Abstract
The aim of this study was to analyse the organization of the deep fascia of the pectoral region and of the thigh. Six unembalmed cadavers (four men, two women, age range 48–93 years old) were studied by dissection and by histological (HE, van Gieson and azan-Mallory) and immunohistochemical (anti S-100) stains; morphometric studies were also performed in order to evaluate the thickness of the deep fascia in the different regions. The pectoral fascia is a thin lamina (mean thickness ± SD: 297 ± 37 μm), adherent to the pectoralis major muscle via numerous intramuscular fibrous septa that detach from its inner surface. Many muscular fibres are inserted into both sides of the septa and into the fascia. The histological study demonstrates that the pectoral fascia is formed by a single layer of undulated collagen fibres, intermixed with many elastic fibres. In the thigh, the deep fascia (fascia lata) is independent from the underlying muscle, separated by the epimysium and a layer of loose connective tissue. The fascia lata presents a mean thickness of 944 μm (±102 μm) and it is formed by bundles of collagen fibres, arranged in two to three layers. In each layer, the fibres are parallel to each other, whereas the orientation of the fibres varies from one layer to the adjacent one. The van Gieson elastic fibres stain highlights the presence of elastic fibres only in the more external layer of the fascia lata. In the thigh the epimysium is easily recognizable under the deep fascia and presents a mean thickness of 48 μm. Both the fascia lata and pectoral fascia result innerved, no specific differences in density or type of innervations is highlighted. The deep fascia of the pectoral region is morphologically and functionally different from that of the thigh: the fascia lata is a relatively autonomous structure with respect to the underlying muscular plane, while the pectoralis fascia acts as an additional insertion for the pectoralis major muscle. Different portions of the pectoralis major muscle are activated according to the glenohumeral joint movements and, consequently, selective portions of the pectoral fascia are stretched, activating specific patterns of proprioceptors. So, the pectoralis muscle has to be considered together with its fascia, and so as a myofascial unit, acting as an integrated control motor system.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, the deep fascia has been object of studies concerning its possible role in motor coordination and in the etiopathogenesis of numerous extra-articular pain syndromes [13, 14, 22–24]. Particularly, the fasciae are considered important for the connections among different muscular groups, explaining the irradiation of pain along specific directions. Langevin et al. [9, 10] affirm that connective tissue functions as a wide range mechanosensitive signaling system and these signals could be altered in some pathological conditions. Rijkelijkhuizen et al. [15] report that the crural fascia has an important role in the transmission of muscle force. Maas et al. [11], Meijer et al. [12] and Yucesoy et al. [31] also demonstrate that myofascial force transmission acts both between adjacent synergic muscles as well as between the more distant agonistic muscles within a body segment thanks to fascial connections. Gerlach and Lierse [4] affirm that connective tissue has a fundamental role in the biomechanics of the lower limbs, proposing to consider a new functional system comprised of bones, muscles, tendons, and fasciae and Fairclough et al. [3] focus on the role of the iliotibial tract in the mechanics of the knee. Vleeming et al. [29] sustain that the thoracolumbar fascia allows the connections between the trunk and the inferior limbs. The results from these studies have often been generalized, attributing the biomechanical characteristics demonstrated for these fasciae to all the deep fasciae. In particular, some Authors [13, 14, 22] affirm that, analogously to the thoracolumbar fascia, also the fasciae of the anterior region of the trunk are implicated in the transmission of traction between the inferior and superior limbs, and between the two upper limbs. Really, in anatomical textbooks [2, 17, 19, 28] the pectoral fascia is described like a thin layer of loose connective tissue and according to Basmajian [1], in the thorax and the abdomen only a thin areolar layer exists, rather than a true deep fascia. Hence, the aim of this study was to contribute to clarify the macroscopic and microscopic differences between the pectoral fascia and the fasciae of the limbs, with particular reference to their relations with the underlying muscles, the amount of collagen and elastic fibres and the type of innervations. Particularly we’ll take as reference the fascia lata, that is one of the most known fascia of the limbs. So, if also our study will demonstrate that the pectoral fascia is really so thin, the biomechanical results obtained for the deep fasciae of the limbs cannot be applicable, but a different concept of the fascial transmissions of the muscular tractions and of the connections among different muscular groups will be think for the pectoral fascia.
