Abstract
Purpose
To characterize the connective tissue found between the subcutaneous adipose tissue and the underlying muscle tissue in different regions and at different stages of human fetal development. We aim to identify its structural similarities to adult deep fascia, and to establish its role in myofascial development.
Methods
Samples from the arm, forearm, low back and thigh regions (from sites topographically homologous to the adult deep fascia) of five fetus body donors were obtained to perform gross anatomy dissection and histologic sections. Sections were stained with hematoxylin-eosin and Masson trichrome stain to observe their overall structure. Antiserum to protein S100 was used to analyze the presence and distribution of nerve fibers, and immunohistochemistry processing with Tcf4 marker was used to ensure fibroblast activity.
Results
Gross anatomy and histological sections of fetal samples showed the presence of connective tissue topographically and morphologically equivalent to adult deep fasciae. Developing blood vessels and nerves were found evenly distributed within the connective tissue during early development and in the portion adjacent to the muscle at later stages. The presence of Tcf4+ fibroblasts was confirmed in all analyzed mesenchymal connective tissue.
Conclusions
Deep fascia is present from week 21 of human development in the lower back and upper and lower limbs. Blood vessels and nerves develop parallel to it and occasionally cross it from the deep to superficial plane. The presence of Tcf4+ fibroblasts in the deep fascia suggests a crucial role for this structure in muscle morphogenesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The deep fascia has been largely studied in recent years due to its involvement in a range of pathologies including iliotibial tract syndrome, low back pain, myofascial pain, compartment syndromes, fibromyalgia, Dupuytren’s contracture and plantar fasciitis or fasciosis [4]. Also, interest in this anatomic structure is increasing as researchers attempt to understand its reactions to myofascial release techniques [10].
According to the definition proposed by Langevin and Huijing [8], the deep fascia is a continuous sheet of mostly dense, irregularly arranged connective tissue that limits the changes in shape of the underlying tissues. It may be continuous with the epimysium and intermuscular septa and it may also contain layers of areolar connective tissue. Furthermore, this continuous sheet of dense connective tissue may have different constitutive properties. Two main types have been described. The first is formed by a single layer of undulated collagen fibers that are continuous/adherent with intramuscular septa, and intermixed with many elastic fibers (for instance, the pectoral deep fascia [13]). The other has been shown to be formed by two or three layers of densely packed collagen bundles, interspersed with thin layers of areolar connective tissue and able to slide over the underlying muscle, sharing only a few myofascial connections with it (the crural deep fascia, thoracolumbar deep fascia, the deep fascia of the sternocleidomastoid caudal ending, the rectus abdominis deep fascia, the rectus femoris deep fascia, and the arm and forearm deep fasciae [5, 13, 14, 16, 17]). The layers of densely packed connective tissue bundles of multi-layered deep fascia are arranged in the same direction within each layer; however, each layer shows a different direction to its adjacent ones. Moreover, between parallel adjacent layers, a persistent angulation in layer orientation of 78°–90° has been shown in thoracolumbar and crural fasciae [3, 16]). The thickness of the deep fasciae, established by histological studies, ranges from 924 (±220) μm in crural deep fascia [16] to 265 μm at the level of the ankle and dorsum of the foot [2].
A wide range of cell populations and structures have been associated with the deep fascia: fibroblasts, arterioles, capillaries, venules, lymphatics, nerves, mast cells, and myofibroblasts [5]. Fibroblasts are the main cell type; they are responsible for extracellular matrix synthesis and also play a key role, along with satellite cells, in the repair process after muscular injury [11]. When located between the deep fascia and the epimysium they have been reported to be responsible for acid hyaluronic biosynthesis, and a specific name for these types of fibroblast has been proposed: fasciacytes [17].
