlies the theory of the persistence of embryonic vessel, which as it seems from the description above, forms in parallel and independently from the proper ACAs.
However, such a theory cannot explain the distribution of the cortical areas supplied by the triplicated anterior cerebral complex [8]. In such a case as ours, it is obvious that the proper ACA irrigates the ‘callosomarginal’ territory, and the additional vessel supplies the ‘pericallosal’ region. This evidence predisposes the support of the early bifurcation of the dominant ACA into callosomarginal and pericallosal branches rather then the parallel development of the median artery of corpus callosum. The variant of the proximal branching of the unpaired pericallosal artery, which actually is the A4–A5 segment of the ACA, was described by Baptista as the ‘bi-hemispheric ACA’; the artery takes off from the A2 segment of the dominant ACA a few centimeters above the anterior communicating region, while the opposite ACA is hypoplastic and irrigates only orbital and frontal territories [1]. In our case, the unpaired distal A4 segment of the dominant ACA has migrated even more proximally—to the Acom complex, leaving precallosal A3 segments to represent the proper marginal callosal arteries (Fig. 3d).
Schematically represented variability of the pericallosal artery with regard to the level of its branching from the main anterior cerebral artery: a the normal bifurcation of the anterior cerebral artery at the level of A3; b bi-hemispheric anterior cerebral artery with unilateral proximal bifurcation at the level of A2; c highly proximal bifurcation of the anterior cerebral artery (A1–A2) with the origin of the medial pericallosal artery from the Acom; d the medial pericallosal artery with double origin from the dAcom (our case). Explanations are in the text above. A1, A2, A3 and A4 the first, second, third and fourth segments of the anterior cerebral artery, respectively; Acom anterior communicating artery, dAcom duplicated anterior communicative artery
The double origin of the median pericallosal artery associated with duplication of the Acom might have an embryological background. The Acom forms during the 6th–7th week of embryological development via the medial sprouting of the middle olfactory arteries, which are the terminal branches of the primordial carotid arteries and the forerunners of the ACAs [11]. Taking into account the recent findings on the genetic regulation of angiogenesis, the hyper-stimulation or the reduced suppression of the endothelial sprouting, which underlies the process of vascular neo-formation and branching, might lead to the development of a double-channeled Acom [5]. The synchronous duplication of the origin of the median pericallosal artery indicates that the receptors triggering the sprouting of the pericallosal artery were originally located on the basal membrane of the growing Acom. Respectively, the genetically determined proximal migration of the receptors initiating the sprouting of the A4 segment of ACA to the Acom complex is one of the possible mechanisms of such developmental formation.
The described anatomical variability of the anterior part of the circle of Willis requires urgent attention of the related specialists. First of all, the distal part of the median pericallosal artery is a common target of aneurismal formations and, if the aneurism is ruptured, the clipping of the single vessel feeding the pericallosal region could lead to danger of acute postoperative ischemia in the dependent area [7]. The duplication of the Acom complex with the median pericallosal artery attached here creates an additional pre-condition for alteration of hemodynamics in the anterior cerebral circulation. Secondly, the multi-channeled arrangement of the Acom region may lead to misidentification of the appearing vessels in the operative field. Thus, the median pericallosal artery could mimic the opposite ACA, and the structures located deeper may be left without proper attention. Despite the wide invention and implementation of visualization techniques and methods, a detailed knowledge of anatomical variability of the vessels and thorough understanding of their embryological background are the reliable tools of practitioners.
Conclusion
The median pericallosal artery ascending from the Acom artery is a clinically important anatomical variation not only because of the high risk of both distal and proximal aneurysmal formations, but also due to potential complications during the endovascular and surgical inventions on the anterior circle of Willis. The double origin of the median pericallosal artery from the duplicated Acom creates a precondition for alteration of hemodynamics at the anterior cerebral circulation, and should be identified before any manipulations in this region. Thorough preventive radiological analysis and interpretation of images reinforced with detailed knowledge of the underlying embryologic processes are crucial in avoiding possible iatrogenic complications.
References
Baptista AG (1963) Studies on the arteries of the brain. The anterior cerebral artery: some anatomic features and their clinical implications. Neurology 13:825–835
Bradac GB (2011) Anterior cerebral artery. Cerebral angiography. Springer, Berlin, pp 47–56
Gunnal SA, Wabale RN, Farooqui MS (2013) Variation of anterior cerebral artery in human. Neurol Asia 18(3):249–259
Harrigan MR, Deveikis JP (2013) Essential neurovascular anatomy. Handbook of cerebrovascular disease and neurointerventional technique, vol 1. Springer, New York, pp 3–98
Herpers RL (2010) Genetic regulation of vascular development. Royal Netherlands Academy of Arts and Sciences (KNAW), Rotterdam
Kardile P, Ughade J, Pandit S, Ughade M (2013) Anatomical variations of anterior communicating artery. J Clin Diagn Res 7(12):2661–2664
Kutsuna M, Monden S, Watanabe K (2006) Two cases of distal anterior cerebral artery aneurysm associated with accessory anterior cerebral artery. No Shinkei Geka 34(2):193–200
Lasjaunias P, Berenstein A, Brugge K (2001) Surgical neuroangiography: clinical vascular anatomy and variations. Springer, Berlin
Makowicz G, Poniatowska R, Lusawa M (2013) Variants of cerebral arteries—anterior circulation. Pol J Radiol 78(3):42–47
Padget DH (1948) The development of the cranial arteries in the human embryo. Contrib Embryol 32:205–261
Vasović L, Trandafilović M, Vlajković S, Jovanović I, Ugrenović S (2014) Anterior cerebral—anterior communicating complex in the postnatal period: from fenestration to the multiplication of arteries. Med Biol 16(1):1–11
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Kornieieva, M.A., Hadidy, A.M. & Hinno, S.H. Median pericallosal artery with double proximal origin: case report and clinical consideration. Surg Radiol Anat 39, 1169–1173 (2017). https://doi.org/10.1007/s00276-017-1852-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00276-017-1852-9