Materials and methods
Collaboration between the Anatomy Departments of the Padua University and of the Paris Descartes University, allowed to perform macroscopic and microscopic analysis of fasciae of the thorax and the thigh in six cadavers (four men, two women, mean age 69 years, age range 48–93 years). These cadavers were neither embalmed nor frozen prior to examination. Each dissection was performed according to the following protocol. A superficial, longitudinal incision was done along the midline of the body from the neck to the pubis, and then a transversal incision along the two clavicles. In the first phase, only the skin in the left side of the trunk was removed in order to display the subcutaneous tissue and the superficial fascia. In a second phase, the superficial fascia was removed and the deep fascia was highlighted, isolated and photographed (Canon EOS 350 digital camera). Its relations with the underlying muscles and with the deep fasciae of the neck, of the serratus anterior, latissimus dorsi, trapezium and deltoid muscles were evaluated. Also the possible continuity with the rectus abdominis sheaths was investigated. Full thickness specimens, from the skin to the muscle planes, and samples of only the deep fascia were removed from each subject for histological analysis. Specimens were taken from the upper third, middle third, and inferior third of the pectoral region of the right side of the trunk, along the midclavicular line. In the thigh, the incision was done along the anterior region of the thigh, from the anterior superior iliac spine to the inferior border of the patella, and then the subcutaneous planes were highlighted in a similarly to the trunk. Specimens were taken from the inguinal region, middle third and distal third of the controlateral thigh, along the line of the rectus femoris muscle (Fig. 1). The specimens were accurately oriented, mounted on a cardboard to avoid deformation artifacts, fixed in 10% formalin solution and then embedded in paraffin. During paraffin embedding, the specimens were oriented paying attention to obtain full thickness section from skin to the deep layer. From the paraffin embedded samples, 10 μm thick sections were obtained and then were stained with hematoxylin and eosin, van Gieson for the elastic fibres and azan-Mallory for the collagen fibres. An immunohistochemical stain (anti-S100 antibody) for nerve structures was also applied, according to a previously described method [20]. All preparations were observed with a Leica DMR microscope. To quantify the elastic component in the fasciae, a morphometric analysis was performed by ImageJ software. Briefly, a picture of the fascia, stained with van Gieson, was acquired. The colors of the picture were analysed, displaying histograms of the distribution of hue, saturation and brightness. The interval of the violet colors, corresponding to the elastic component, was manually selected. The selected interval of colors was converted into white and all the other colors into black. On the processed images, the area corresponding to the deep fascia was selected, and the percentage of the elastic component was automatically measured.
Full thickness specimens were removed from each subject, together with specific deep fascia specimens for histological analysis. In the thigh (a), specimens were taken from the inguinal region, middle third and distal third of the thigh, along the line of the rectus femoris muscle. Specimens were taken from the upper third, middle third, and inferior third of the pectoral region, along the midclavicular line (b)
Morphometric measurements of the deep fascia and, where present, of the epimysium were made in the full thickness specimens. The mean values and standard deviations of these measurements were calculated. The differences in thickness between the regions were analysed by appropriate statistical tests (Kurskal–Wallis test and Dunn’s Multiple Comparison Test).
Further anatomical evaluations were performed on specimens from the anatomical collection of the Anatomy Institute of the University of Padova, consisting of transverse, 5–10 cm-thick, slices of three whole human bodies (two males, one female; ages: 3, 56, 62 years), preserved in Kaiserling’s solution. In these specimens, types of connection between each fascia and underlying muscle were analysed.
Results
Macroscopic examination
In all of the examined cases, the fascia of the pectoralis major muscle appears as a thin lamina, adherent to the muscle. The pectoral fascia envelops the pectoralis major muscle, and so superficial and deep layers of this fascia have been recognized. Medially, the pectoral fascia passes over the sternum and continues with the controlateral pectoral fascia. Proximally, the pectoral fascia is continuous with the superficial lamina of the deep fascia of the neck,Footnote 1 laterally with the deltoid fascia and more caudally passes over the serratus anterior muscle to continue with the fascia of the trapezium and latissimus dorsi muscles. The fasciae of the latissimus dorsi, trapezium and deltoid muscles are not separable from the corresponding muscles and many intramuscular septa are recognizable. Distally, the pectoral fascia presents some fibrous expansions that extend to the ipsilateral and controlateral rectus abdominis sheaths. In this way, a clearly visible, criss–cross interweaving of fibres coming from the two sides of the pectoralis major muscle forms over the xyphoid process (Fig. 2a). The cadaver transverse slices confirm the relationship of the pectoral fascia and of underlying muscle (Fig. 3a): the two layers of the pectoral fascia envelope the pectoralis major muscle, then fuse on the lateral margin of the pectoralis major muscle to cover the serratus anterior muscle. Hence the deep fascia appears to be thicker. The same fascia divides itself again in the dorsal region to envelope the latissimus dorsi muscle. Inside the pectoralis major muscle, many intramuscular septa can be recognized. They present a radiate disposition, converging toward the humeral insertion of the pectoralis major muscle. Particularly, we can distinguish some main septa, that allow the delimitation between the pectoralis major and the deltoid muscles, and between the costal and clavicular parts of the same muscle (marginal septa), and many secondary septa, that subdivide the pectoralis major muscle in numerous portions (intramuscular septa). Many muscular fibres are inserted into both sides of the intramuscular septa and into the inner surface of the fascia, resulting the relationships between the different muscular bundles.