Although adult human fasciae have been widely described, very few studies of fasciae ontogeny are available. At an embryological level, the deep fascia surrounding the developing muscles has been referred to as mesenchymal undifferentiated connective tissue [1, 12]. Accordingly, Tcf4-positive fibroblasts in mesenchymal connective tissue have been described as a key factor in muscle morphogenesis in developmental mouse and chick models [6, 9].
Our purpose is to study the morphometric parameters and morphological characteristics of the mesenchymal connective tissue found between the adipose and connective subcutaneous tissue (the latter also known in adults as superficial fascia) and underlying muscle tissue during human fetal development. We also hypothesize that this connective tissue plays an important role in muscle morphogenesis, as previously observed in mouse and chick models. The data obtained are further discussed considering previous literature on adult human and animal models.
Materials and methods
Samples from the arm, forearm, low back and thigh regions (from topographically homologous sites to adult deep fascia) were obtained from five fetal cadaveric body donors (21–39 weeks of gestation). Donations were made to the dissection room of the University of Barcelona. Fetuses were cryopreserved, not embalmed. Specimens were processed by dissection on one side, and on the contralateral side histological samples were obtained.
Gross anatomy
To study the disposition of the different anatomic layers, dissections were made using the classical anatomic approach. First a longitudinal incision was made, followed by two transverse incisions at the cranial and distal aspects of the longitudinal incision. Then, the skin was carefully dissected apart from the subcutaneous adipose tissue and associated connective tissue, which was also carefully removed to reveal the deep fascia. Qualitative analysis of fascia continuity was performed.
Histological and immunohistochemical study
Histological samples were obtained by en bloc removal of the region of study with a scalpel, using straight cuts. After removal the samples were mounted on a petri dish with a base of paraffin using needles to avoid deformation artifacts, and fixed in a 4 % formaldehyde solution. A small piece of paper indicating sample orientation was placed on the sample to guide the histological slice. Samples were embedded in paraffin and processed to obtain histological slices, which were further deparaffinized and stained using various methods. Hematoxylin-eosin (HE) and Masson trichrome staining were used for morphological and morphometrical analysis of the samples, including the number of layers and mean thickness (average of five measures along the histological sample) of the connective tissue between the subcutaneous adipose tissue and the underlying muscle tissue, the morphological pattern of collagen fiber organization (areolar/dense, regular/irregular) and to assess the presence of blood vessels.
Immunostaining for S-100 protein and the transcription factor TcF4 (also known as TCF7L2) were performed to verify the presence of neural structures and connective tissue fibroblast, respectively. After deparaffinization, sections were boiled for 20 min in a citric acid/citrate buffer (10 mM) at pH 6. Then, slices were left to cool at room temperature for at least 2 hours before the endogenous peroxidase activity was blocked by immersing the slides in 10 % methanol and 6 % H2O2 for 15 min. After washing with phosphate buffered saline (PBS) the slices were incubated with PBS containing 20 % goat normal serum and 0.05 % gelatin for at least 1 hour at room temperature to avoid nonspecific binding (blocking solution). Slices were incubated overnight at 4 °C with the primary antibodies, anti-S100 rabbit polyclonal antibody (DAKO, 1:500 dilution) and anti-TCF7L2 rabbit monoclonal antibody (Abcam, 1:200 dilution), in PBS containing 1 % goat normal serum and 0.05 % gelatin (alternatively, this step was carried out at room temperature for 1 h with similar results). After washing with PBS, slices were incubated for 30 min at room temperature with EnVision + System-HRP Labelled Polymer anti-Rabbit (DAKO). After washing with PBS, slices were counterstained with hematoxylin, dehydrated and mounted. Negative controls were obtained by performing the protocol as specified but omitting the primary antibody. All preparations were observed with a Leica DMD 108 microscope. HE stained slices were also observed under fluorescence microscopy with a Nikon E800 microscope.