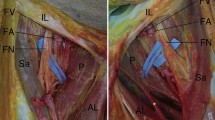
a The crossing of fibres in front of xyphoid process. The different muscles expansions are evident, in particular we can recognize the pectoral major muscle on the right and its fibrous expansions extended into the ipsilateral and controlateral rectus abdominis sheaths. b Fascia lata and iliotibial tract. During dissection, it was very difficult to separate the iliotibial tract from the deep fascia, while the fascia lata is easily separable from all the muscles of the thigh
a Photograph of a cadaveric axial section of the thigh showing the fascia lata (FL), the iliotibial tract (ITT) and the lateral intermuscular septum (LIS), and their relationship with the muscles of the thigh. BM biceps femori muscle, STM semitendinosus muscle, SMM semimembranosus muscle, GM gracilis muscle, SM sartorius muscle, VMM vastus medialis muscle, VLM vastus lateralis muscle. b Photograph of a cadaveric axial section of the trunk showing the superficial layer of the deep fascia of the trunk (PF) enveloping the pectoralis major muscle (PMa) and the latissimus dorsi muscle (LD) and covering the serratus anterior muscle (SA); PMi pectoralis minor muscle, TM teres major muscle, SM subscapularis muscle. c Schematization of the relations of the fascia lata and the muscles of the thigh. The fascia lata splits into two layers to enclose the tensor fascia lata muscle, the iliotibial tract and the sartorius muscle, while all the other muscles of the thigh are under the fascia lata and separated from it by loose connective tissue. d Schematization of the relation of the superficial layer of the deep fascia of the trunk and the underlying muscles. In the trunk, the pectoralis major, latissimus dorsi and trapezius muscles are eveloped by the same fascia and form an additional myofascial layer with respect to the other muscular planes of the trunk
In the lower limb, the deep fascia of the thigh (also known as fascia lata) covers all muscles and appears as a thick, whitish layer of connective tissue, similar to an aponeurosis. It progressively increases in thickness in a proximal to distal direction. It continues distally with the crural fascia. Laterally, it splits into two layers to enclose the tensor fascia lata muscle and the iliotibial tract. To separate the iliotibial tract from the deep fascia is very difficult by dissection (Fig. 2b), while the fascia lata is easily separable from all the muscles of the thigh. Indeed there is a virtually uninterrupted plane of sliding between the fascia lata and the muscle bellies, surrounded by their epimysium, and a little layer of loose connective tissue is found between fascia and epimysium. Only few strong intermuscular septa origin from the inner surface of the fascia lata and extend between the muscle bellies dividing the thigh into different compartments. Also the analysis of the cadaver transverse slices (Fig. 3b) confirmed the relations between the fascia lata, the iliotibial tract and the underlying muscles.
Microscopic examination
Morphometrical analysis of the histological specimens (Table 1) confirms that the deep fascia of the pectoral region is a thin connective lamina (mean thickness and SD: 297 ± 37 μm) closely connected with the pectoralis major muscle. In particular, the thickness of the pectoral fascia in the inferior thorax region (579 ± 42 μm) is significantly higher (P < 0.001) than in the subclavicular region (131 ± 19 μm) and mammary region (182 ± 87 μm). An epimysial fascia, or epimysium, is not distinguished between this fascia and the underlying muscle, but many muscular fibres of the pectoralis major muscle are in continuity with the pectoral fascia itself. Undulated collagen fibres, set at right angles to underlying muscular fibres, form the pectoral fascia. At van Gieson stain (Fig. 4a) the elastic fibres are 14 ± 1.2% and the collagen fibres are 86 ± 1.2%.
a: Deep fascia of pectoral region (Van Gieson stain, magnification ×200). It is formed by a single layer of connective tissue with many elastic fibres, forming an irregular mesh. It is evident the close relation and connection with the pectoralis major muscle (PM). b Fascia lata (Hematoxylin-eosin stain, no magnification); it appears as a lamina of connective tissue similar to an aponeurosis formed by thick fibrous bundles. A thin lamina of loose connective tissue separates the fascia lata (FL) from the underlying muscles (QM quadriceps femoris muscle). c Fascia lata (azan-Mallory stain, ×25), well-defined bundles of muscular fibres were evident (black arrows) between the connective laminae of the fascia lata. d Innervation of the pectoral fascia (immunohistochemical stain anti-S100, ×25). The black arrows evidence two small nerves
The deep fascia of the femoral region is a lamina of connective tissue similar to an aponeurosis and presenting a mean thickness of 944 ± 102 μm (Fig. 4b). At the morphometric measurements, its thickness is 541 ± 23 μm in the inguinocrural region, 874 ± 62 μm in the middle third of the thigh and 1419 ± 105 μm in the distal third of the thigh. The different thicknesses in the three regions were statistically significant (P < 0.05). The collagen fibres form thick fibrous bundles arranged in two or three layers. In each layer, the fibres are parallel to each other, whereas, from one layer to the adjacent one, the orientation of the fibres varies. A thin lamina of loose connective tissue separates each layer. In one anatomical specimen, well-defined bundles of muscular fibres were found between these connective laminae (Fig. 4c). At the internal and external surfaces of the fascia lata two thin laminae (mean thickness: 23 ± 4 μm) of undulated collagen fibres, not grouped into bundles, are present. At the van Gieson stain, elastic fibres are present only in these two thin external layers, whereas lacking between the aponeurotic layers of the fascia lata. The histological stains demonstrate that the epimysial fascia (or epimysium) is a mono-layered structure formed by connective tissue and elastic fibres. At the morphometric measurements, it presents a mean thickness of 48 μm. Some differences are evident in the different regions of the thigh: in the inguinocrural region it presents a mean thickness of 43 ± 9.5 μm, in the anterior thigh region of 19 ± 2.6 μm and the distal region of the thigh of 82 ± 7.7 μm.
At the anti S-100 immunohistochemical stain some small nerve fibres are evidenced both in the fascia lata and in the pectoral fascia, no specific differences among the density or in the type of innervations have been highlighted (Fig. 4d).
Discussion
The review of the Literature showed that the term “fascia” is used to describe various anatomical structures with different macro and microscopical characteristics. From our study, it is evident that the pectoralis fascia is thinner than the femoral fascia and presents macroscopic and microscopic specificities. The pectoral fascia shows a mean thickness of 297 μm, value comparable to that reported by Jinde [7] (0.2–1.14 mm). At the contrary, our results differ from the description of the pectoral fascia by Graf [5], Tebbetts [27] and Hwang [6], that describe the pectoral fascia as a thick connective layer that becomes thin and feeble at the inferior border of the PM (sixth intercostal space). These differences are probably deriving from a different use of the term “pectoral fascia”, indeed for the plastic surgeons it is usually referred to the superficial fascia, while for the anatomists is referred to the fascia enveloping the pectoralis major muscle [17]. Our dissections also demonstrate that the pectoral fascia does not end at the level of the clavicle and the sternum but continues with the deep cervical fascia, the controlateral pectoral fascia and laterally with the fasciae of the latissimus dorsi and deltoid muscles. Therefore, all these muscles are comprised within two layers of the superficial lamina of the deep fascia, and are not separable from the same (Fig. 3d). This myofascial relation better explains the description of the fasciae of the trunk by Sato [16], which reports that the pectoralis major, latissimus dorsi and trapezius muscles form an additional myofascial layer with respect to the underlying muscular planes. From the developmental point of view, these muscles originate as members of the limbs muscles, but then they develope in the trunk just until the connection with the midline (with the interspinous processes of the spine in the back, with the sternum in the thorax) [8, 25]. In this way an additional muscular plan in the trunk is formed. The development of these muscles in the trunk is probably determined by the necessity to connect firmly the limbs to the trunk, but also the superior and inferior limbs and the superior limbs of the opposite sides. The superficial layer of the deep fascia, enveloping all these muscles, probably reinforces these connections. Indeed in our study numerous expansions of the muscles into the fascia have been found. Particularly, the expansions of the pectoralis major muscles into the rectus abdominis sheaths connect the muscles of the thorax and abdomen and the two emisomes, whereas the superficial layer of the pectoral fascia passes over the sternum permits the connections between the two pectoralis major muscles.