Results
Gross anatomy
Similar findings were obtained during dissection of all the specimens. The skin was very easily dissected from the subcutaneous adipose tissue, which constituted a very thin layer. Adipose tissue was mostly located around nerves and showed a granular pattern. Nerves were easily observed and easy to dissect. A continuous deep fascia-like tissue was found underlying to the subcutaneous adipose tissue; it was continuous in all regions of study, packing all the muscles (Fig. 1).
Anatomic view of the thoracolumbar region at week 39. a Skin and subcutaneous adipose tissue and associated connective tissue have been removed; the integrity of the underlying layer of connective tissue is maintained. Nerves (arrowheads) are observed by transparency, below a thin layer of connective tissue, running parallel to the underlying muscle. b The connective tissue sheet is reflected from the midline and nerves (arrowheads) are clearly observed longitudinally between the connective tissue sheet (reflected) and the underlying muscle. LDM latissimus dorsi muscle, ESM erector spinae muscle, TLF thoracolumbar fasciae
In the low back region we recognized the posterior branches of spinal nerves piercing the deep fascia-like connective tissue and reaching the subcutaneous adipose tissue. The deep fascia-like tissue was very easily separated from the underlying muscles in all regions of study, especially between the thoracolumbar fascia and the erector spinae muscles, where few myofascial expansions were seen (Fig. 1).
Histological study
A continuous sheet of connective tissue was observed between the subcutaneous adipose tissue and the underlying muscle tissue across all samples and in all the regions of study, albeit with slight differences.
-
Weeks 22–23: in all samples a single layer of dense irregular connective tissue was observed between the subcutaneous adipose tissue and muscle tissue (Fig. 2) under both optical and fluorescent microscopy. This layer of connective tissue extended superficially to organize the adipose tissue, as in adult superficial fascia, and deeply to embed muscle tissue. HE and Masson trichrome staining together with immunostaining demonstrated the presence of a high number of mesenchymal cells, isolated groups of adipocytes, blood vessels and neural structures within this layer.
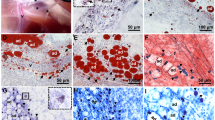
Fig. 2 Deep fascia development. The top row displays specimens at week 22 of development. A layer of dense irregular connective tissue between the subcutaneous adipose tissue and muscle tissue is observed. The middle row displays specimens at week 31 of development. Forearm and low back regions show dense irregular connective tissue and a thin layer of less compact dense irregular connective tissue adjacent to muscle tissue (epimysium). In the thigh a multi-layered structure is observed. Below this multi-layered structure is another layer of less compact connective tissue, with expansions into the muscle tissue (epimysium). The lower row displays specimens at week 39 of development. All samples show a well-differentiated, deep fascia-like structure with underlying dense irregular connective tissue (epimysium). Scale bars 100 μm. Arrow points to deep fascia-like connective tissue, mu skeletal muscle tissue, ad adipose tissue, ad* location of adipose tissue, not visible due to sample processing
-
Week 31: arm, forearm and low back samples showed dense irregular connective tissue. No sublayers were distinguished and in some cases small aggregates of adipose tissue were found within this structure. Also, a thin layer of less compact dense irregular connective tissue adjacent to the muscle tissue (epimysium) was observed (Fig. 2). In the thigh sample a structure organized in three different layers with different directions could be distinguished. The middle layer was the most prominent and was made of several compact bundles of collagen fibers while the outermost layers were woven-like and very thin (Fig. 3). Below the described structure a thin layer of less compact dense irregular tissue was present in contact with the muscle tissue (epimysium) (Fig. 2). Other histological features were found as described in samples from previous weeks (Fig. 3a–c).