Our study shows the presence of many intramuscular septa originating from the pectoral fascia. Numerous muscular fiber bundles insert on these septa and on the internal surface of the fascia itself. It could be hypothesize that this close relationship between fascia and muscle allows selective spatial stretching of the pectoral fascia during the contraction of the pectoralis major muscle, permitting that the zonal activation of the muscle corresponds to zonal stretching of the fascia. Besides, the particular relationship between the muscular fibres and the intramuscular septa permits the fine orientation of the vectorial forces created by the activation of the muscle: different portions of the pectoralis major muscle are activated according to the degree of movement at the glenohumeral joint and, consequently, different portions of the pectoral fascia are stretched (Fig. 5a).
a The contraction of the pectoralis major muscle stretches the pectoral fascia directly, thanks to the muscular fibres inserted onto the fascia or its septa. So, different portions of the pectoralis major muscle are stretched according to the glenohumeral joint movements and, consequently different patterns of receptors are activated. b The fascia lata is a relatively autonomous structure respect to the underlying muscles. So, it feels the contractions of the muscles only thanks to some muscular expansions. In this figure are represented the expansions of the gluteus maximus (E1), the gluteus medium (E2) and the tensor of the fascia lata (E3) muscles into the fascia lata
The fascia lata shows a completely different structure. It is very consistent and thick, like an aponeurotic-type tissue [17], easily separable from the underlying muscles due to the presence of the epimysium, which permits to the muscles to slide independently from the fascia lata. Between the epimysium and the fascia lata, a thin layer of loose connective tissue, relatively rich in adipocytes, further facilitates the sliding between the fascia and the muscular planes (Fig. 3c). Thus, the femoral fascia, as compared to the pectoral fascia, is a relatively autonomous structure with respect to the underlying muscular plane. It perceives the state of contraction of the thigh muscles only due to some aponeurotic expansions that the muscles extend to the fascia (Fig. 5b). Moreover it is probable that the aponeurotic expansions of the gluteus maximus, tensor fascia lata, gluteus medius can explain the different orientation of the collagen fiber bundles within the fascia lata [17, 23].
The different relationships between deep fasciae and muscles in the trunk and limbs suggest that the pectoral fascia is stretched in a more precise manner than the fascia lata, with different activation of the proprioceptors. This strong relationship between the deep muscular fascia and muscles could give evidence of the anatomical base of the concept of myofascial units (MFU), term that indicates the muscles and fascia of a specific region [22–24], subtending not only a morphological characterization, but also a precise functional organization. Indeed the MFU could be at the bases of peripheral motor coordination [13, 14, 21–23, 29] and dynamic proprioception [18, 20, 23, 26, 30]. Further studies will be necessary to better clarify the possible alterations of the fasciae in pathological conditions.
Notes
The Authors assumed the Anglo–Saxon classification of the fasciae of the neck. Indeed, the term “superficial fascia”, used in the French and Italian classifications, referred to the fascia covering the sternocleidomastoid and the trapezius muscles, could misunderstand with the “true” superficial fascia, that is the fibroadipose lamina enveloping the platysma muscle, and that shows completely different macro and microscopic characteristics.