Fig. 3 a, b Display thigh samples from the same specimen after 31 weeks of development, stained with HE and Masson trichrome, respectively. This comparison enables to clearly differentiate specific regions where the muscle tissue is developing from the connective tissue, independently of its compact or lax constitution. b Connective tissue is interspersed between all the other more specialized tissues (adipose; ad and muscle; mu), compartmentalizing and compacting them. c The same sample as in (a) and (b) observed under fluorescent microscopy. A prominent middle layer formed by compact bundles of dense connective tissue is observed, with two adjacent thin layers of woven-like connective tissue (asterisk) in different directions. d S-100 immunostaining of the thigh at week 31 of development. A small myelinated neural structure; arrow, is observed below the compact dense connective tissue bundles. e S-100 staining of the arm at week 21 of development. Transverse section of a nerve (arrow) found within the dense irregular connective tissue, which is organized as a single layered sheet. f. Tcf4 immunostaining of the arm at week 21 of development. All connective tissue is Tcf4+ (arrows). Neither muscle nor adipose tissues are stained with Tcf4. Scale bars: a, e, f 200 μm; b 100 μm; c, d 50 μm
-
Week 39: all samples showed a well-differentiated deep fascia-like structure of 2–3 layers of dense regular connective tissue with areolar connective tissue in between. Between all deep fasciae and muscle tissue, an epimysium was present (Fig. 2). The connective tissue of the thigh region showed the most organized pattern: its middle layer comprised several non-aligned dense connective tissue bundles, all in the same direction (Figs. 2, 3). Few cells remained in the areolar connective tissue found between deep fascia bundles and in the epimysium. Blood vessels and nerves were observed below the deep fascia or within the subcutaneous adipose tissue. No adipocytes were observed within the deep fascia at this point.
Morphometric thickness parameters of the connective tissue structure found between the subcutaneous adipose tissue and the underlying muscle are shown for all samples in Table 1. The connective tissue under study in the low back region showed the least growth in thickness throughout development with the lowest standard deviation, followed by the upper limb and the thigh region; the thigh region had the most growth in thickness throughout the study (Table 1).
Immunohistochemical analysis
Evidence of neural structures was observed related to deep fascia-like connective tissue in all specimens and regions studied, from 22 to 39 weeks of gestation (Fig. 3d, e). Major differences in distribution were observed between samples with less dense and irregular connective tissue and those with denser and compact connective tissue. In the former, the nerves were found randomly distributed longitudinally within the whole layer of the connective tissue structure studied (Fig. 3d), while in the latter, nerves were also found longitudinally, but underlying the more compact bundles of dense connective tissue (Fig. 3e). In the samples analyzed, no nerves were observed crossing from the deep plane to the subcutaneous plane.
Tcf4+ fibroblasts/cells were found in all samples, not only in the connective tissue directly adjacent to muscle, but also in the dermis and the connective tissue trabeculae that compartmentalize the subcutaneous adipose tissue (Fig. 3f). In fact, in all specimens observed, the dermis was the region with the most Tcf4+ fibroblasts. As mentioned, Tcf4+ fibroblasts were also found in the connective tissue packing the subcutaneous adipose tissue, and also in the connective tissue surrounding and compartmentalizing the muscle tissue, but not in the adipose or muscle tissue itself. In addition to connective tissue structures, the walls of the developing blood vessels were also stained, mainly in the cutaneous and subcutaneous layers (not shown). However, besides the dermis, the aforementioned tissues and structures were not stained in all regions or specimens. No specific pattern was noted for this differential distribution, and further studies are required to elucidate this question.
Discussion
During fetal development (weeks 22–39), a deep fascia-like structure appears as a connective tissue sheet which develops simultaneously beneath the subcutaneous adipose tissue and around muscles.
Morphologic characteristics of the deep fascia-like tissue in the different regions studied show similar features. Major differences are observable at a microscopic rather than a macroscopic level. In weeks 21–23, a rather lax but dense irregular connective tissue (although they are not compact, a predominance of collagen fibers is observed) runs parallel to skin below the subcutaneous adipose tissue showing projections that extend superficially to organize adipose tissue and deeply to embed muscle tissue. At this point, the distribution pattern of the connective tissue studied is already concordant with the general definition of fasciae proposed in the literature [4, 7, 8, 15]. By week 39 of development, it condenses to form different layers (2–3 layers) of compact dense regular connective tissue with areolar connective tissue remaining between layers, as described in deep musculoskeletal fasciae in adult humans [5, 13, 14, 16, 17]. Also at this point a notable decrease in cell density suggests a more mature form of connective tissue.
The nerves between the subcutaneous adipose tissue and the underlying muscle were always found running within the less dense irregular connective tissue and therefore beneath the more compact dense regular connective tissue in the later stages of development. Although we did not find any nerves crossing the deep fascia or running towards the subcutaneous adipose tissue in the histological slices, they were observed during dissection. This confirms that subcutaneous nerves cross the deep fascia only at specific locations, a feature widely accepted in gross anatomy.
When comparing adult to fetal deep fascia thickness, histological analysis (Table 2) indicates a proportional increase of fascia thickness (about 500 %) across regions.
In view of the morphometric parameters and the morphologic characteristics obtained, we propose that deep fasciae should be anatomically and histologically considered as such already in embryonic stages of the myofascial system development.
Tcf4+ fibroblasts have been shown to play an active role in chick and mouse limb muscle morphogenesis by establishing a prepattern structure of muscles, to which muscle precursor cells migrate and differentiate [6]. During myogenesis, high levels of tcf4+ expressing fibroblasts are found within the connective tissue of the myofascial unit but tcf4+ is not expressed by muscle differentiating cells in mice [9]. Tcf4+ expressing fibroblasts have also been found to play a key role in adult mice myofascial regeneration along with satellite cells, by promoting fibrosis of the tissue discontinuity and guiding distribution and growth of satellite cells into the scar tissue [11]. In the present study Tcf4+ fibroblasts were found within the connective tissue that compartmentalizes muscle tissue, specifically in the epimysium but also in the perimysium. We also found Tcf4+ fibroblasts in immature deep fasciae, but none were found when the fasciae matured into compact collagen bundles. Therefore, the present evidence suggests that muscle-related deep fascia is an important component in myogenesis and muscle morphogenesis in the human fetus as well. The presence of Tcf4+ expressing fibroblasts in the connective tissue organizing subcutaneous adipose tissue suggests a more extensive role of this transcription factor in tissue morphogenesis and development than previously thought.
To conclude, deep fascia appears to be present at least from week 21 of human development in the low back, upper and lower limbs. It matures along with underlying muscle tissue, as demonstrated by an increase in thickness, increase in packed connective tissue bundles organized in 2–3 layers, and a decrease in cell number. Blood vessels and nerves also develop beneath and cross the deep fascia to reach the subcutaneous adipose tissue and the connective tissue organizing it. In addition, Tcf4+ fibroblasts are present in the deep fascia throughout development, strongly suggesting that it will play a crucial role in muscle morphogenesis. The present report is one of the few studies of comparative embryonic human fascia, and as far as we know, the first to consider the deep fascia and related structures. Additional data in this field would help to establish a stronger correlation between fetal and adult deep fascia and would broaden our understanding of the myofascial system.