References
Basmajian JW (1989) Grant’s method of anatomy, 11th edn. Williams & Wilkins, Baltimore, pp 359–371
Chiarugi G (1975) Istituzioni di Anatomia dell’Uomo, vol 1. Società editrice libraria, Milano, p 146
Fairclough J, Hayashi K, Toumi H, Lyons K, Bydder G, Phillips N, Best TM, Benjamin M (2006) The functional anatomy of the iliotibial band during flexion and extension of the knee: implications for understanding iliotibial band syndrome. J Anat 208:309–316
Gerlach UJ, Lierse W (1990) Functional construction of the superficial and deep fascia system of the lower limb in man. Acta Anat 139:11–25
Graf RM, Bernardes A, Auersvald A, Damasio RC (2000) Subfascial endoscopica transaxillary augmentation mammaplasty. Aesthetic Plast Surg 24:216–220
Hwang K, Kim DJ (2005) Anatomy of pectoral fascia in relation to subfascial mammary augmentation. Ann Plast Surg 55:576–579
Jinde L, Jianlianq S, Xiaopinq C, Xiaoyan T, Jiaqinq L, Qun M, Bo L (2006) Anatomy and clinical significance of pectoral fascia. Plast Reconstr Surg 118:1557–1560
Kent GC (1978) Comparative anatomy of the vertebrates. Mosby Co., Saint Louis
Langevin HM (2006) Connective tissue: a body-wide signalling network? Med Hypotheses 66:1074–1077
Langevin HM, Sherman KJ (2007) Pathophysiological model for chronic low back pain integrating connective tissue and nervous system mechanisms. Med Hypotheses 68:74–80
Maas H, Meijer JM, Huijing PA (2005) Intermuscular interactions between synergists in rat originates from both intermuscular and extramuscular myofascial force transmission. Cells Tissues Organs 181:38–50
Meijer HJ, Baan GC, Huijing PA (2006) Myofascial force transmission is increasingly important at lower forces: firing frequency related length-force characteristics of rat extensor digitorum longus. Acta Physiol 186:185–195
Myers TW (2001) Anatomy trains. Churchill Livingstone, Oxford, pp 171–194
Paoletti S (2002) Les Fascias. Rôle des tissus dans la mécanique humaine. Sully, Vannes, pp 193–199
Rijkelijkhuizen JM, Meijer HJM, Baan GC, Huijing PA (2007) Myofascial force transmission also occurs beween antagonistic muscles located within opposite compartments of the rat lower hind limb. J Electromyogr Kines 17:690–697
Sato T, Hashimoto M (1984) Morphological analysis of the fascial lamination of the trunk. Bull Tokyo Med Dent Univ 31:21–32
Standring S, Ellis H, Healy J, Johnson D, Williams A (2005) Gray’s anatomy, 39th edn. Churchill Livingstone, London, pp 817–852
Staubersand J, Li Y (1996) Zum Feinbau der Fascia cruris mit besonderer intrafaszialer nerven. Manuelle Medizin, vol 34. Springer, Heidelberg, pp 196–200
Stecco C, Porzionato A, Macchi V, Tiengo C, Parenti A, Aldegheri R, Delmas V, De Caro R (2006) Histological characteristics of the deep fascia of the upper limb. Ital J Anat Embryol 111:105–110
Stecco C, Gagey O, Belloni A, Pozzuoli A, Porzionato A, Macchi V, Aldegheri R, De Caro R, Delmas V (2007) Anatomy of the deep fascia of the upper limb second part: study of innervation. Morphologie 91:38–43
Stecco C, Porzionato A, Macchi V, Parenti A, Aldegheri R, Delmas V, De Caro R (2008) The expansions of the pectoral girdle muscles onto the brachial fascia: morphological aspects and spatial disposition. Cell Tissues Organ 19 (Epub ahead of print)
Stecco L (1996) La Manipolazione Neuroconnettivale. Marrapese, Roma, pp 45–62
Stecco L (2004) Fascial manipulation for musculoskeletal pain. Piccin, Padova, pp 123–130
Stecco L, Stecco C (2007) Manipolazione fasciale. Parte pratica. Piccin, Padova, pp 3–29
Stefanelli A (1968) Anatomia comparata: morfologia dei vertebrati. Ed. dell’Ateneo, Roma
Stilwell D (1957) Regional variations in the innervation of deep fasciae and aponeuroses. Anat Rec 23:94–104
Tebbetts JB (2004) Does fascia provide additional, meaningful coverage over a breast implant? Plast Reconstr Surg 113:777–779
Testut JL, Jacob O (1905) Précis d’anatomie topographique avec applications medico-chirurgicales, vol III. Gaston Doin et Cie, Paris, p 302
Vleeming A, Stoeckart R, Snijders CJ (1995) The posterior layer of the thoracolumbar fascia. Spine 20:753–758
Yahia H, Rhalmi S, Newman N (1992) Sensory innervation of human thoracolumbar fascia, an immunohistochemical study. Acta Orthop Scand 63:195–197
Yucesoy CA, Maas H, Koopman B, Grootenboer HJ, Huijing PA (2006) Mechanisms causing effects of muscle position on proximo-distal muscle force differences in extra-muscular myofascial force transmission. Med Eng Phys 28:214–226
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stecco, A., Macchi, V., Masiero, S. et al. Pectoral and femoral fasciae: common aspects and regional specializations. Surg Radiol Anat 31, 35–42 (2009). https://doi.org/10.1007/s00276-008-0395-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00276-008-0395-5