References
Abe S, Suzuki M, Cho KH, Murakami G, Cho BH, Ide Y (2011) CD34-positive developing vessels and other structures in human fetuses: an immunohistochemical study. Surg Radiol Anat 33(10):919–927. doi:10.1007/s00276-011-0854-2
Abu-Hijleh MF, Harris PF (2007) Deep fascia on the dorsum of the ankle and foot: extensor retinacula revisited. Clin Anat 20(2):186–195. doi:10.1002/ca.20298
Benetazzo L, Bizzego A, de Caro R, Frigo G, Guidolin D, Stecco C (2011) 3D reconstruction of the crural and thoracolumbar fasciae. Surg Radiol Anat 33(10):855–862. doi:10.1007/s00276-010-0757-7
Benjamin M (2009) The fascia of the limbs and back—a review. J Anat 214(1):1–18. doi:10.1111/j.1469-7580.2008.01011.x
Bhattacharya V, Barooah PS, Nag TC, Chaudhuri GR, Bhattacharya S (2010) Detail microscopic analysis of deep fascia of lower limb and its surgical implication. Indian J Plast Surg 43(2):135–140. doi:10.4103/0970-0358.73424
Kardon G, Harfe BD, Tabin CJ (2003) A Tcf4-positive mesodermal population provides a prepattern for vertebrate limb muscle patterning. Dev Cell 5(6):937–944 pii S1534580703003605
Kumka M, Bonar J (2012) Fascia: a morphological description and classification system based on a literature review. J Can Chiropr Assoc 56(3):179–191
Langevin HM, Huijing PA (2009) Communicating about fascia: history, pitfalls, and recommendations. Int J Ther Massage Bodyw 2(4):3–8
Mathew SJ, Hansen JM, Merrell AJ, Murphy MM, Lawson JA, Hutcheson DA, Hansen MS, Angus-Hill M, Kardon G (2011) Connective tissue fibroblasts and Tcf4 regulate myogenesis. Development 138(2):371–384. doi:10.1242/dev.057463
Mckenney K, Elder AS, Elder C, Hutchins A (2013) Myofascial release as a treatment for orthopaedic conditions: a systematic review. J Athl Train 48(4):522–527. doi:10.4085/1062-6050-48.3.17
Murphy MM, Lawson JA, Mathew SJ, Hutcheson DA, Kardon G (2011) Satellite cells, connective tissue fibroblasts and their interactions are crucial for muscle regeneration. Development 138(17):3625–3637. doi:10.1242/dev.064162
Sato T, Koizumi M, Kim JH, Wang BJ, Murakami G, Cho BH (2011) Fetal development of deep back muscles in the human thoracic region with a focus on transversospinalis muscles and the medial branch of the spinal nerve posterior ramus. J Anat 219(6):756–765. doi:10.1111/j.1469-7580.2011.01430.x
Stecco A, Macchi V, Masiero S, Porzionato A, Tiengo C, Stecco C, Delmas V, de Caro R (2009) Pectoral and femoral fasciae: common aspects and regional specializations. Surg Radiol Anat 31(1):35–42. doi:10.1007/s00276-008-0395-5
Stecco C, Gagey O, Belloni A, Pozzuoli A, Porzionato A, Macchi V, Aldegheri R, de Caro R, Delmas V (2007) Anatomy of the deep fascia of the upper limb. Second part: study of innervation. Morphologie 91(292):38–43. doi:10.1016/j.morpho.2007.05.002
Stecco C, Macchi V, Porzionato A, Duparc F, de Caro R (2011) The fascia: the forgotten structure. Ital J Anat Embryol 116(3):127–138
Stecco C, Pavan PG, Porzionato A, Macchi V, Lancerotto L, Carniel EL, Natali AN, de Caro R (2009) Mechanics of crural fascia: from anatomy to constitutive modelling. Surg Radiol Anat 31(7):523–529. doi:10.1007/s00276-009-0474-2
Stecco C, Stern R, Porzionato A, Macchi V, Masiero S, Stecco A, de Caro R (2011) Hyaluronan within fascia in the etiology of myofascial pain. Surg Radiol Anat 33(10):891–896. doi:10.1007/s00276-011-0876-9
Acknowledgments
The authors are grateful to the body donors and their families. We also thank Nieves Cayuela and Eva Sanchez for their assistance in the laboratory. Supported by Grants ACESB09/10 (Faculty of Medicine, C. Bellvitge, University of Barcelona) and 2009SGR152 (AGAUR, Generalitat de Catalunya, Spain).
Conflict of interest
The authors have no conflicts of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Blasi, M., Blasi, J., Domingo, T. et al. Anatomical and histological study of human deep fasciae development. Surg Radiol Anat 37, 571–578 (2015). https://doi.org/10.1007/s00276-014-1396-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00276-014-1396-1